In Silico Studies to Support Vaccine Development
Abstract
1. Introduction
2. Materials and Methods
3. Results
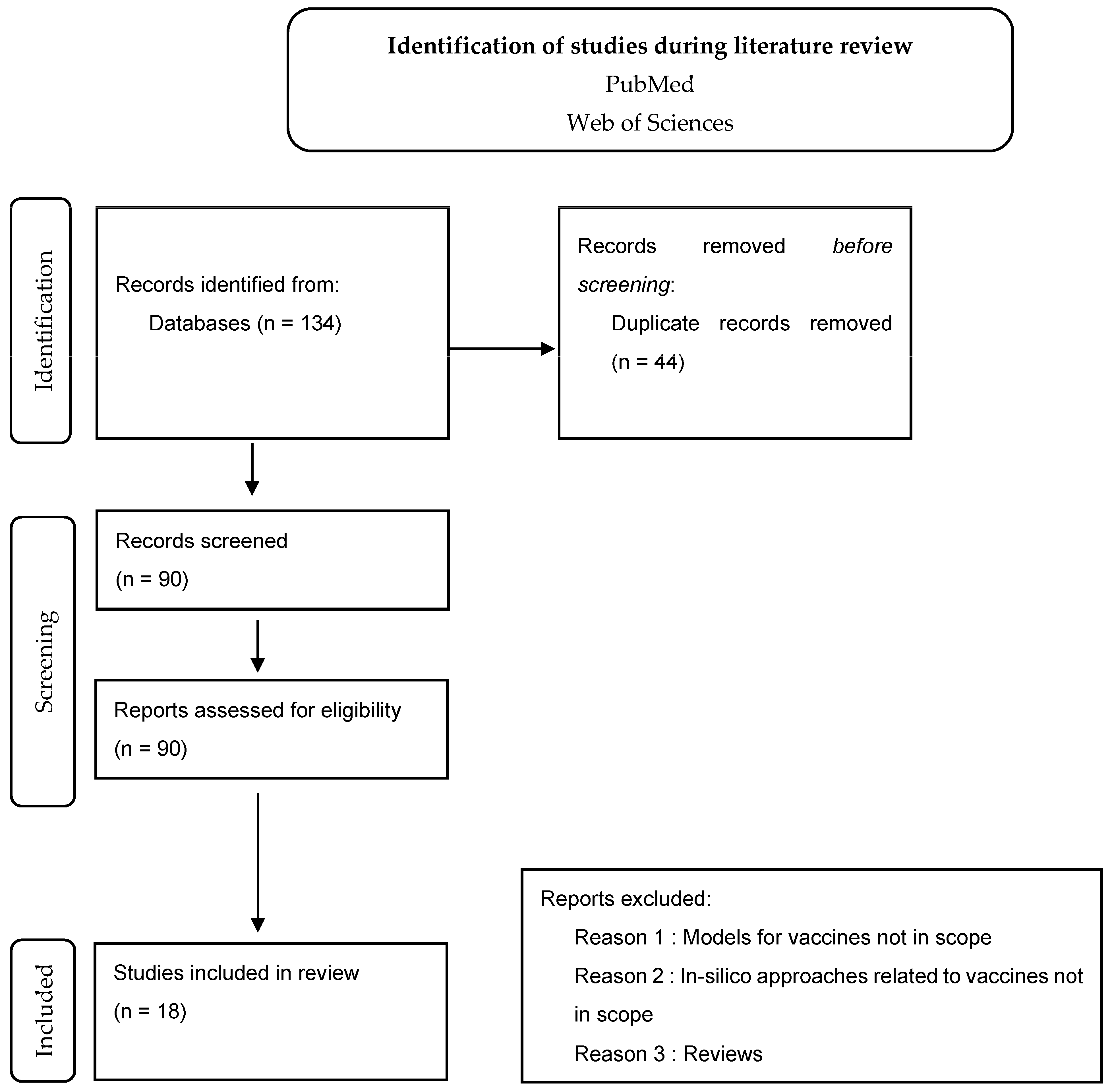
Results from Phase 1 (Literature Review and Screening)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos Trans. R. Soc. Lond B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef] [PubMed]
- Bilusic, P.J.D.M. Cancer Vaccines. Hematol. Oncol. Clin. N. Am. 2019, 33, 199–214. [Google Scholar]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. Editorial: In silico Methods for Drug Design and Discovery. Front. Chem. 2020, 8, 612. [Google Scholar] [CrossRef]
- EMA. EMA Implements New Measures to Minimise Animal Testing during Medicines Development. 2021. Available online: https://www.ema.europa.eu/en/news/ema-implements-new-measures-minimise-animal-testing-during-medicines-development (accessed on 6 January 2022).
