Design and Evaluation of Pegylated Large 3D Pore Ferrisilicate as a Potential Insulin Protein Therapy to Treat Diabetic Mellitus
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis
2.1.1. Synthesis of Ferrisilicate Using Hydrothermal Technique
2.1.2. Synthesis of Iron-Impregnated Structured Silica (10 wt% Fe/KIT-6 and 10 wt% Fe/MSU-F) Using Impregnation Technique
2.1.3. Insulin/Ferrisilicate
2.1.4. Insulin/Ferrisilicate/PEGylation
2.2. Characterization Techniques
2.3. Insulin Entrapment Efficiency and Loading Capacity
2.4. Insulin Release Study
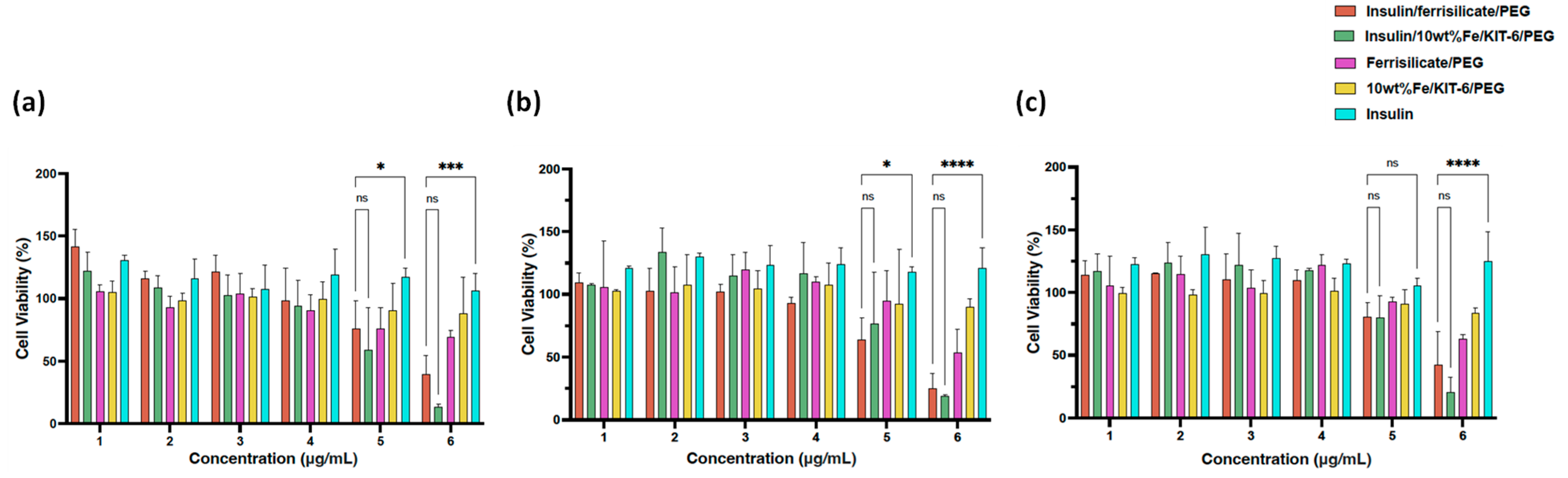
2.5. Cytotoxicity of Insulin/Ferrisilicate Nanoformulation against HFF-1 Cells
