Freeze-Drying of Pharmaceuticals in Vials Nested in a Rack System—Part I: Freezing Behaviour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
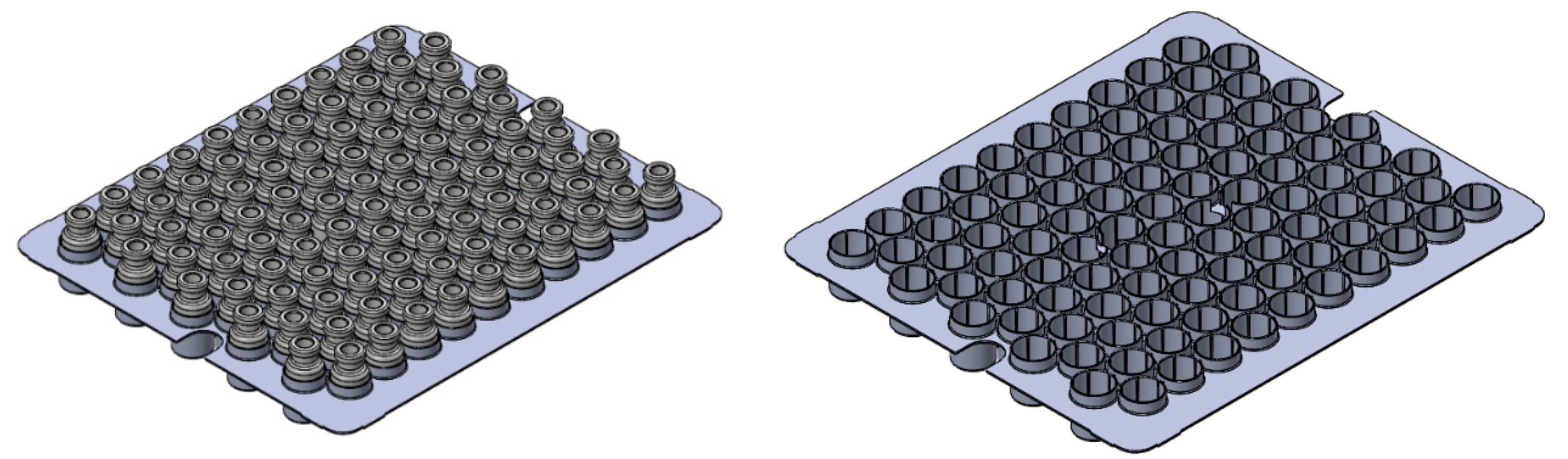
2.2. Experimental Set-Up
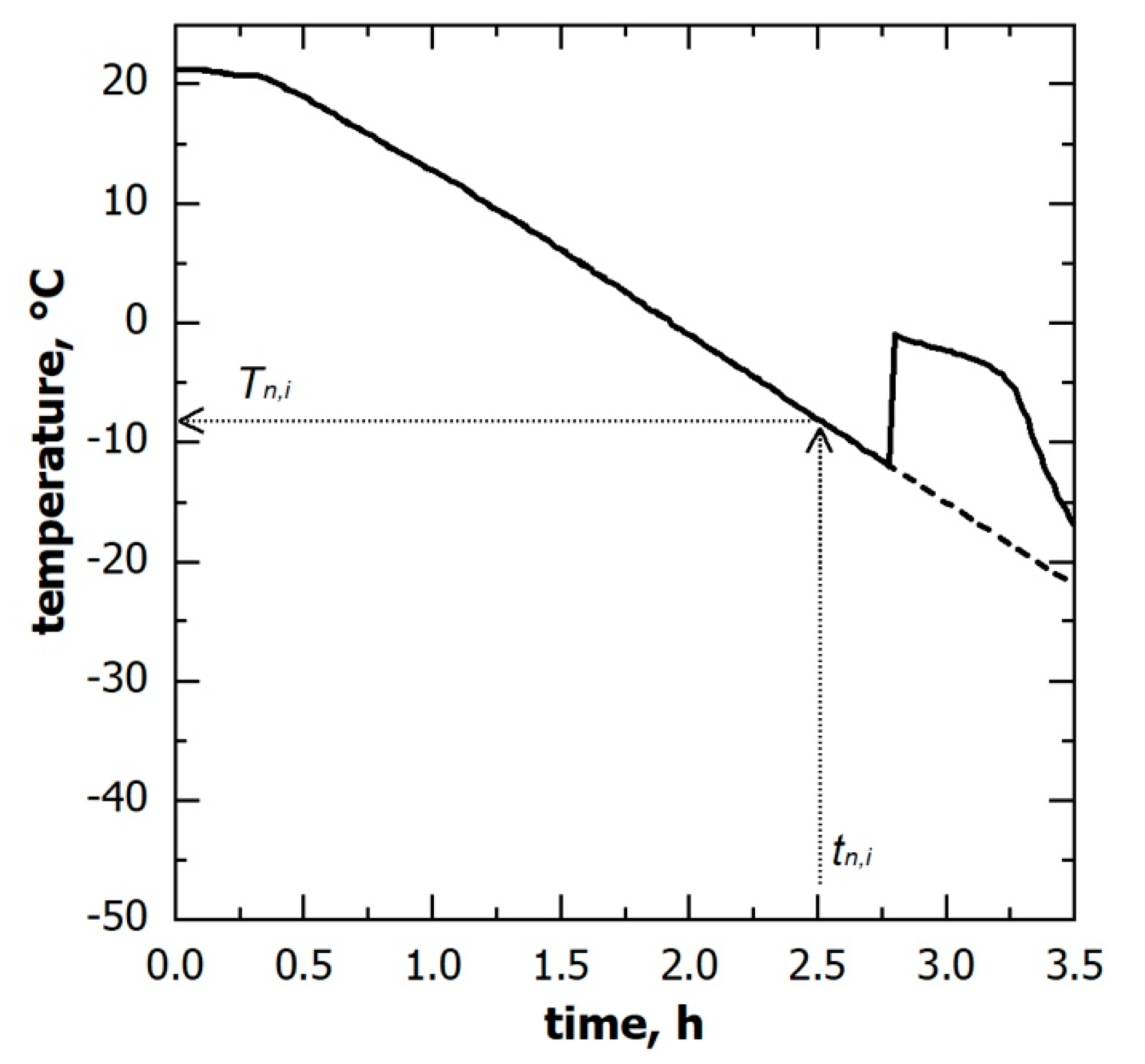
2.3. Determination of the Ice Nucleation Time Distribution
2.4. Determination of the Overall Equipment-to-Vial Heat Transfer Coefficient
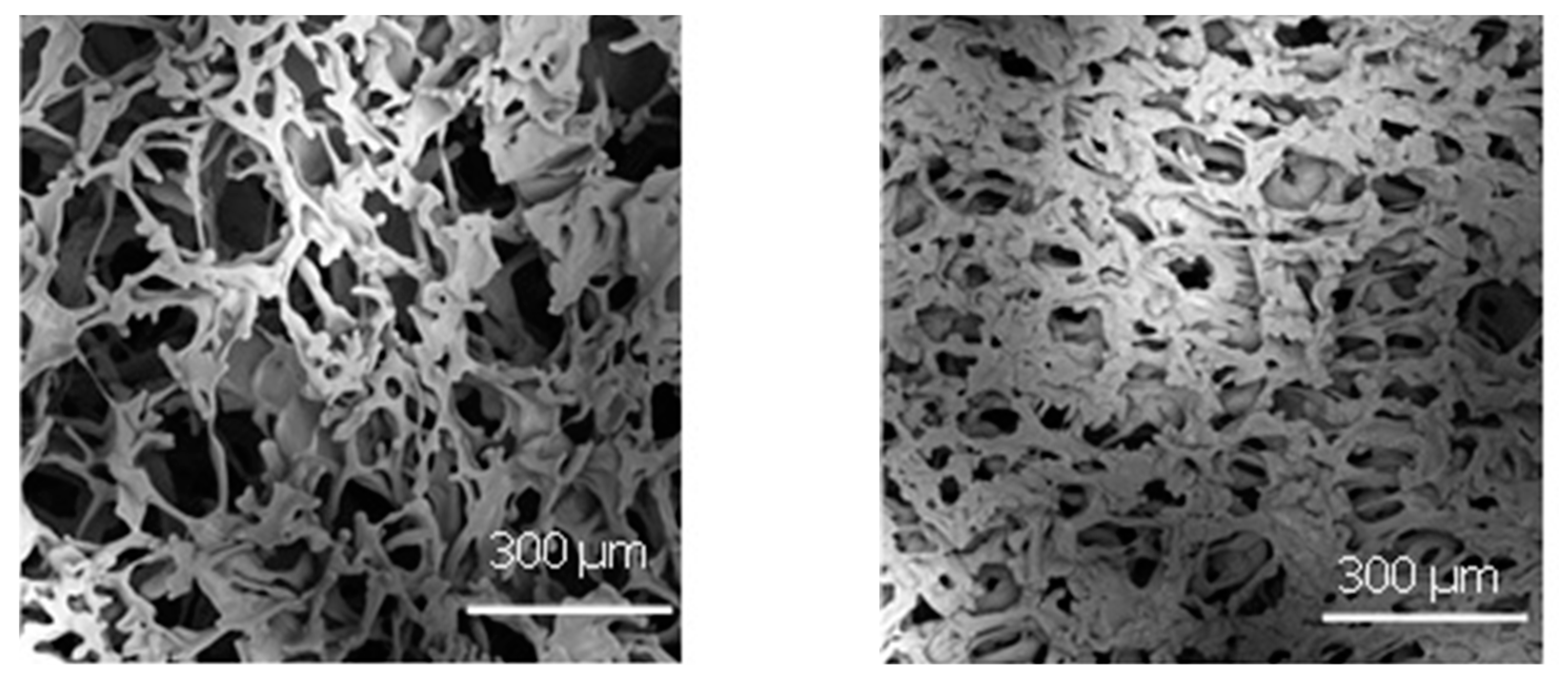
2.5. Lyophilised Product Morphology Characterisation
2.6. Residual Biological Activity of Lactate Dehydrogenase
3. Results
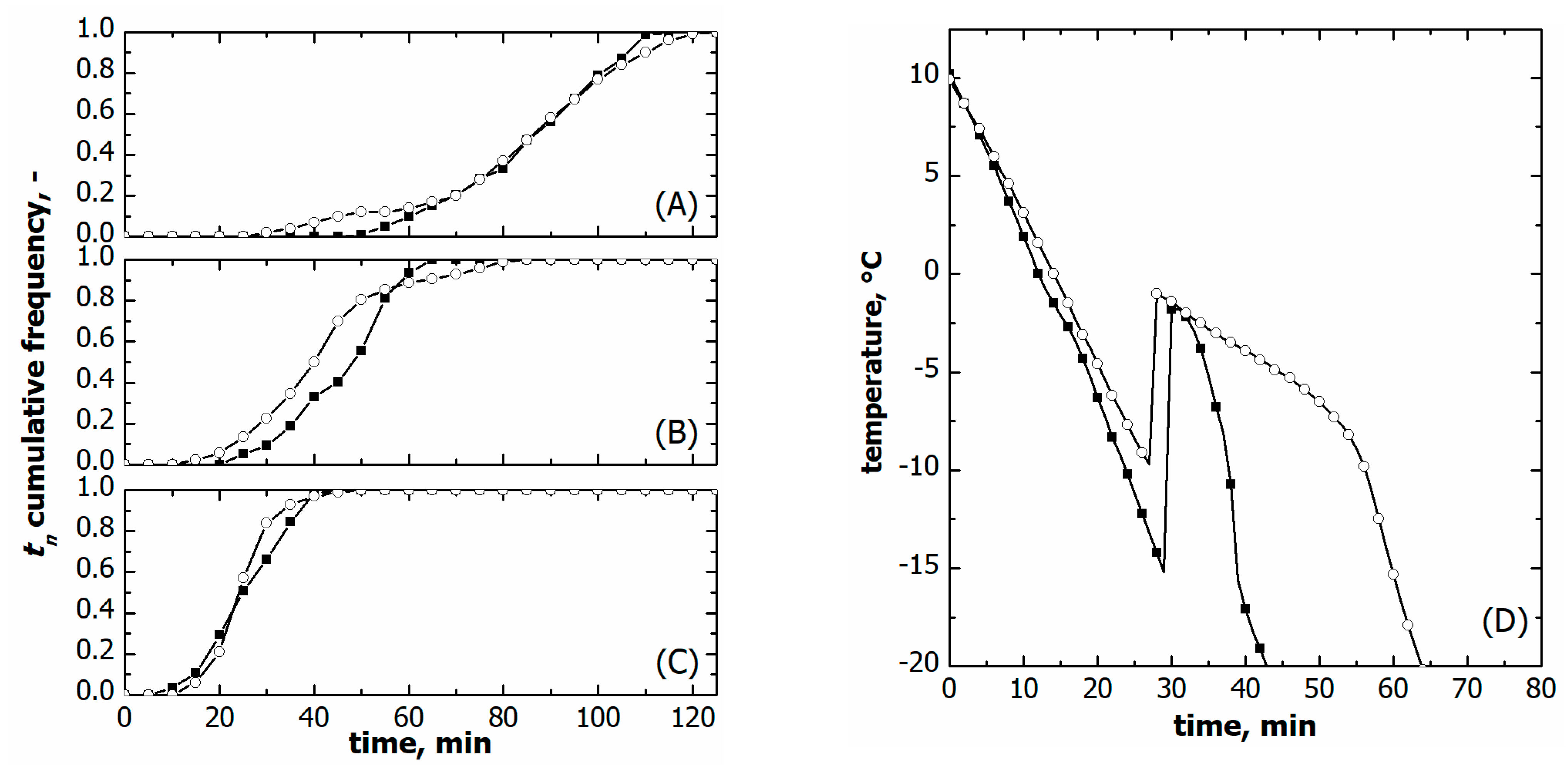
3.1. Vials in Direct Contact with the Shelf
3.2. Vials Nested in a Rack System
3.3. Comparison between Vials in Direct Contact and Nested in a Rack System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cross-sectional area of the vial, m2 | |
| specific heat capacity of the liquid being frozen, J kg−1K−1 | |
| cumulative fraction of nucleated vials, – | |
| heat flow rate, W | |
| Nj | number of vials of j-class, – |
| total number of monitored vials, – | |
| pressure, Pa | |
| saturation pressure, Pa | |
| time, s | |
| 10th percentile of the nucleation time data, min | |
| median nucleation time, min | |
| 90th percentile of the nucleation time data, min | |
| ice nucleation time, min | |
| 10th percentile of the nucleation temperature data, °C | |
| median nucleation temperature, °C | |
| 90th percentile of the nucleation temperature data, °C | |
| temperature of the liquid being frozen, °C | |
| ice nucleation temperature, °C | |
| shelf temperature, °C | |
| overall heat transfer coefficient, Wm−2K−1 | |
| volume of the liquid being frozen, m−3 | |
| Greek letters | |
| mass density of the liquid being frozen, kg m−3 | |
| Abbreviations | |
| PVDF | polyvinylidene fluoride |
