The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Fibril Preparation
2.2. Monitoring Fibril Growth Using a ThT Fluorescence Assay
2.3. Preparation of HEWL Fibril–Polysaccharide Mixtures
2.4. CD Spectroscopy
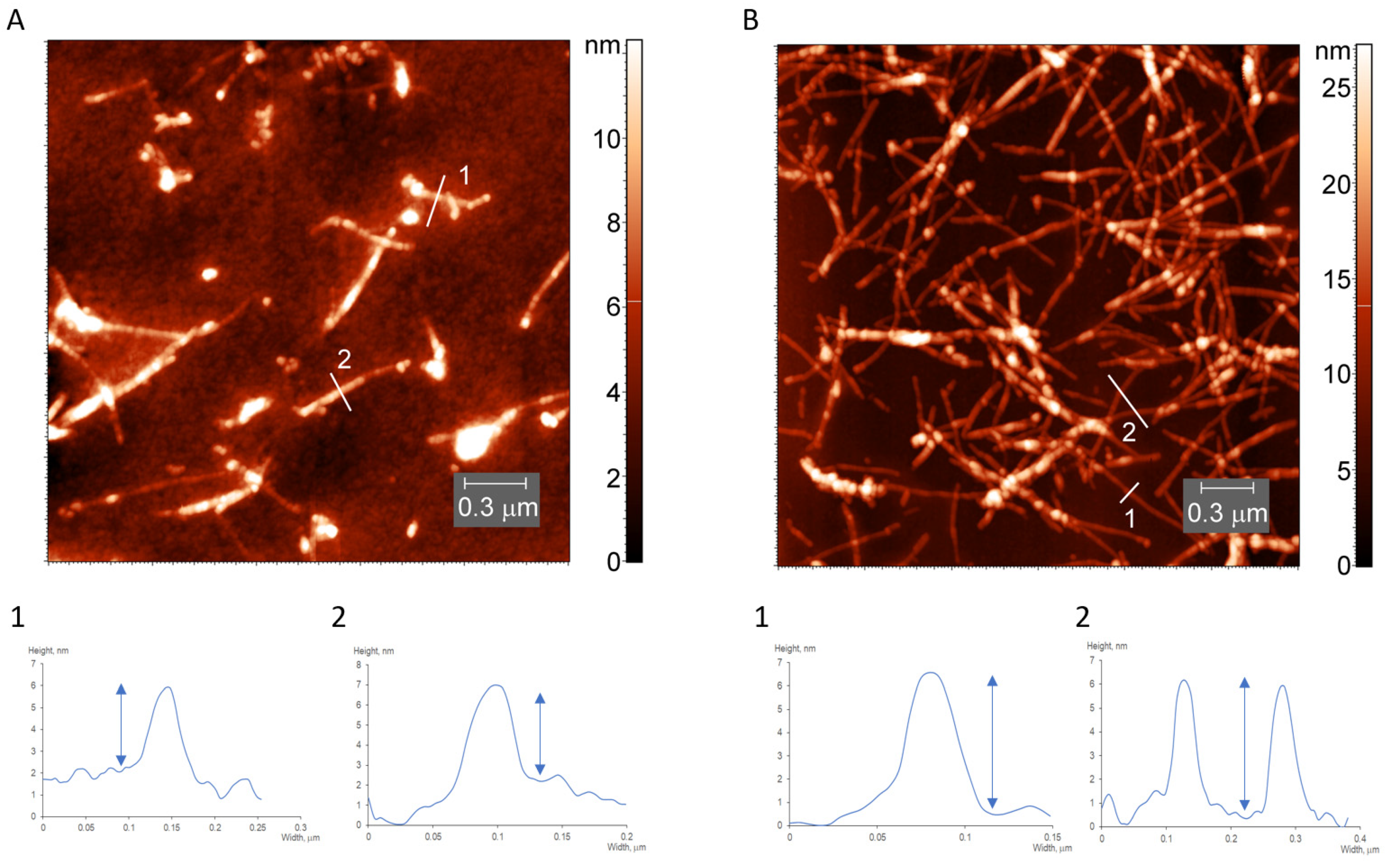
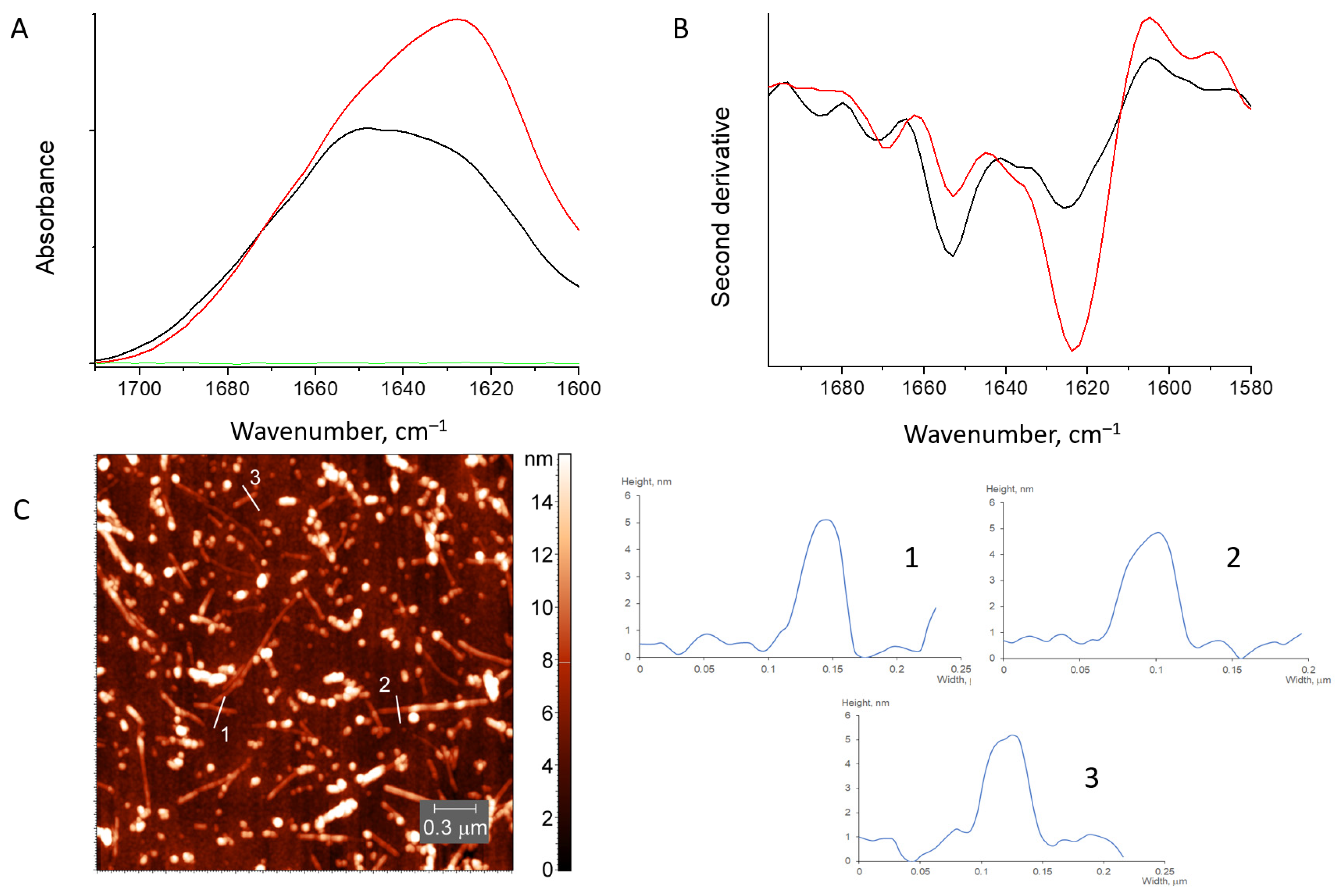
2.5. AFM Measurements
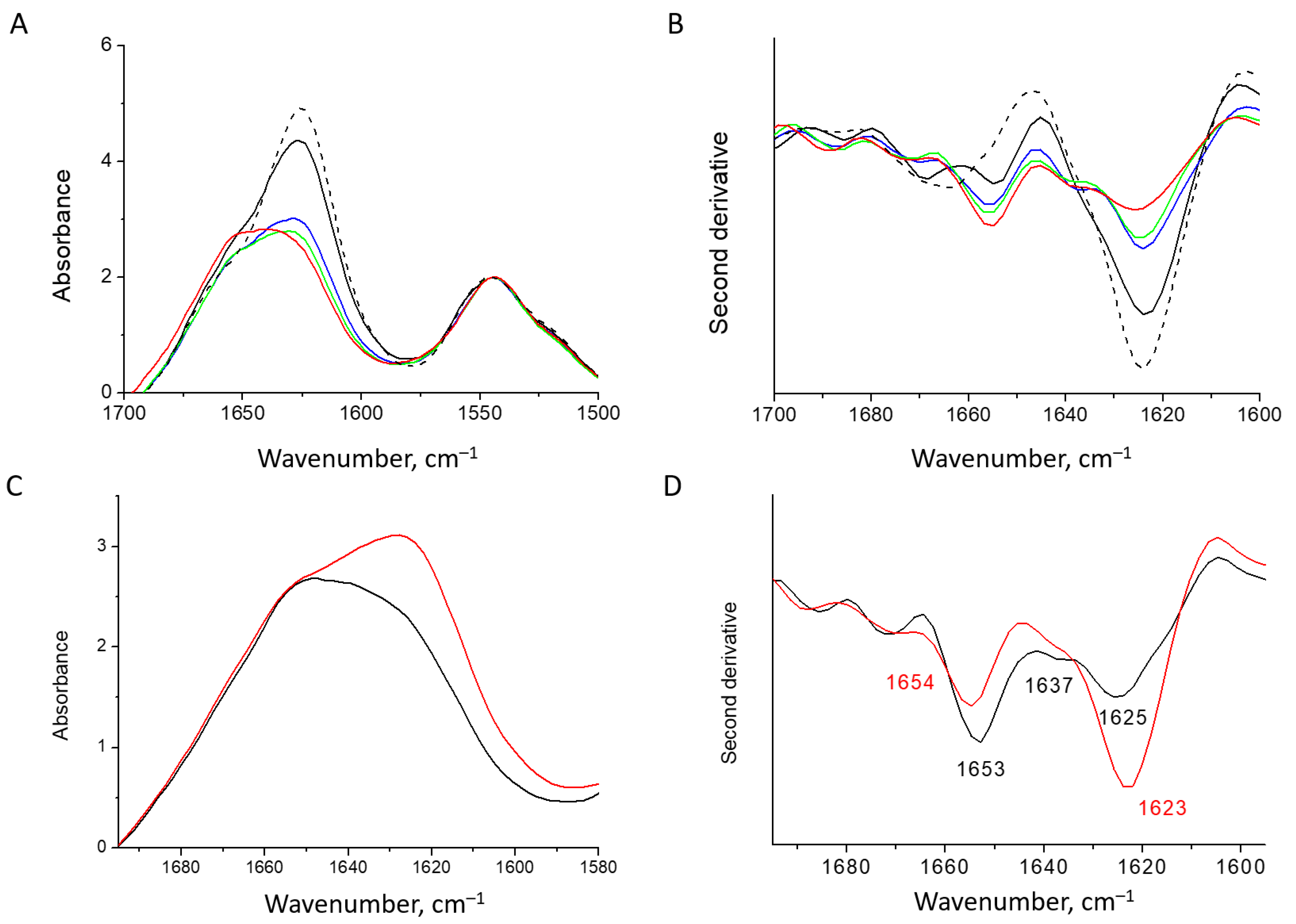
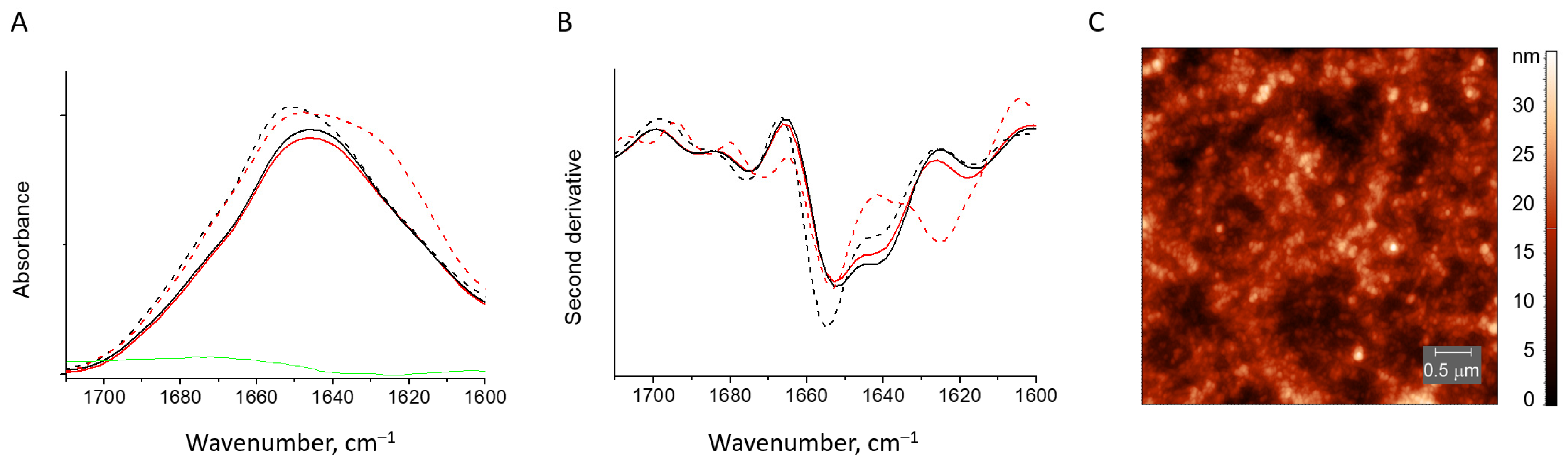
2.6. FTIR Spectroscopy
3. Results
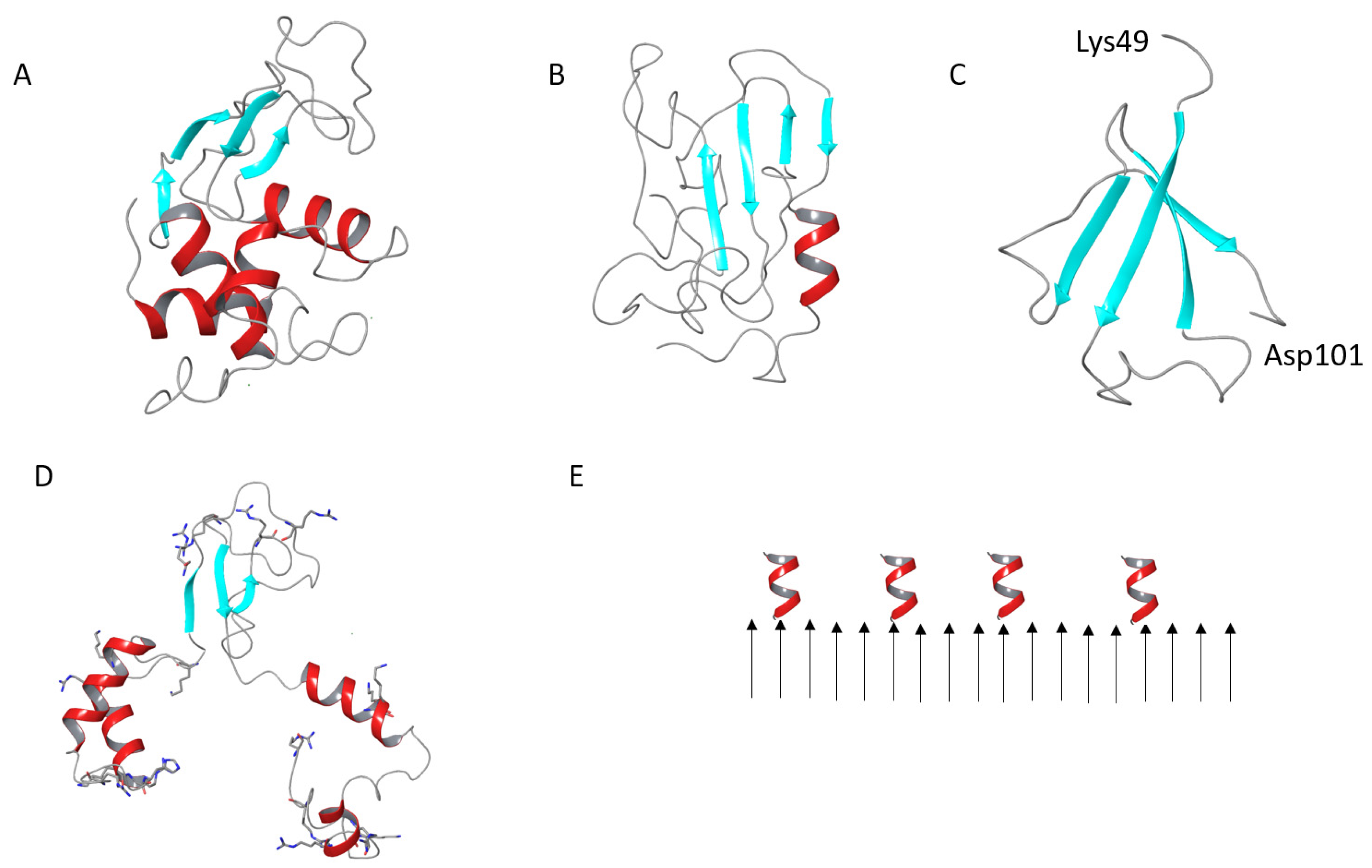
3.1. Characterization of Water-Soluble HEWL Fibrils
3.2. HEWL Fibril–Polysaccharide Mixtures
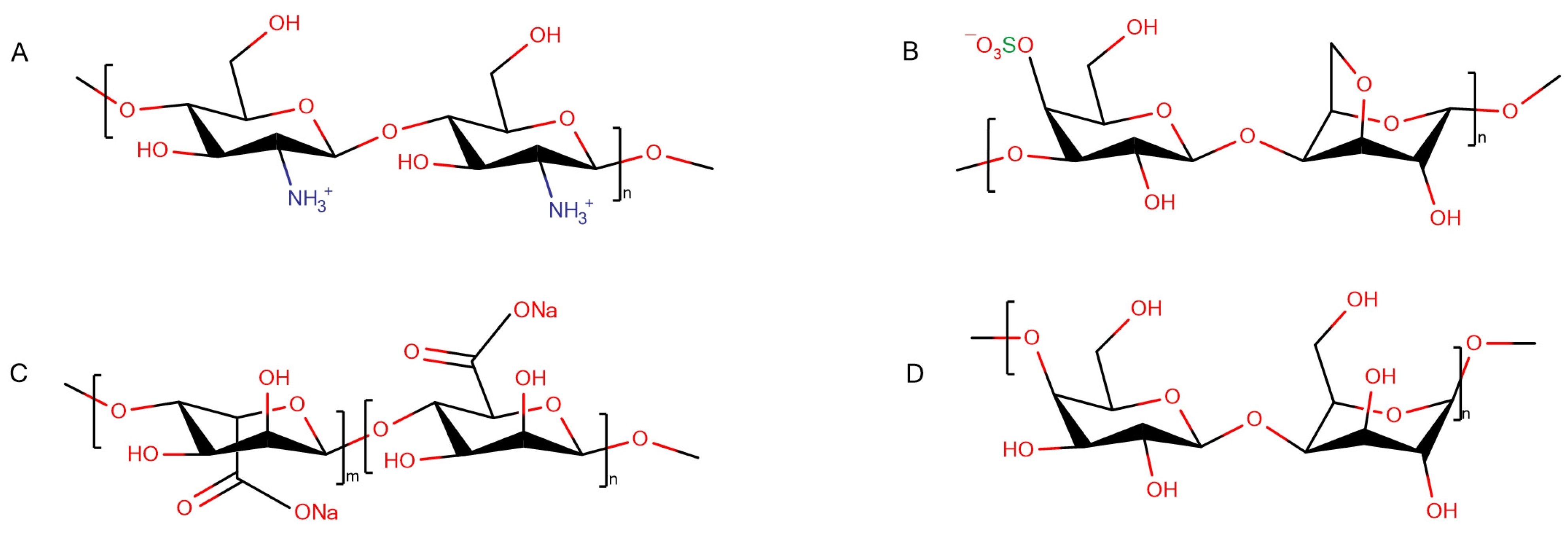
3.2.1. HEWL Fibril–Chitosan Mixtures
3.2.2. HEWL Fibril–β-(1,4)-Galactan Mixtures
3.2.3. HEWL Fibril–κ-Carrageenan Gels
3.2.4. HEWL Fibril–Sodium Alginate Gels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boersema, P.J.; Melnik, A.; Hazenberg, B.P.C.; Rezeli, M.; Marko-Varga, G.; Kamiie, J.; Portelius, E.; Blennow, K.; Zubarev, R.A.; Polymenidou, M.; et al. Biology/Disease-Driven Initiative on Protein-Aggregation Diseases of the Human Proteome Project: Goals and Progress to Date. J. Proteome Res. 2018, 17, 4072–4084. [Google Scholar] [CrossRef] [PubMed]
- Debnath, K.; Sarkar, A.K.; Jana, N.R.; Jana, N.R. Inhibiting Protein Aggregation by Small Molecule-Based Colloidal Nanoparticles. Acc. Mater. Res. 2022, 3, 54–66. [Google Scholar] [CrossRef]
- Wu, L.; Velander, P.; Brown, A.M.; Wang, Y.; Liu, D.; Bevan, D.R.; Zhang, S.; Xu, B. Rosmarinic Acid Potently Detoxifies Amylin Amyloid and Ameliorates Diabetic Pathology in a Transgenic Rat Model of Type 2 Diabetes. ACS Pharmacol. Transl. Sci. 2021, 4, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Azzam, S.K.; Jang, H.; Choi, M.C.; Alsafar, H.; Lukman, S.; Lee, S. Inhibition of Human Amylin Aggregation and Cellular Toxicity by Lipoic Acid and Ascorbic Acid. Mol. Pharm. 2018, 15, 2098–2106. [Google Scholar] [CrossRef]
- Tran, C.H.; Saha, R.; Blanco, C.; Bagchi, D.; Chen, I.A. Modulation of A-Synuclein Aggregation in Vitro by a DNA Aptamer. Biochemistry 2022, 61, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Makshakova, O.N.; Bogdanova, L.R.; Faizullin, D.A.; Ermakova, E.A.; Zuev, Y.F.; Sedov, I.A. Interaction-Induced Structural Transformation of Lysozyme and Kappa-Carrageenan in Binary Complexes. Carbohydr. Polym. 2021, 252, 117181. [Google Scholar] [CrossRef] [PubMed]
- Makshakova, O.N.; Zuev, Y.F. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels 2022, 8, 287. [Google Scholar] [CrossRef]
- Ghahghaei, A.; Faridi, N. Review: Structure of Amyloid Fibril in Diseases. J. Biomed. Sci. Eng. 2009, 2, 345–358. [Google Scholar] [CrossRef]