2.6. Statistics
2.7. In Vivo Study
3. Results and Discussion
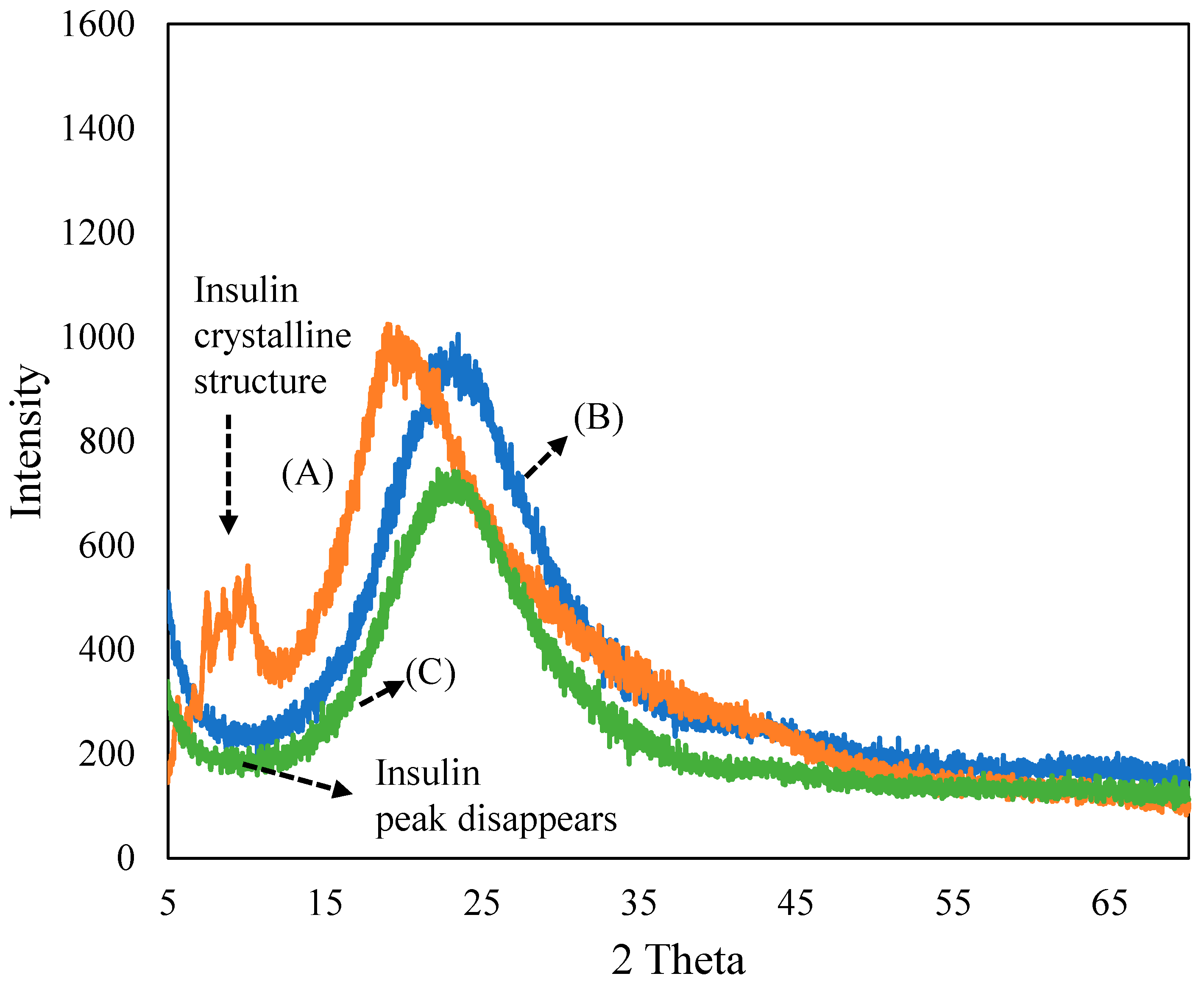
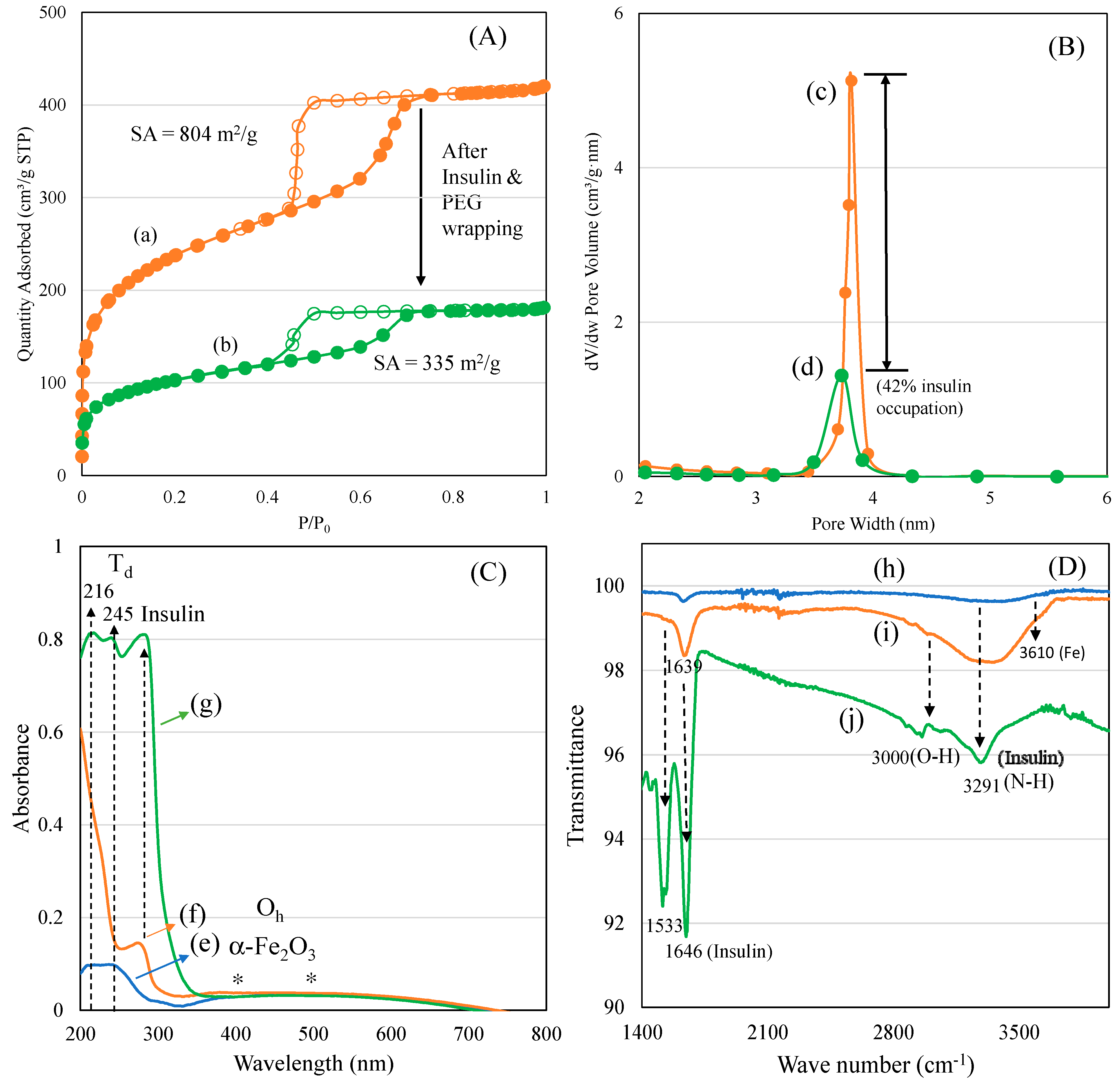
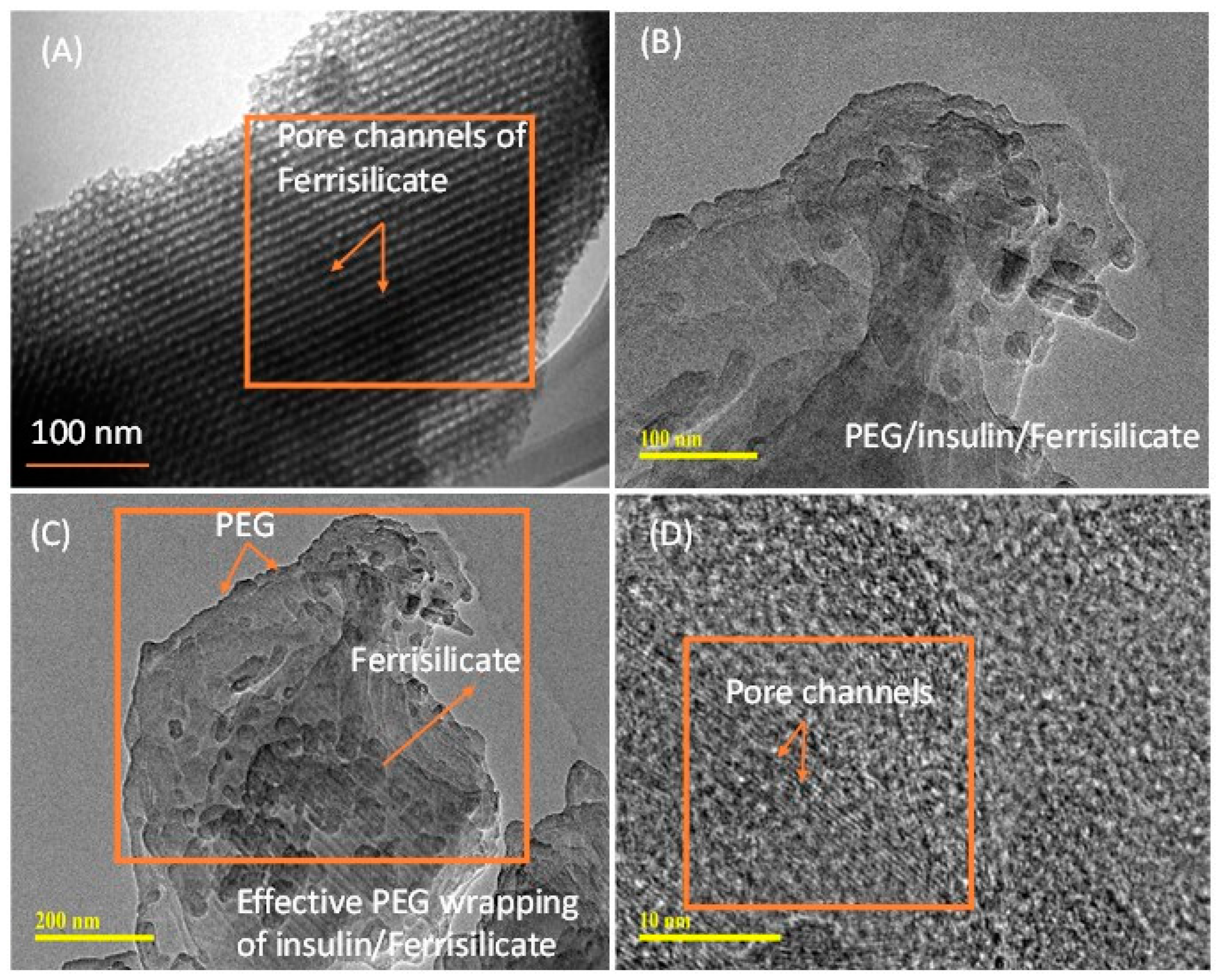
3.1. Characterization of Ferrisilicate/Insulin/PEG
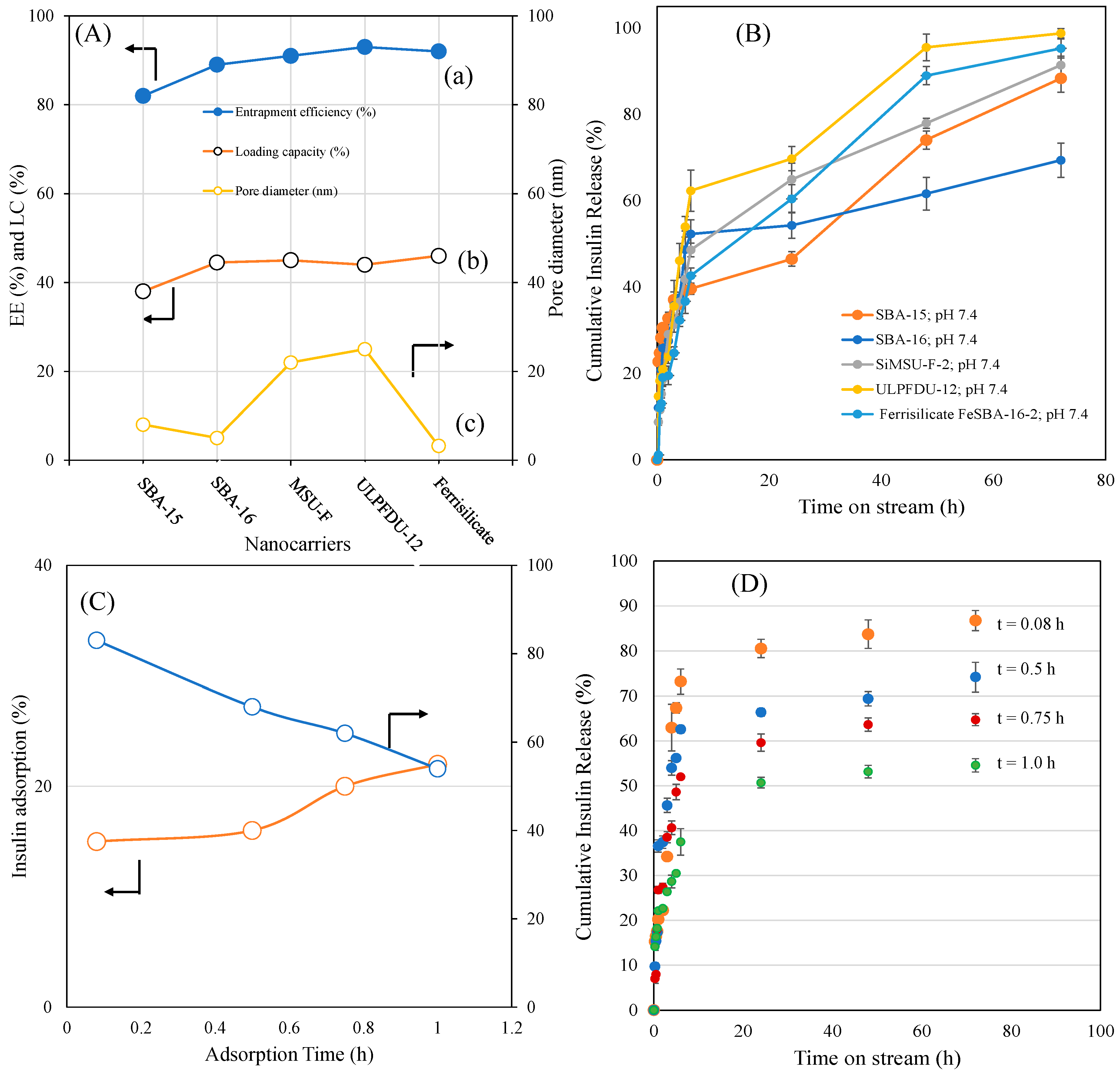
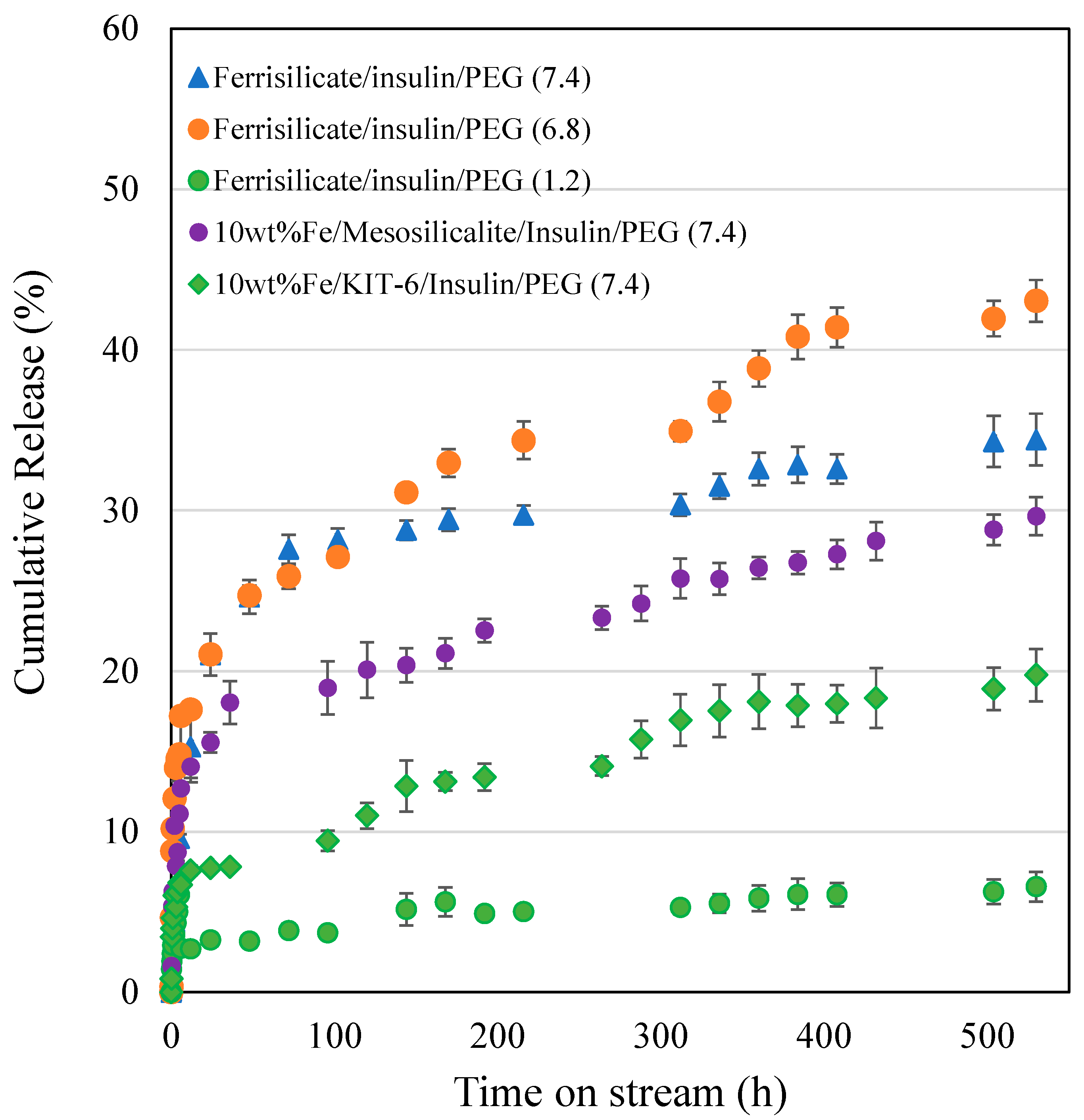
3.2. Kinetics of Different Ferrisilicate/Insulin Nanoformulation Drug Release Using the Korsmeyer–Peppas Model
3.3. In Vitro Study
3.4. In Vivo Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Scholten, B.J.; Kreiner, F.F.; Gough, S.C.L.; von Herrath, M. Current and future therapies for type 1 diabetes. Diabetologia 2021, 64, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A review of current trends with Type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab. Syndr. Obes. 2021, 14, 3567. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas. Available online: https://diabetesatlas.org (accessed on 20 January 2023).