- Pappalardo, F.; Russo, G.; Tshinanu, F.M.; Viceconti, M. In silico clinical trials: Concepts and early adoptions. Brief Bioinform. 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- EMA. Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation. 2018. Available online: https://www.ema.europa.eu/en/reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation-scientific-guideline (accessed on 13 January 2022).
- Van Tilbeurgh, M.; Lemdani, K.; Beignon, A.S.; Chapon, C.; Tchitchek, N.; Cheraitia, L.; Marcos-Lopez, E.; Pascal, Q.; Le Grand, R.; Maisonnasse, P.; et al. Predictive Markers of Immunogenicity and Efficacy for Human Vaccines. Vaccines 2021, 9, 579. [Google Scholar] [CrossRef]
- Mitkus, R.J.; Hess, M.A.; Schwartz, S.L. Pharmacokinetic modeling as an approach to assessing the safety of residual formaldehyde in infant vaccines. Vaccine 2013, 31, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Tegenge, M.A.; Mitkus, R.J. A physiologically-based pharmacokinetic (PBPK) model of squalene-containing adjuvant in human vaccines. J. Pharmacokinet. Pharmacodyn. 2013, 40, 545–556. [Google Scholar] [CrossRef]
- Saylor, K.; Zhang, C. A simple physiologically based pharmacokinetic model evaluating the effect of anti-nicotine antibodies on nicotine disposition in the brains of rats and humans. Toxicol. Appl. Pharmacol. 2016, 307, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Tegenge, M.A.; Mitkus, R.J. A first-generation physiologically based pharmacokinetic (PBPK) model of alpha-tocopherol in human influenza vaccine adjuvant. Regul. Toxicol. Pharmacol. 2015, 71, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Badhan, R.K.S.; Khadke, S.; Perrie, Y. Application of Pharmacokinetics Modelling to Predict Human Exposure of a Cationic Liposomal Subunit Antigen Vaccine System. Pharmaceutics 2017, 9, 57. [Google Scholar] [CrossRef]
- Capuani, S.; Hernandez, N.; Paez-Mayorga, J.; Dogra, P.; Wang, Z.; Cristini, V.; Chua, C.Y.X.; Nichols, J.E.; Grattoni, A. Localization of drug biodistribution in a 3D-bioengineered subcutaneous neovascularized microenvironment. Mater. Today Bio 2022, 16, 100390. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, J.; Ziemys, A.; Dogra, P.; Ferrari, M. A modeling platform for the lymphatic system. J. Theor. Biol. 2020, 493, 110193. [Google Scholar] [CrossRef]
- Pennisi, M.; Russo, G.; Sgroi, G.; Bonaccorso, A.; Parasiliti Palumbo, G.A.; Fichera, E.; Mitra, D.K.; Walker, K.B.; Cardona, P.J.; Amat, M.; et al. Predicting the artificial immunity induced by RUTI® vaccine against tuberculosis using universal immune system simulator (UISS). BMC Bioinform. 2019, 20, 504. [Google Scholar] [CrossRef]
- Russo, G.; Sgroi, G.; Parasiliti Palumbo, G.A.; Pennisi, M.; Juarez, M.A.; Cardona, P.-J.; Motta, S.; Walker, K.B.; Fichera, E.; Viceconti, M.; et al. Moving forward through the in silico modeling of tuberculosis: A further step with UISS-TB. BMC Bioinform. 2020, 21, 458. [Google Scholar] [CrossRef]
- Russo, G.; Pappalardo, F.; Juarez, M.A.; Pennisi, M.; Cardona, P.J.; Coler, R.; Fichera, E.; Viceconti, M. Evaluation of the efficacy of RUTI and ID93/GLA-SE vaccines in tuberculosis treatment: In silico trial through UISS-TB simulator. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 2197–2201. [Google Scholar]
- Russo, G.; Pennisi, M.; Fichera, E.; Motta, S.; Raciti, G.; Viceconti, M.; Pappalardo, F. In silico trial to test COVID-19 candidate vaccines: A case study with UISS platform. BMC Bioinform. 2020, 21, 527. [Google Scholar] [CrossRef]