- Ke, P.C.; Zhou, R.; Serpell, L.C.; Riek, R.; Knowles, T.P.J.; Lashuel, H.A.; Gazit, E.; Hamley, I.W.; Davis, T.P.; Fandrich, M.; et al. Half a Century of Amyloids: Past, Present and Future. Chem. Soc. Rev. 2020, 49, 5473–5509. [Google Scholar] [CrossRef]
- Giorgetti, S.; Greco, C.; Tortora, P.; Aprile, F.A. Targeting Amyloid Aggregation: An Overview of Strategies and Mechanisms. Int. J. Mol. Sci. 2018, 19, 2677. [Google Scholar] [CrossRef] [Green Version]
- Holubová, M.; Štěpánek, P.; Hrubý, M. Polymer Materials as Promoters/Inhibitors of Amyloid Fibril Formation. Colloid Polym. Sci. 2021, 299, 343–362. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Mezzenga, R. Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nian, Y.; Zhang, Z.; Chen, Q.; Zeng, X.; Hu, B. High Internal Phase Emulsions Stabilized with Amyloid Fibrils and Their Polysaccharide Complexes for Encapsulation and Protection of Β-Carotene. Colloids Surf. B Biointerfaces 2019, 183, 110459. [Google Scholar] [CrossRef] [PubMed]
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.I.; Merlini, G.; Saraiva, M.J.; Westermark, P. Amyloid Fibril Proteins and Amyloidosis: Chemical Identification and Clinical Classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 2016, 23, 209–213. [Google Scholar] [CrossRef]
- Eanes, E.D.; Glenner, G.G. X-Ray Diffraction Studies on Amyloid Filaments. J. Histochem. Cytochem. 1968, 16, 673–677. [Google Scholar] [CrossRef]
- Willbold, D.; Strodel, B.; Schroder, G.F.; Hoyer, W.; Heise, H. Amyloid-Type Protein Aggregation and Prion-Like Properties of Amyloids. Chem. Rev. 2021, 121, 8285–8307. [Google Scholar] [CrossRef]
- Rambaran, R.N.; Serpell, L.C. Amyloid Fibrils: Abnormal Protein Assembly. Prion 2008, 2, 112–117. [Google Scholar] [CrossRef]
- Langkilde, A.E.; Vestergaard, B. Methods for Structural Characterization of Prefibrillar Intermediates and Amyloid Fibrils. FEBS Lett. 2009, 583, 2600–2609. [Google Scholar] [CrossRef]
- Swaminathan, R.; Ravi, V.K.; Kumar, S.; Kumar, M.V.; Chandra, N. Lysozyme: A Model Protein for Amyloid Research. Adv. Protein Chem. Struct. Biol. 2011, 84, 63–111. [Google Scholar] [CrossRef]
- Lara, C.; Adamcik, J.; Jordens, S.; Mezzenga, R. General Self-Assembly Mechanism Converting Hydrolyzed Globular Proteins into Giant Multistranded Amyloid Ribbons. Biomacromolecules 2011, 12, 1868–1875. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Irace, G.; Sirangelo, I. The Effect of Glycosaminoglycans (Gags) on Amyloid Aggregation and Toxicity. Molecules 2015, 20, 2510–2528. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.D.; Cummings, J.A.; Lake, T. The Unifying Hypothesis of Alzheimer’s Disease: Heparan Sulfate Proteoglycans/Glycosaminoglycans Are Key as First Hypothesized over 30 Years Ago. Front. Aging Neurosci. 2021, 13, 710683. [Google Scholar] [CrossRef] [PubMed]