- Vecchio, I.; Tornali, C.; Bragazzi, N.L.; Martini, M. The discovery of insulin: An important milestone in the history of medicine. Front. Endocrinol. 2018, 9, 613. [Google Scholar] [CrossRef]
- Holleman, F.; Gale, E.A. Nice insulins, pity about the evidence. Diabetologia 2007, 50, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Manikandan, S.; Jayanthi, M. Practitioners section-Newer insulin analogues and inhaled insulin. Indian J. Med. Sci. 2006, 60, 117–123. [Google Scholar] [CrossRef]
- Jonasson, J.; Ljung, R.; Talbäck, M.; Haglund, B.; Gudbjörnsdòttir, S.; Steineck, G. Insulin glargine use and short-term incidence of malignancies—A population-based follow-up study in Sweden. Diabetologia 2009, 52, 1745–1754. [Google Scholar] [CrossRef]
- Najmeddine, A.A.; Saeed, M.; Beadham, I.G.; ElShaer, A. Efficacy and safety of glucose sensors for delivery of insulin: A Systematic Review. PharmaNutrition 2021, 18, 100280. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhai, J.; Xiao, Q.; Zhao, S.; Li, C. Polysaccharide/mesoporous silica nanoparticle-based drug delivery systems: A review. Int. J. Biol. Macromol. 2021, 193, 457–473. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, P.; Chen, M.; Jin, T.; Wu, H.; Hei, M.; Wang, C.; Xu, Y.; Qian, X.; Zhu, W. On-demand transdermal insulin delivery system for type 1 diabetes therapy with no hypoglycemia risks. J. Colloid Interface Sci. 2022, 605, 582–591. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Ren, S.; Pan, J.; Wang, Y.; Shen, Y.; Zeng, Z.; Cui, H.; Zhao, X. Versatile Oral Insulin Delivery Nanosystems: From Materials to Nanostructures. Int. J. Mol. Sci. 2022, 23, 3362. [Google Scholar] [CrossRef]
- Tong, X.; Li, Z.; Chen, W.; Wang, J.; Li, X.; Mu, J.; Tang, Y.; Li, L. Efficient catalytic ozonation of diclofenac by three-dimensional iron (Fe)-doped SBA-16 mesoporous structures. J. Colloid Interface Sci. 2020, 578, 461–470. [Google Scholar] [CrossRef]
- Kunde, G.B.; Yadav, G.D. Green strategy for the synthesis of mesoporous, free-standing MAl2O4 (M = Fe, Co, Ni, Cu) spinel films by sol–gel method. Mater. Sci. Eng. B 2021, 271, 115244. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Jermy, B.R.; Alomari, M.; Ravinayagam, V.; Almofty, S.A.; Akhtar, S.; Borgio, J.F.; AbdulAzeez, S. SPIONs/3D SiSBA-16 based Multifunctional Nanoformulation for target specific cisplatin release in colon and cervical cancer cell lines. Sci. Rep. 2019, 9, 14523. [Google Scholar] [CrossRef]
- Balasamy, R.J.; Ravinayagam, V.; Alomari, M.; Ansari, M.A.; Almofty, S.A.; Rehman, S.; Dafalla, H.; Marimuthu, P.R.; Akhtar, S.; Al Hamad, M. Cisplatin delivery, anticancer and antibacterial properties of Fe/SBA-16/ZIF-8 nanocomposite. RSC Adv. 2019, 9, 42395–42408. [Google Scholar] [CrossRef]
- Jose, S.; Senthilkumar, M.; Elayaraja, K.; Haris, M.; George, A.; Raj, A.D.; Sundaram, S.J.; Bashir, A.K.; Maaza, M.; Kaviyarasu, K. Preparation and characterization of Fe doped n-hydroxyapatite for biomedical application. Surf. Interfaces 2021, 25, 101185. [Google Scholar] [CrossRef]
- Janarthanan, G.; Noh, I. Recent trends in metal ion based hydrogel biomaterials for tissue engineering and other biomedical applications. J. Mater. Sci. Technol. 2021, 63, 35–53. [Google Scholar] [CrossRef]
- Jermy, B.R.; Kim, S.Y.; Bineesh, K.V.; Selvaraj, M.; Park, D.W. Easy route for the synthesis of Fe-SBA-16 at weak acidity and its catalytic activity in the oxidation of cyclohexene. Microporous Mesoporous Mater. 2009, 121, 103–113. [Google Scholar] [CrossRef]
- Andreani, T.; de Souza, A.L.R.; Kiill, C.P.; Lorenzon, E.N.; Fangueiro, J.F.; Calpena, A.C.; Chaud, M.V.; Garcia, M.L.; Gremiao, M.P.D.; Silva, A.M.; et al. Preparation and characterization of PEG-coated silica nanoparticles for oral insulin delivery. Int. J. Pharm. 2014, 473, 627–635. [Google Scholar] [CrossRef]