- Silva, L.d.L.e.; Xavier, M.P.; Santos, R.W.d.; Lobosco, M.; Reis, R.F. Uncertain Quantification of Immunological Memory to Yellow Fever Virus. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Republic of Korea, 16–19 December 2020; pp. 1281–1288. [Google Scholar]
- Tegenge, M.A.; Von Tungeln, L.S.; Mitkus, R.J.; Anderson, S.A.; Vanlandingham, M.M.; Forshee, R.A.; Beland, F.A. Pharmacokinetics and biodistribution of squalene-containing emulsion adjuvant following intramuscular injection of H5N1 influenza vaccine in mice. Regul. Toxicol. Pharmacol. 2016, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Naidoo, L.; Zhang, L.; Carpp, L.N.; Rudnicki, E.; Randhawa, A.; Gonzales, P.; McDermott, A.; Ledgerwood, J.; Lorenzo, M.M.G.; et al. Pharmacokinetics and predicted neutralisation coverage of VRC01 in HIV-uninfected participants of the Antibody Mediated Prevention (AMP) trials. EBioMedicine 2021, 64, 103203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gilbert, P.B.; Capparelli, E.; Huang, Y. Simulation-Based Pharmacokinetics Sampling Design for Evaluating Correlates of Prevention Efficacy of Passive HIV Monoclonal Antibody Prophylaxis. Stat. Biopharm. Res. 2022, 14, 611–625. [Google Scholar] [CrossRef]
- Lőrincz, O.; Tóth, J.; Molnár, L.; Miklós, I.; Pántya, K.; Megyesi, M.; Somogyi, E.; Csiszovszki, Z.; Tőke, E.R. In Silico Model Estimates the Clinical Trial Outcome of Cancer Vaccines. Cells 2021, 10, 3048. [Google Scholar] [CrossRef]
- Linderman, J.J.; Cilfone, N.A.; Pienaar, E.; Gong, C.; Kirschner, D.E. A multi-scale approach to designing therapeutics for tuberculosis. Integr. Biol. 2015, 7, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Russo, G.; Parasiliti Palumbo, G.A.; Pappalardo, F. In silico design of recombinant multi-epitope vaccine against influenza A virus. BMC Bioinform. 2022, 22, 617. [Google Scholar] [CrossRef]
- Zhuang, X.; Lu, C. PBPK Modeling and Simulation in Drug Research and Development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef]
- FDA. How Simulation Can Transform Regulatory Pathways. 2018. Available online: https://www.fda.gov/science-research/about-science-research-fda/how-simulation-can-transform-regulatory-pathways (accessed on 6 January 2022).
- El-Khateeb, E.; Burkhill, S.; Murby, S.; Amirat, H.; Rostami-Hodjegan, A.; Ahmad, A. Physiological-based pharmacokinetic modeling trends in pharmaceutical drug development over the last 20-years; in-depth analysis of applications, organizations, and platforms. Biopharm. Drug Dispos. 2021, 42, 107–117. [Google Scholar] [CrossRef]
- Kabiri Chimeh, M.; Heywood, P.; Pennisi, M.; Pappalardo, F.; Richmond, P. Parallelisation strategies for agent based simulation of immune systems. BMC Bioinform. 2019, 20, 579. [Google Scholar] [CrossRef]
- Truong, V.T.; Baverel, P.G.; Lythe, G.D.; Vicini, P.; Yates, J.W.T.; Dubois, V.F.S. Step-by-step comparison of ordinary differential equation and agent-based approaches to pharmacokinetic-pharmacodynamic models. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 133–148. [Google Scholar] [CrossRef]
- FDA. Population Pharmacokinetics Guidance for Industry. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics (accessed on 13 January 2022).
- EMA. Guideline on Reporting the Results of Population Pharmacokinetics Analysis. 2007. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-results-population-pharmacokinetic-analyses_en.pdf (accessed on 13 January 2022).