- Magnus, J.H.; Husby, G.; Kolset, S.O. Presence of Glycosaminoglycans in Purified Aa Type Amyloid Fibrils Associated with Juvenile Rheumatoid Arthritis. Ann. Rheum. Dis. 1989, 48, 215–219. [Google Scholar] [CrossRef]
- Torres-Bugeau, C.M.; Ávila, C.L.; Raisman-Vozari, R.; Papy-Garcia, D.; Itri, R.; Barbosa, L.R.; Cortez, L.M.; Sim, V.L.; Chehín, R.N. Characterization of Heparin-Induced Glyceraldehyde-3-Phosphate Dehydrogenase Early Amyloid-Like Oligomers and Their Implication in A-Synuclein Aggregation. J. Biol. Chem. 2012, 287, 2398–2409. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Siedlak, S.L.; Fortino, A.E.; Perry, G.; Ghetti, B.; Smith, M.A. Chitin-Like Polysaccharides in Alzheimer’s Disease Brains. Curr. Alzheimer Res. 2005, 2, 419–423. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G.; Smith, M.A. The Role of Novel Chitin-Like Polysaccharides in Alzheimer Disease. Neurotox. Res. 2007, 12, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Arimon, M.; Valle-Delgado, J.J.; García, R.; Durany, N.; Castel, S.; Cruz, M.; Ventura, S.; Fernàndez-Busquets, X. Sulfated Polysaccharides Promote the Assembly of Amyloid Beta(1-42) Peptide into Stable Fibrils of Reduced Cytotoxicity. J. Biol. Chem. 2008, 283, 32471–32483. [Google Scholar] [CrossRef]
- Takase, H.; Tanaka, M.; Yamamoto, A.; Watanabe, S.; Takahashi, S.; Nadanaka, S.; Kitagawa, H.; Yamada, T.; Mukai, T. Structural Requirements of Glycosaminoglycans for Facilitating Amyloid Fibril Formation of Human Serum Amyloid A. Amyloid 2016, 23, 67–75. [Google Scholar] [CrossRef]
- Cohlberg, J.A.; Li, J.; Uversky, V.N.; Fink, A.L. Heparin and Other Glycosaminoglycans Stimulate the Formation of Amyloid Fibrils from Alpha-Synuclein in Vitro. Biochemistry 2002, 41, 1502–1511. [Google Scholar] [CrossRef]
- Mehra, S.; Ghosh, D.; Kumar, R.; Mondal, M.; Gadhe, L.G.; Das, S.; Anoop, A.; Jha, N.N.; Jacob, R.S.; Chatterjee, D.; et al. Glycosaminoglycans Have Variable Effects on A-Synuclein Aggregation and Differentially Affect the Activities of the Resulting Amyloid Fibrils. J. Biol. Chem. 2018, 293, 12975–12991. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ojha, B.; Morris, C.; Jiang, M.; Wojcikiewicz, E.P.; Rao, P.P.; Du, D. Positively Charged Chitosan and N-Trimethyl Chitosan Inhibit Aβ40 Fibrillogenesis. Biomacromolecules 2015, 16, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hou, W.; Sun, Y.; Gao, Z.; Zhu, S.; Jiang, Z. Chitosan Oligosaccharides Inhibit/Disaggregate Fibrils and Attenuate Amyloid Beta-Mediated Neurotoxicity. Int. J. Mol. Sci. 2015, 16, 10526–10536. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Ghormade, V.; Kolge, H.; Paknikar, K.M. Dual Effect of Chitosan-Based Nanoparticles on the Inhibition of Β-Amyloid Peptide Aggregation and Disintegration of the Preformed Fibrils. J. Mater. Chem. B 2019, 7, 3362–3373. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Wang, H.; Cui, Z.B.; Yu, W.G.; Lu, X.Z. Chitosan Oligosaccharides Attenuate Amyloid Formation of Hiapp and Protect Pancreatic Beta-Cells from Cytotoxicity. Molecules 2020, 25, 1314. [Google Scholar] [CrossRef]
- Ow, S.Y.; Bekard, I.; Blencowe, A.; Qiao, G.G.; Dunstan, D.E. A Generic Class of Amyloid Fibril Inhibitors. J. Mater. Chem. B 2015, 3, 1350–1359. [Google Scholar] [CrossRef]
- Choudhary, S.; Save, S.N.; Vavilala, S.L. Unravelling the Inhibitory Activity of Chlamydomonas Reinhardtii Sulfated Polysaccharides against Alpha-Synuclein Fibrillation. Sci. Rep. 2018, 8, 5692. [Google Scholar] [CrossRef]
- Liang, Y.; Ueno, M.; Zha, S.; Okimura, T.; Jiang, Z.; Yamaguchi, K.; Hatakeyama, T.; Oda, T. Sulfated Polysaccharide Ascophyllan Prevents Amyloid Fibril Formation of Human Insulin and Inhibits Amyloid-Induced Hemolysis and Cytotoxicity in Pc12 Cells. Biosci. Biotechnol. Biochem. 2021, 85, 2281–2291. [Google Scholar] [CrossRef]
- Meng, F.; Raleigh, D.P. Inhibition of Glycosaminoglycan-Mediated Amyloid Formation by Islet Amyloid Polypeptide and Proiapp Processing Intermediates. J. Mol. Biol. 2011, 406, 491–502. [Google Scholar] [CrossRef]
- Kim, B.; Ko, Y.H.; Runfola, M.; Rapposelli, S.; Ortore, G.; Chiellini, G.; Kim, J.H. Diphenyl-Methane Based Thyromimetic Inhibitors for Transthyretin Amyloidosis. Int. J. Mol. Sci. 2021, 22, 3488. [Google Scholar] [CrossRef]
- Cohen, S.I.A.; Arosio, P.; Presto, J.; Kurudenkandy, F.R.; Biverstal, H.; Dolfe, L.; Dunning, C.; Yang, X.; Frohm, B.; Vendruscolo, M.; et al. A Molecular Chaperone Breaks the Catalytic Cycle That Generates Toxic Aβ Oligomers. Nat. Struct. Mol. Biol. 2015, 22, 207–213. [Google Scholar] [CrossRef]
- Sedov, I.; Khaibrakhmanova, D. Molecular Mechanisms of Inhibition of Protein Amyloid Fibril Formation: Evidence and Perspectives Based on Kinetic Models. Int. J. Mol. Sci. 2022, 23, 13428. [Google Scholar] [CrossRef] [PubMed]