| tIQR | interquartile range of the nucleation time distribution, min |
| TIQR | interquartile range of the nucleation temperature distribution, °C |
References
- Chen, X.; Fernando, G.J.; Crichton, M.L.; Flaim, C.; Yukiko, S.R.; Fairmaid, E.J.; Corbetta, H.J.; Primiero, C.A.; Ansaldo, A.B.; Frazer, I.H.; et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilisation. J. Control. Release 2011, 152, 349–355. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Immunization Supply Chain and Logistics: A Neglected but Essential System for National Immunization Programmes; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Bhatnagar, B.S.; Bogner, R.H.; Pikal, M.J. Protein stability during freezing: Separation of stresses and mechanisms of protein stabilization. Pharm. Dev. Technol. 2007, 12, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Privalov, P.L. Cold denaturation of proteins. Crit. Rev. Biochem. Mol. Biol. 1990, 25, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Graziano, G.; Catanzano, F.; Riccio, A.; Barone, G. A reassessment of the molecular origin of cold denaturation. J. Biochem. 1997, 122, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.F.; Darst, R.K.; Rossky, P.J. Mechanistic elements of protein cold denaturation. J. Phys. Chem. B 2008, 112, 5961–5967. [Google Scholar] [CrossRef] [PubMed]
- Strambini, G.B.; Gabellieri, E. Proteins in frozen solutions: Evidence of ice-induced partial unfolding. Biophys. J. 1996, 70, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; Pisano, R. The ice-water interface and protein stability: A review. J. Pharm. Sci. 2020, 109, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; McCarty, J.; Pisano, R.; Shea, J.