- Jones, H.M.; Dickins, M.; Youdim, K.; Gosset, J.R.; Attkins, N.J.; Hay, T.L.; Gurrell, I.K.; Logan, Y.R.; Bungay, P.J.; Jones, B.C.; et al. Application of PBPK modelling in drug discovery and development at Pfizer. Xenobiotica 2012, 42, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.A.; Campbell, J.L.; Pithawalla, Y.B.; Pourhashem, H.; Muhammad-Kah, S.R.; Sarkar, M.A.; Liu, J.; McKinney, W.J.; Gentry, R.; Gogova, M. A comprehensive physiologically based pharmacokinetic (PBPK) model for nicotine in humans from using nicotine-containing products with different routes of exposure. Sci. Rep. 2022, 12, 1091. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, F.; Russo, G.; Pennisi, M.; Sgroi, G.; Palumbo, G.; Motta, S.; Maimone, D.; Chiacchio, F. Agent based modeling of relapsing multiple sclerosis: A possible approach to predict treatment outcome. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; p. 41. [Google Scholar]
- Douglas, G.; Samant, B. The Vaccine Industry. In Plotkin’s Vaccines, 7th ed.; Plotkin, S., Orenstein, W., Offit, P., Edwards, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 4, pp. 41–50. [Google Scholar]
- Abbas, A.; Lichtman, H.; Pillai, S. Immunity to Microbes. In Cellular and Molecular Immunology, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 365–388. [Google Scholar]
- Hamley, I.W. Peptides for Vaccine Development. ACS Appl. Bio Mater. 2022, 5, 905–944. [Google Scholar] [CrossRef]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. Vaccine Design. In Methods in Molecular Biology; Thomas, S., Ed.; Humana: New York, NY, USA, 2021; Volume 2412, pp. 145–178. [Google Scholar]
- Matić, Z.; Šantak, M. Current view on novel vaccine technologies to combat human infectious diseases. Appl. Microbiol. Biotechnol. 2022, 106, 25–56. [Google Scholar] [CrossRef] [PubMed]
- CDC. Varicella. In Epidemiology and Prevention of Vaccine-Preventable Diseases; CDC: Atlanta, GA, USA, 2021. [Google Scholar]
- Wolf, J.; Bruno, S.; Eichberg, M.; Jannat, R.; Rudo, S.; VanRheenen, S.; Coller, B.A. Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. Npj Vaccines 2020, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2020, 589, 16–18. [Google Scholar] [CrossRef]
- Rowland, M.; Peck, C.; Tucker, G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev. Pharm. Toxicol. 2011, 51, 45–73. [Google Scholar] [CrossRef]
- Jones, H.M.; Chen, Y.; Gibson, C.; Heimbach, T.; Parrott, N.; Peters, S.A.; Upetri, V.V.; Zheng, M.; Hall, S.D. Physiologically based pharmacokinetic modeling in drug discovery and development: A pharmaceutical industry perspective. Clin. Pharmacol. Ther. 2015, 97, 247–262. [Google Scholar] [CrossRef]
- Plotkin, S.A. Increasing Complexity of Vaccine Development. J. Infec. Dis. 2015, 212, 12–16. [Google Scholar] [CrossRef]
- Trinchieri, G. Cancer Immunity: Lessons From Infectious Diseases. J. Infect. Dis. 2015, 212, S67–S73. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Gwin, W.R.; Mitchell, D.A. Vaccine Therapies for Cancer: Then and Now. Targ Oncol. 2021, 16, 121–152. [Google Scholar] [CrossRef] [PubMed]
- Six, A.; Bellier, B.; Vaslin, T.V.; Klatzmann, D. Systems biology in vaccine design. Microb. Biotechnol. 2012, 5, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Cohn, M.; Mata, J. Quantitative modeling of immune responses. Immunol. Rev. 2007, 216, 5–8. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Kenza, A.; Samer, C.F.; Yvonne, G.; Jules, A.D.; Youseff, D. Reviewing Data Integrated for PBPK Model Development to Predict Metabolic Drug-Drug Interactions: Shifting Perspectives and Emerging Trends. Front. Pharmacol. 2021, 12, 708299. [Google Scholar]
- Shekhani, R.; Steinacher, L.; Swen, J.J.; Sundberg, I.M. Evaluation of Current Regulation and Guidelines of Pharmacogenomic Drug Labels: Opportunities for Improvements. Clin. Pharmacol. Ther. 2020, 107, 1240–1255. [Google Scholar] [CrossRef]
- Manolis, E.; Pons, G. Proposals for Model-Based Paediatric Medicinal Development within the Current European Union Regulatory Framework. Br. J. Clin. Pharmacol. 2009, 68, 493–501. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef]
- WHO. How Are Vaccines Developed? Available online: https://www.who.int/news-room/feature-stories/detail/how-are-vaccines-developed (accessed on 22 January 2023).