- Törner, R.; Kupreichyk, T.; Gremer, L.; Debled, E.C.; Fenel, D.; Schemmert, S.; Gans, P.; Willbold, D.; Schoehn, G.; Hoyer, W.; et al. Structural Basis for the Inhibition of Iapp Fibril Formation by the Co-Chaperonin Prefoldin. Nat. Commun. 2022, 13, 2363. [Google Scholar] [CrossRef]
- Usuelli, M.; Germerdonk, T.; Cao, Y.; Peydayesh, M.; Bagnani, M.; Handschin, S.; Nystrom, G.; Mezzenga, R. Polysaccharide-Reinforced Amyloid Fibril Hydrogels and Aerogels. Nanoscale 2021, 13, 12534–12545. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, Y.; Li, K.; Zhong, C. Biofilm-Inspired Amyloid-Polysaccharide Composite Materials. Appl. Mater. Today 2022, 27, 101497. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to Measure and Predict the Molar Absorption Coefficient of a Protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Dichroweb, an Online Server for Protein Secondary Structure Analyses from Circular Dichroism Spectroscopic Data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular Structure and Properties of Kappa-Carrageenan-Gelatin Gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Safarova, E.R.; Zuev, Y.F. Structural Insights in Interactions between Rnase from Bacillus Intermedius and Rhamnogalacturonan I from Potato. Carbohydr. Polym. 2021, 251, 117038. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Faizullin, D.A.; Zuev, Y.F. Interplay between Secondary Structure and Ion Binding Upon Thermoreversible Gelation of Kappa-Carrageenan. Carbohydr. Polym. 2020, 227, 115342. [Google Scholar] [CrossRef] [PubMed]
- Mahdavimehr, M.; Meratan, A.A.; Ghobeh, M.; Ghasemi, A.; Saboury, A.A.; Nemat-Gorgani, M. Inhibition of Hewl Fibril Formation by Taxifolin: Mechanism of Action. PLoS ONE 2017, 12, e0187841. [Google Scholar] [CrossRef]
- Patel, P.; Parmar, K.; Patel, D.; Kumar, S.; Trivedi, M.; Das, M. Inhibition of Amyloid Fibril Formation of Lysozyme by Ascorbic Acid and a Probable Mechanism of Action. Int. J. Biol. Macromol. 2018, 114, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Arnaudov, L.N.; de Vries, R. Thermally Induced Fibrillar Aggregation of Hen Egg White Lysozyme. Biophys. J. 2005, 88, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Lara, C.; Usov, I.; Adamcik, J.; Mezzenga, R. Sub-Persistence-Length Complex Scaling Behavior in Lysozyme Amyloid Fibrils. Phys. Rev. Lett. 2011, 107, 238101. [Google Scholar] [CrossRef] [PubMed]
- Brudar, S.; Hribar-Lee, B. The Role of Buffers in Wild-Type Hewl Amyloid Fibril Formation Mechanism: A Methodological Approach. Methods Mol. Biol. 2023, 2551, 285–296. [Google Scholar] [CrossRef]