-E. Heightened cold-denaturation of proteins at the ice-water interface. J. Am. Chem. Soc. 2020, 142, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Pikal, M.J. Mechanisms of protein stabilisation during freeze-drying storage: The relative importance of thermodynamic stabilisation and glassy state relaxation dynamics. In Freeze-Drying/Lyophilization of Pharmaceutical and Biological Products, 2nd ed.; Rey, L., May, J.C., Eds.; Informa Healthcare: London, UK, 2010; pp. 198–232. [Google Scholar]
- Chang, B.S.; Kendrick, B.S.; Carpenter, J.F. Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. J. Pharm. Sci. 1996, 85, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Nail, S.L. Effect of process conditions on recovery of protein activity after freezing and freeze-drying. Eur. J. Pharm. Biopharm. 1998, 45, 249–257. [Google Scholar] [CrossRef]
- Sarciaux, J.M.; Mansour, S.; Hageman, M.J.; Nail, S.L. Effects of buffer composition and processing conditions on aggregation of bovine IgG during freeze-drying. J. Pharm. Sci. 1999, 88, 1354–1361. [Google Scholar] [CrossRef]
- Eckhardt, B.M.; Oeswein, J.Q.; Bewley, T.A. Effect of freezing on aggregation of human growth hormone. Pharm. Res. 1991, 8, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Tanaka, K.; Mudhivarthi, V.; Bogner, R.H.; Pikal, M.J. Effect of controlled ice nucleation on stability of lactate dehydrogenase during freeze-drying. J. Pharm. Sci. 2018, 107, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Bald, W.B. On crystal size and cooling rate. J. Microsc. 1986, 143, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; Marenco, L.; Pisano, R. A model-based approach for the rational design of the freeze-thawing of a protein-based formulation. Pharm. Dev. Technol. 