- Nakai, M.; Miyake, K.; Inoue, R.; Ono, K.; Al Jabri, H.; Hirota, Y.; Uchida, Y.; Miyamoto, M.; Nishiyama, N. Synthesis of high silica* BEA type ferrisilicate (Fe-Beta) by dry gel conversion method using dealuminated zeolites and its catalytic performance on acetone to olefins (ATO) reaction. Microporous Mesoporous Mater. 2019, 273, 189–195. [Google Scholar] [CrossRef]
- Bhaumik, A.; Samanta, S.; Mal, N.K. Iron oxide nanoparticles stabilized inside highly ordered mesoporous silica. Pramana 2005, 65, 855–862. [Google Scholar] [CrossRef]
- Hinds, K.D.; Kim, S.W. Effects of PEG conjugation on Insulin properties. Adv. Drug Deliv. Rev. 2002, 54, 505–530. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Feng, Y.; Tang, X.; Yi, H.; Zhao, S.; Gao, F.; Zhou, Y.; Zhang, Y.; Zhuang, R. A novel ferrisilicate MEL zeolite with bi-functional adsorption/catalytic oxidation properties for non-methane hydrocarbon removal from cooking oil fumes. Microporous Mesoporous Mater. 2020, 309, 110509. [Google Scholar] [CrossRef]
- Massaro, M.; Cavallaro, G.; Colletti, C.G.; D’Azzo, G.; Guernelli, S.; Lazzara, G.; Pieraccini, S.; Riela, S. Halloysite nanotubes for efficient loading, stabilization and controlled release of insulin. J. Colloid Interface Sci. 2018, 524, 156–164. [Google Scholar] [CrossRef]
- Kim, M.K.; Ki, D.H.; Na, Y.G.; Lee, H.S.; Baek, J.S.; Lee, J.Y.; Lee, H.K.; Cho, C.W. Optimization of Mesoporous Silica Nanoparticles through Statistical Design of Experiment and the Application for the Anticancer Drug. Pharmaceutics 2021, 13, 184. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, H.; Wang, L.; Shen, D.; Chen, X.; Wang, N. Synthesis and testing of polymer grafted mesoporous silica as glucose-responsive insulin release drug delivery systems. Eur. Polym. J. 2021, 157, 110651. [Google Scholar] [CrossRef]
- Gaballa, H.; Theato, P. Glucose-responsive polymeric micelles via boronic acid–diol complexation for insulin delivery at neutral pH. Biomacromolecules 2019, 20, 871–881. [Google Scholar] [CrossRef]
- Luo, L.; Song, R.; Chen, J.; Zhou, B.; Mao, X.; Tang, S. Fluorophenylboronic acid substituted chitosan for insulin loading and release. React. Funct. Polym. 2020, 146, 104435. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Q.; He, D.; Liu, G.; Tang, D.; Liu, L.; Wu, J. A new Glucose-Responsive delivery system based on Sulfonamide-phenylboronic acid for subcutaneous insulin injection. Eur. Polym. J. 2021, 157, 110648. [Google Scholar] [CrossRef]
- Lin, K.; Yi, J.; Mao, X.; Wu, H.; Zhang, L.M.; Yang, L. Glucose-sensitive hydrogels from covalently modified carboxylated pullulan and concanavalin A for smart controlled release of insulin. React. Funct. Polym. 2019, 139, 112–119. [Google Scholar] [CrossRef]
- Yin, R.; Bai, M.; He, J.; Nie, J.; Zhang, W. Concanavalin A-sugar affinity based system: Binding interactions, principle of glucose-responsiveness, and modulated insulin release for diabetes care. Int. J. Biol. Macromol. 2019, 124, 724–732. [Google Scholar] [CrossRef]
- Xu, M.; Huang, J.; Jiang, S.; He, J.; Wang, Z.; Qin, H.; Guan, Y.Q. Glucose sensitive konjac glucomannan/concanavalin A nanoparticles as oral insulin delivery system. Int. J. Biol. Macromol. 2022, 202, 296–308. [Google Scholar] [CrossRef]
- Chai, Z.; Dong, H.; Sun, X.; Fan, Y.; Wang, Y.; Huang, F. Development of glucose oxidase-immobilized alginate nanoparticles for enhanced glucose-triggered insulin delivery in diabetic mice. Int. J. Biol. Macromol. 2020, 159, 640–647. [Google Scholar] [CrossRef]
- Wu, J.Z.; Williams, G.R.; Li, H.Y.; Wang, D.; Wu, H.; Li, S.D.; Zhu, L.M. Glucose-and temperature-sensitive nanoparticles for insulin delivery. Int. J. Nanomed. 2017, 12, 4037. [Google Scholar] [CrossRef]