- Rapin, N.; Lund, O.; Castiglione, F. Immune system simulation online. Bioinformatics 2011, 27, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
| Construct | Strings | No. of Articles Identified | ||
|---|---|---|---|---|
| Web of Science | vaccin* (All fields) | AND | “Physiological based pharmacokinetic” (All fields) | 1 |
| vaccin* (All fields) | AND | “pbpk” (All fields) | 18 | |
| vaccin* (All fields) | AND | “Population Pharmacokinetics” (All fields) | 50 | |
| vaccin* (All fields) | AND | “poppk” (All fields) | 2 | |
| vaccin* (All fields) | AND | “in silico trial*” (All fields) | 18 | |
| Total articles | 89 | |||
| PubMed | vaccin* (All fields) | AND | “Physiological based pharmacokinetic” (All fields) | 1 |
| vaccin* (All fields) | AND | “pbpk” (All fields) | 14 | |
| vaccin* (All fields) | AND | “Population Pharmacokinetics” (All fields) | 15 | |
| vaccin* (All fields) | AND | “poppk” (All fields) | 2 | |
| vaccin* (All fields) | AND | “in silico trial*” (All fields) | 13 | |
| Total articles | 45 | |||
| Reason 1 | Models for vaccines not in scope |
| Reason 2 | In silico approaches related to vaccines not in scope |
| Reason 3 | Review articles instead of vaccine model preparation |
| Product in Scope | Type of Model | Aim of Model | Comments | Software | Authors; Year |
|---|---|---|---|---|---|
| Formaldehyde-containing vaccines | PBPK | To assess the safety of residual formaldehyde in infant vaccines. | This model was used to predict formaldehyde disposition after an intramuscular injection. | CMATRIX | Robert J. Mitkus Maureen A.Hess Sorell L. Schwartz; 2013 [13] |
| Squalene-containing adjuvant vaccines | PBPK | To provide an estimation, quantitatively, of the squalene distribution in tissue following intramuscular injection. | This model was used to predict distribution after following intramuscular injection in humans. | Vensim PLE Plus (Ventana Systems, Inc., Harvard, MA, USA) | Million A. Tegenge Robert J. Mitkus; 2013 [14] |
| Nicotine vaccines | PBPK | To simulate and evaluate the efficacy of a nicotine vaccine. | The aim of the model is to predict the role of anti-nicotine antibodies on the nicotine disposition brain of humans and rats. | SimBiology | Kyle Saylor Chenming Zhang; 2016 [15] |
| α-tocopherol in emulsified-influenza vaccine adjuvant | PBPK | This model has two main goals. First, it is a PBPK model that will assess the in vivo fate of novel vaccine adjuvants; Secondly, it will predict the distribution of α-tocopherol in humans after a single dose of squalene-containing adjuvant vaccine | The aim of this model is to predict in vivo fate of α-tocopherol in adjuvanted influenza vaccine in humans after an intramuscular injection. | Vensim Professional® (Ventana Systems, Inc., Harvard, MA, USA) | Million A.Tegenge Robert J. Mitkus; 2015 [16] |
| Cationic liposomal subunit antigen vaccine | PBPK | To predict human exposure to a cationic liposomal subunit antigen vaccine system. | The aim of the model is to predict the in-vivo fate of dimethyldioctadecylammonium bromide (DDA) and the immunostimulatory agent trehalose 6,6-dibehenate (TDB) (8:1 molar ratio) combined with the Ag85B-ESAT-6 (H1) in humans. Additionally, it aims to demonstrate what is the consequence of the formulation degradation and fraction escaping the depot site and what are the depot’s effects on the site of administration. | MATLAB (The MathWorks Inc., Natick, MA, USA, 2015) | Raj K. S. Badhan Swapnil Khadke Yvonne Perrie; 2017 [17] |
| Cancer vaccine | PBPK | To represent the distribution of certain molecules eluted through a 3D-printed implantable system named ‘NICHE’ | The NICHE platform aims to study immunomodulation for cell therapeutics and cancer vaccines. It is a two-compartment model composed of a vascularized tissue reservoir and a surrounding refillable drug reservoir. The PBPK model was able to recapitulate the biodistribution of the molecules in scope, and together with NICH, they represent a flexible, adaptable platform to investigate local immunomodulation for biomedical applications. | Simbiology (MATLAB 2021b, Mathworks) | Simone Capuani et al.; 2022 [18] |