2020, 25, 823–831. [Google Scholar] [CrossRef]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The ice nucleation temperature determines the primary drying rate of lyophilisation for samples frozen on a temperature-controlled shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Pisano, R. Looking inside the ‘black box’: Freezing engineering to ensure the quality of freeze-dried biopharmaceuticals. Eur. J. Pharm. Biopharm. 2018, 129, 58–65. [Google Scholar] [CrossRef]
- Kasper, J.C.; Friess, W.F. The freezing step in lyophilisation: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur. J. Pharm. Biopharm. 2011, 78, 248–263. [Google Scholar] [CrossRef]
- Pisano, R. Alternative methods of controlling nucleation in freeze drying. In Lyophilization of Pharmaceuticals and Biologicals: New Technologies and Approaches, 1st ed.; Ward, K.R., Matejtschuk, P., Eds.; Springer: New York, NY, USA, 2019; pp. 79–111. [Google Scholar]
- Pisano, R.; Arsiccio, A.; Nakagawa, K.; Barresi, A.A. Tuning, measurement and prediction of the impact of freezing on product morphology: A step toward improved design of freeze-drying cycles. Drying Technol. 2019, 37, 579–599. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Trout, B.L.; Pisano, R. From batch to continuous: Freeze-drying of suspended vials for pharmaceuticals in unit-doses. Ind. Eng. Chem. Re.s 2019, 58, 1635–1649. [Google Scholar] [CrossRef]
- Deck, L.-T.; Ochsenbein, D.R.; Mazzotti, M. Stochastic shelf-scale modeling framework for the freezing stage in freeze-drying processes. Int. J. Pharm. 2022, 613, 121276. [Google Scholar] [CrossRef]
- Deck, L.-T.; Ochsenbein, D.R.; Mazzotti, M. Stochastic ice nucleation governs the freezing process of biopharmaceuticals in vials. Int. J. Pharm. 2022, 625, 122051. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; Barresi, A.A.; Pisano, R. Prediction of ice crystal size distribution after freezing of pharmaceutical solutions. Cryst. Growth Des. 2017, 17, 4573–4581. [Google Scholar] [CrossRef]