- Ow, S.Y.; Dunstan, D.E. The Effect of Concentration, Temperature and Stirring on Hen Egg White Lysozyme Amyloid Formation. Soft Matter 2013, 9, 9692–9701. [Google Scholar] [CrossRef]
- Mikalauskaite, K.; Ziaunys, M.; Smirnovas, V. Lysozyme Amyloid Fibril Structural Variability Dependence on Initial Protein Folding State. Int. J. Mol. Sci. 2022, 23, 5421. [Google Scholar] [CrossRef]
- Zurdo, J.; Guijarro, J.I.; Dobson, C.M. Preparation and Characterization of Purified Amyloid Fibrils. J. Am. Chem. Soc. 2001, 123, 8141–8142. [Google Scholar] [CrossRef] [PubMed]
- Sarroukh, R.; Goormaghtigh, E.; Ruysschaert, J.M.; Raussens, V. Atr-Ftir: A “Rejuvenated” Tool to Investigate Amyloid Proteins. Biochim. Biophys. Acta 2013, 1828, 2328–2338. [Google Scholar] [CrossRef]
- Islam, Z.; Ali, M.H.; Popelka, A.; Mall, R.; Ullah, E.; Ponraj, J.; Kolatkar, P.R. Probing the Fibrillation of Lysozyme by Nanoscale-Infrared Spectroscopy. J. Biomol. Struct. Dyn. 2021, 39, 1481–1490. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Y.; Hao, W.; Hu, X.; Ma, G. Parallel Β-Sheet Fibril and Antiparallel Β-Sheet Oligomer: New Insights into Amyloid Formation of Hen Egg White Lysozyme under Heat and Acidic Condition from Ftir Spectroscopy. J. Phys. Chem. B 2013, 117, 4003–4013. [Google Scholar] [CrossRef]
- Xing, L.; Fan, W.; Chen, N.; Li, M.; Zhou, X.; Liu, S. Amyloid Formation Kinetics of Hen Egg White Lysozyme under Heat and Acidic Conditions Revealed by Raman Spectroscopy. J. Raman Spectrosc. 2019, 50, 629–640. [Google Scholar] [CrossRef]
- Fujiwara, S.; Matsumoto, F.; Yonezawa, Y. Effects of Salt Concentration on Association of the Amyloid Protofilaments of Hen Egg White Lysozyme Studied by Time-Resolved Neutron Scattering. J. Mol. Biol. 2003, 331, 21–28. [Google Scholar] [CrossRef]
- Mari, E.; Ricci, C.; Pieraccini, S.; Spinozzi, F.; Mariani, P.; Ortore, M.G. Trehalose Effect on the Aggregation of Model Proteins into Amyloid Fibrils. Life 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Gustot, A.; Raussens, V.; Dehousse, M.; Dumoulin, M.; Bryant, C.E.; Ruysschaert, J.M.; Lonez, C. Activation of Innate Immunity by Lysozyme Fibrils Is Critically Dependent on Cross-Β Sheet Structure. Cell Mol. Life Sci. 2013, 70, 2999–3012. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.R.; Sunde, M.; Bellotti, V.; Robinson, C.V.; Hutchinson, W.L.; Fraser, P.E.; Hawkins, P.N.; Dobson, C.M.; Radford, S.E.; Blake, C.C.; et al. Instability, Unfolding and Aggregation of Human Lysozyme Variants Underlying Amyloid Fibrillogenesis. Nature 1997, 385, 787–793. [Google Scholar] [CrossRef]