| Immune vaccines | PBPK through ordinary differential equations (ODEs) | To simulate and predict the distribution of different therapeutic agents and interactions with the immune system and its redistribution across lymphoid compartments. Furthermore, it allows the study of the infiltration into tumor tissues. | The aim of the model is to study the biodistribution of therapeutic agents and cells in blood and lymphatics, representing a PBPK novel model with tumor compartment properties enabling the study of key biological factors in the field. | Mathematical modeling | Javier Ruiz-Ramírez et al.; 2020 [19] |
| RUTI® vaccine against tuberculosis | Agent-based model (ABM) | To predict the artificial immunity induced by RUTI® vaccines using UISS. | The aim of the model is to predict the immune system’s complex dynamics by simulating mechanisms related to the infection and predicting how therapeutic strategies could face the infection. | Universal Immune System Simulator (UISS) | Marzio Pennisi et al.; 2019 [20] |
| Agent-based model (ABM) | To assess and simulate the response of the combination of a standard anti-TB therapy strategy with a potential therapeutic vaccine, such as RUTI. | The model simulates the disease activities and their interaction within the immune system. Additionally, it allows the prediction of the efficacy of the combination of isoniazid and RUTI vaccine in a certain digital population cohort. | Universal Immune System Simulator (UISS) | Giulia Russo et al.; 2020 [21] | |
| Specific tuberculosis vaccines: RUTI and ID93/GLA-SE | Agent-Based Model (ABM) | This is an EU—funded STriTuVaD project computational platform. It allows the prediction of immunity provided by RUTI and ID93/GLA-SE. | A multi-scale (cellular and molecular level), multi-compartment, polyclonal agent-based simulator that predicts the ability to predict the immunity induced by RUTI and ID93/GLA-SE (both tuberculosis vaccines). | Universal Immune System Simulator (UISS) | Giulia Russo et al.; 2019 [22] |
| COVID-19 candidate vaccines | Agent-based model (ABM) | This model aids the testing and designing of therapeutics against SARS-CoV-2. Its intention is to allow a boost in vaccine development to predict any failures and minimize side effects. | A model to predict the efficacy of therapy against COVID-19. | Universal Immune System Simulator (UISS) | Giulia Russo et al.; 2020 [23] |
| Yellow fever vaccine | Ordinary differential equations (ODE) | These mathematical models allow the study of primary and secondary responses to the yellow fever virus. | A model integrated by ordinary differential equations, which aim is to study responses to the yellow fever virus in five populations: yellow fever virus, three types of B cells (naive, active, and memory), and antibodies. | Mathematical models | Larissa de L. e Silva et al.; 2020 [24] |
| Squalene-containing emulsion vaccine adjuvants | PopPK | Estimating PK parameters are important to the study of squalene properties after intramuscular administration of influenza vaccines. | The aim of the study is to simulate PK parameters that are properties after intramuscular injection. Results aim to contribute to the knowledge of an informed benefit-risk assessment of a vaccine containing squalene as an adjuvant. | NONMEM® 7.3, Hanover, MD | Million A.Tegenge et al.; 2016 [25] |
| HIV vaccine | PopPK | To demonstrate the pharmacokinetics properties and predict HIV-1 neutralization. | This model aims to assess and predict VRC01 serum concentration and serum neutralization titer to panels of HIV-1 isolates in order to validate a potential biomarker to support an HIV vaccine development. | NONMEM software system (version 7·4, ICON Development Solutions). | Yunda Huang et al.; 2021 [26] |
| HIV vaccine | PopPK | This model supports the estimation of individual-specific VRC01 concentrations as correlates of protection (CoP). It assesses the association between the value of VRC01 concentration and the instantaneous rate of HIV infection. | To simulate population characteristics and study visits data, R version 3.5.1 R Core Team (2016) was used. With the NONMEM software system (Version 7.4, ICON Development Solutions), it was possible to model concentration data. | R version 3.5.1 R Core Team (2016) NONMEM software system (Version 7.4, ICON Development Solutions) | Lily Zhang et al.; 2021 [27] |