- Pisano, R.; Capozzi, L.C. Prediction of product morphology of lyophilised drugs in the case of Vacuum Induced Surface Freezing. Chem. Eng. Res. Des. 2017, 125, 119–129. [Google Scholar] [CrossRef]
- Colucci, D.; Fissore, D.; Barresi, A.A.; Braatz, R.D. A new mathematical model for monitoring the temporal evolution of the ice crystal size distribution during freezing in pharmaceutical solutions. Eur. J. Pharm. Biopharm. 2020, 148, 148–159. [Google Scholar] [CrossRef]
- Buceta, J.P.; Tréléa, I.C.; Scutellà, B.; Bourlés, E.; Fonseca, F.; Passot, S. Heat transfer during freeze-drying using a high-throughput vial system in view of process scale-up to serum vials. J. Pharm. Sci. 2021, 110, 1323–1336. [Google Scholar] [CrossRef]
- Matejčíková, A.; Tichý, E.; Rajniak, P. Experimental investigation of inhomogeneities of primary drying during lyophilisation: Impact of the vials packing density. J. Drug Deliv. Sci. Technol. 2022, 74, 103550. [Google Scholar] [CrossRef]
- Palmkron, S.B.; Gustavsson, L.; Wahlgren, M.; Bergensthål, B.; Fureby, A.M. Temperature and heat transfer control during freeze drying. Effect of vial holders and influence of pressure. Pharm. Res. 2022, 39, 2597–2606. [Google Scholar] [CrossRef]
- Ehlers, S.; Schroeder, R.; Friess, W. Trouble with the neighbor during freeze-drying: Rivalry about energy. J. Pharm. Sci. 2021, 110, 1219–1226. [Google Scholar] [CrossRef]
- Arsiccio, A.; Pisano, R. Application of the Quality by Design approach to the freezing step of freeze-drying: Building the design space. J. Pharm. Sci. 2018, 107, 1586–1596. [Google Scholar] [CrossRef]