- Hill, S.E.; Robinson, J.; Matthews, G.; Muschol, M. Amyloid Protofibrils of Lysozyme Nucleate and Grow Via Oligomer Fusion. Biophys. J. 2009, 96, 3781–3790. [Google Scholar] [CrossRef]
- Chalapathi, D.; Kumar, A.; Behera, P.; Sathi, S.N.; Swaminathan, R.; Narayana, C. Insights on Aggregation of Hen Egg-White Lysozyme from Raman Spectroscopy and Md Simulations. Molecules 2022, 27, 7122. [Google Scholar] [CrossRef]
- Zein, H.F.; Alam, I.; Asanithi, P.; Sutthibutpong, T. Molecular Dynamics Study on the Effects of Charged Amino Acid Distribution under Low Ph Condition to the Unfolding of Hen Egg White Lysozyme and Formation of Beta Strands. PLoS ONE 2022, 17, e0249742. [Google Scholar] [CrossRef]
- Ermakova, E.; Makshakova, O.; Zuev, Y.; Sedov, I. Beta-Rich Intermediates in Denaturation of Lysozyme: Accelerated Molecular Dynamics Simulations. J. Biomol. Struct. Dyn. 2021, 40, 13953–13964. [Google Scholar] [CrossRef]
- Frare, E.; Polverino De Laureto, P.; Zurdo, J.; Dobson, C.M.; Fontana, A. A Highly Amyloidogenic Region of Hen Lysozyme. J. Mol. Biol. 2004, 340, 1153–1165. [Google Scholar] [CrossRef]
- Sziegat, F.; Wirmer-Bartoschek, J.; Schwalbe, H. Characteristics of Human Lysozyme and Its Disease-Related Mutants in Their Unfolded States. Angew. Chem. Int. Ed. 2011, 50, 5514–5518. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, E.A.; Makshakova, O.N.; Zuev, Y.F.; Sedov, I.A. Fibril Fragments from the Amyloid Core of Lysozyme: An Accelerated Molecular Dynamics Study. J. Mol. Graph. Model. 2021, 106, 107917. [Google Scholar] [CrossRef] [PubMed]
- Kurouski, D.; Lu, X.; Popova, L.; Wan, W.; Shanmugasundaram, M.; Stubbs, G.; Dukor, R.K.; Lednev, I.K.; Nafie, L.A. Is Supramolecular Filament Chirality the Underlying Cause of Major Morphology Differences in Amyloid Fibrils? J. Am. Chem. Soc. 2014, 136, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, D.; Ebrahim-Habibi, A.; Moosavi-Movahedi, A.A.; Nemat-Gorgani, M. Chemical Modification of Lysine Residues in Lysozyme May Dramatically Influence Its Amyloid Fibrillation. Biochim. Biophys. Acta 2010, 1804, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Capocefalo, A.; Deckert-Gaudig, T.; Brasili, F.; Postorino, P.; Deckert, V. Unveiling the Interaction of Protein Fibrils with Gold Nanoparticles by Plasmon Enhanced Nano-Spectroscopy. Nanoscale 2021, 13, 14469–14479. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Antosova, A.; Gambucci, M.; Bednarikova, Z.; Albonetti, C.; Valle, F.; Sassi, P.; Latterini, L.; Gazova, Z.; Bystrenova, E. Effect of Metallic Nanoparticles on Amyloid Fibrils and Their Influence to Neural Cell Toxicity. Nano Res. 2020, 13, 1081–1089. [Google Scholar] [CrossRef]