- Jana, B.A.; Shinde, U.; Wadhwani, A. Synthetic enzyme-based nanoparticles act as smart catalyst for glucose responsive release of insulin. J. Biotechnol. 2020, 324, 1–6. [Google Scholar] [CrossRef]
- Ma, R.; Yang, H.; Li, Z.; Liu, G.; Sun, X.; Liu, X.; An, Y.; Shi, L. Phenylboronic acid-based complex micelles with enhanced glucose-responsiveness at physiological pH by complexation with glycopolymer. Biomacromolecules 2012, 13, 3409–3417. [Google Scholar] [CrossRef]

| Nanocarriers | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| Ferrisilicate | 804 | 0.65 | 3.2 |
| Insulin/Ferrisilicate/PEG | 335 | 0.28 | 3.3 |
| Insulin/Fe-Mesosilicalite/PEG | 360 | 0.38 | 4.2 |
| Insulin/Fe-KIT-6/PEG | 560 | 0.84 | 5.9 |
| Drug Formulations | k (h−n) | n | n−0.45 | R2 |
|---|---|---|---|---|
| SBA-15; pH 7.4 | 25.2086 ± 3.4391 | 0.2671 ± 0.0596 | 0.1829 | 0.9088 |
| SBA-16; pH 7.4 | 21.9335 ± 3.2175 | 0.2882 ± 0.0638 | 0.1618 | 0.9102 |
| MSU-F; pH 7.4 | 18.0398 ± 2.4209 | 0.4153 ± 0.0587 | 0.0347 | 0.9613 |
| ULPFDU-12; pH 7.4 | 18.0954 ± 2.9981 | 0.4337 ± 0.0714 | 0.0163 | 0.9482 |
| Ferrisilicate FeSBA-16; pH 7.4 | 13.7013 ± 2.1190 | 0.4879 ± 0.0670 | 0.0379 | 0.9634 |
| Ferrisilicate; Adsorp time = 5 min | 23.5404 ± 4.6002 | 0.3746 ± 0.0832 | 0.0754 | 0.9097 |
| Ferrisilicate; Adsorp time = 30 min | 27.6661 ± 5.4414 | 0.2840 ± 0.0837 | 0.1660 | 0.8512 |
| Ferrisilicate; Adsorp time = 45 min | 21.1912 ± 3.7614 | 0.3351 ± 0.0761 | 0.1149 | 0.9058 |
| Ferrisilicate; Adsorp time = 60 min | 20.1574 ± 1.2871 | 0.2555 ± 0.0288 | 0.1945 | 0.9750 |
| Insulin/Ferrisilicate ratio 0.125-11-7.4 | 21.6909 ± 2.1147 | 0.2558 ± 0.0507 | 0.1942 | 0.9353 |
| Insulin/Ferrisilicate ratio 0.25-12-7.4 | 26.0871 ± 3.4667 | 0.2814 ± 0.0680 | 0.1686 | 0.9067 |
| Insulin/Ferrisilicate ratio 0.75-13-7.4 | 21.9251 ± 4.1966 | 0.4021 ± 0.0955 | 0.0479 | 0.9097 |
| Insulin/Ferrisilicate ratio 1.0-14-7.4 | 36.3453 ± 3.6093 | 0.2102 ± 0.0516 | 0.2398 | 0.9040 |
| Ferrisilicate/insulin/PEG (7.4) | 1.4153 ± 0.5882 | 0.5722 ± 0.0837 | 0.1222 | 0.9013 |
| Ferrisilicate/insulin/PEG (6.8) | 7.8563 ± 1.3468 | 0.2728 ± 0.0381 | 0.1772 | 0.9093 |
| Ferrisilicate/insulin/PEG (1.2) | 2.6017 ± 0.1705 | 0.1791 ± 0.0151 | 0.2709 | 0.9631 |
| 10 wt% Fe/Mesosilicalite/insulin/PEG (7.4) | 5.4089 ± 0.9689 | 0.2771 ± 0.0385 | 0.1729 | 0.9016 |
| 10 wt % Fe/KIT-6/insulin/PEG (7.4) | 3.7004 ± 0.4118 | 0.2533 ± 0.0241 | 0.1967 | 0.9490 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jermy, B.R.; Salahuddin, M.; Tanimu, G.; Dafalla, H.; Almofty, S.; Ravinayagam, V. Design and Evaluation of Pegylated Large 3D Pore Ferrisilicate as a Potential Insulin Protein Therapy to Treat Diabetic Mellitus. Pharmaceutics 2023, 15, 593. https://doi.org/10.3390/pharmaceutics15020593
Jermy BR, Salahuddin M, Tanimu G, Dafalla H, Almofty S, Ravinayagam V. Design and Evaluation of Pegylated Large 3D Pore Ferrisilicate as a Potential Insulin Protein Therapy to Treat Diabetic Mellitus. Pharmaceutics. 2023; 15(2):593. https://doi.org/10.3390/pharmaceutics15020593
Chicago/Turabian StyleJermy, B. Rabindran, Mohammed Salahuddin, Gazali Tanimu, Hatim Dafalla, Sarah Almofty, and Vijaya Ravinayagam. 2023. "Design and Evaluation of Pegylated Large 3D Pore Ferrisilicate as a Potential Insulin Protein Therapy to Treat Diabetic Mellitus" Pharmaceutics 15, no. 2: 593. https://doi.org/10.3390/pharmaceutics15020593
APA StyleJermy, B. R., Salahuddin, M., Tanimu, G., Dafalla, H., Almofty, S., & Ravinayagam, V. (2023). Design and Evaluation of Pegylated Large 3D Pore Ferrisilicate as a Potential Insulin Protein Therapy to Treat Diabetic Mellitus. Pharmaceutics, 15(2), 593. https://doi.org/10.3390/pharmaceutics15020593