| Loading Config. | Cooling Rate, °C min−1 | t10, min | t50, min | t90, min | (t90-t10), min | tIQR, min | T10, °C | T50, °C | T90, °C | (T90-T10), °C | TIQR, °C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct | 0.25 | 50 | 87 | 107 | 57 | 28 | −12 | −18 | −23 | −11 | −6 |
| Direct | 0.50 | 30 | 47 | 57 | 27 | 25 | −11 | −19 | −23 | −12 | −7 |
| Direct | 1.00 | 15 | 25 | 36 | 21 | 24 | −13 | −20 | −29 | −16 | −9 |
| Nested | 0.25 | 45 | 86 | 110 | 65 | 37 | −6 | −16 | −22 | −16 | −6 |
| Nested | 0.50 | 23 | 40 | 65 | 42 | 15 | −7 | −14 | −22 | −15 | −8 |
| Nested | 1.00 | 18 | 22 | 34 | 16 | 6 | −10 | −16 | −22 | −12 | −6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisano, R.; Artusio, F.; Adami, M.; Barresi, A.A.; Fissore, D.; Frare, M.C.; Zanetti, F.; Zunino, G. Freeze-Drying of Pharmaceuticals in Vials Nested in a Rack System—Part I: Freezing Behaviour. Pharmaceutics 2023, 15, 635. https://doi.org/10.3390/pharmaceutics15020635
Pisano R, Artusio F, Adami M, Barresi AA, Fissore D, Frare MC, Zanetti F, Zunino G. Freeze-Drying of Pharmaceuticals in Vials Nested in a Rack System—Part I: Freezing Behaviour. Pharmaceutics. 2023; 15(2):635. https://doi.org/10.3390/pharmaceutics15020635
Chicago/Turabian StylePisano, Roberto, Fiora Artusio, Marco Adami, Antonello A. Barresi, Davide Fissore, Maria Chiara Frare, Francesco Zanetti, and Gabriele Zunino. 2023. "Freeze-Drying of Pharmaceuticals in Vials Nested in a Rack System—Part I: Freezing Behaviour" Pharmaceutics 15, no. 2: 635. https://doi.org/10.3390/pharmaceutics15020635
APA StylePisano, R., Artusio, F., Adami, M., Barresi, A. A., Fissore, D., Frare, M. C., Zanetti, F., & Zunino, G. (2023). Freeze-Drying of Pharmaceuticals in Vials Nested in a Rack System—Part I: Freezing Behaviour. Pharmaceutics, 15(2), 635. https://doi.org/10.3390/pharmaceutics15020635