- Kumar Ban, D.; Paul, S. Functionalized Gold and Silver Nanoparticles Modulate Amyloid Fibrillation, Defibrillation and Cytotoxicity of Lysozyme Via Altering Protein Surface Character. Appl. Surf. Sci. 2019, 473, 373–385. [Google Scholar] [CrossRef]
- Wang, M.; Kakinen, A.; Pilkington, E.H.; Davis, T.P.; Ke, P.C. Differential Effects of Silver and Iron Oxide Nanoparticles on Iapp Amyloid Aggregation. Biomater. Sci. 2017, 5, 485–493. [Google Scholar] [CrossRef]
- Semenyuk, P.I.; Moiseeva, E.V.; Stroylova, Y.Y.; Lotti, M.; Izumrudov, V.A.; Muronetz, V.I. Sulfated and Sulfonated Polymers Are Able to Solubilize Efficiently the Protein Aggregates of Different Nature. Arch. Biochem. Biophys. 2015, 567, 22–29. [Google Scholar] [CrossRef]
- Evstafyeva, D.B.; Izumrudov, V.A.; Muronetz, V.I.; Semenyuk, P.I. Tightly Bound Polyelectrolytes Enhance Enzyme Proteolysis and Destroy Amyloid Aggregates. Soft Matter 2018, 14, 3768–3773. [Google Scholar] [CrossRef]
- Balasco, N.; Diaferia, C.; Morelli, G.; Vitagliano, L.; Accardo, A. Amyloid-Like Aggregation in Diseases and Biomaterials: Osmosis of Structural Information. Front. Bioeng. Biotechnol. 2021, 9, 641372. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Roberts, S.J.; Meade, S.J.; Gerrard, J.A. Amyloid Fibrils as a Nanoscaffold for Enzyme Immobilization. Biotechnol. Prog. 2010, 26, 93–100. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makshakova, O.; Bogdanova, L.; Faizullin, D.; Khaibrakhmanova, D.; Ziganshina, S.; Ermakova, E.; Zuev, Y.; Sedov, I. The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein. Pharmaceutics 2023, 15, 624. https://doi.org/10.3390/pharmaceutics15020624
Makshakova O, Bogdanova L, Faizullin D, Khaibrakhmanova D, Ziganshina S, Ermakova E, Zuev Y, Sedov I. The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein. Pharmaceutics. 2023; 15(2):624. https://doi.org/10.3390/pharmaceutics15020624
Chicago/Turabian StyleMakshakova, Olga, Liliya Bogdanova, Dzhigangir Faizullin, Diliara Khaibrakhmanova, Sufia Ziganshina, Elena Ermakova, Yuriy Zuev, and Igor Sedov. 2023. "The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein" Pharmaceutics 15, no. 2: 624. https://doi.org/10.3390/pharmaceutics15020624
APA StyleMakshakova, O., Bogdanova, L., Faizullin, D., Khaibrakhmanova, D., Ziganshina, S., Ermakova, E., Zuev, Y., & Sedov, I. (2023). The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein. Pharmaceutics, 15(2), 624. https://doi.org/10.3390/pharmaceutics15020624