| Cancer vaccines | In silico model population (MP) | This model will support the prediction of clinical outcomes for cancer vaccines. | With the in silico modeling, it was possible to predict the frequency of vaccine-specific HLA-binding epitopes in order to calculate the immune response rate (IRR) for the model population. | Immune Epitope Database (IEDB) | Orsolya Lőrincz et al.; 2021 [28] |
| Designing therapeutics for vaccines | Agent-based model (ABM) | To provide a description of the cellular behavior of the immune system and dynamics. | These three model pieces are linked to cross-information in all scales. It is a mathematical and also multi-scale model (including both cellular- and molecular-level events). | ABM is constructed using the C++ programming language, Boost libraries (distributed under the Boost Software License: http://www.boost.org), and the Qt framework for visualization (distributed under GPL: http://www.qt.digia.com). T; Simulations performed on Nyx/Flux computing cluster available at the Center for Advanced Computing at the University of Michigan | Jennifer J. Linderman, Nicholas A. Cilfone, Elsje Pienaar, Chang Gong, Denise E. Kirschner; 2015 [29] |
| Ordinary differential equations (ODEs) | To record events related to receptor–ligand binding, trafficking, and intracellular signaling. | ||||
| Relevant partial differential equations | To describe the diffusion of certain ligands, cytokines, and other components. | ||||
| Recombinant multi-epitope vaccine against influenza A virus | Computational vaccine design | Retrieving influenza protein sequences and multiple alignments | The NCBI database and Jalview software were used to expose the amino acid sequences and to perform the multiple alignments, respectively. | NCBI database and Jalview software | Avisa Malek et al.; 2021 [30] |
| B-cell epitopes prediction | This is an important step in synthetic peptide vaccine development. These epitopes should be capable of evoking antibodies in order to neutralize the pathogen. | SVMTriP IEDB Analysis | |||
| CTL epitopes prediction | NetCTL 1.2 server was used to identify MHC class I epitopes. | NetCTL 1.2 server | |||
| CD4 T-cell epitopes prediction | NetMHCIIpan 4.0 was used to identify MHC class 2 epitopes. | NetMHCIIpan–4.0 | |||
| Antigenicity and allergenicity prediction of CTL, CD4 T-cell, and B-cell epitopes | In order to verify the antigenicity of the peptides, the VaxiJen v2.0 was used. In parallel, to evaluate their allergenicity, the software AllerTOP v2.0 was used. The toxicity of peptides was assessed with ToxinPred. | VaxiJen v2.0 AllerTOP v2.0 TxinPred | |||
| Human population coverage analysis | To verify and assess human population coverage, IEDB was used. | IEDB | |||
| Recombinant multi-epitope vaccine | Analyses were made of three vaccine adjuvants in order to select the candidate for the final vaccine formulation. | BCEPS web server | |||
| Evaluation of physicochemical properties and solubility | To reveal the physicochemical properties of the vaccine, ProtParam was used. The solubility was assessed with the Protein-sol server. | ProtParam Protein-sol server | |||
| Secondary structure prediction of the recombinant vaccine | The secondary structure of the final formulation and its properties was predicted with the RaptorX Property web server. | PSIPRED 4.0 web server RaptorX Property web server | |||
| Codon adaption and in silico cloning of the recombinant vaccine | Reverse translation and codon optimization for candidates were conducted with JAVA Codon Adaptation Tool (JCat). | JAVA Codon Adaptation Tool (JCat) | |||
| Agent-based model (ABM) | In silico trial simulation of the immune system | Immune response and immunogenicity were assessed with UISS. | Universal Immune System Simulator (UISS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldanha, L.; Langel, Ü.; Vale, N. In Silico Studies to Support Vaccine Development. Pharmaceutics 2023, 15, 654. https://doi.org/10.3390/pharmaceutics15020654
Saldanha L, Langel Ü, Vale N. In Silico Studies to Support Vaccine Development. Pharmaceutics. 2023; 15(2):654. https://doi.org/10.3390/pharmaceutics15020654
Chicago/Turabian StyleSaldanha, Leonor, Ülo Langel, and Nuno Vale. 2023. "In Silico Studies to Support Vaccine Development" Pharmaceutics 15, no. 2: 654. https://doi.org/10.3390/pharmaceutics15020654
APA StyleSaldanha, L., Langel, Ü., & Vale, N. (2023). In Silico Studies to Support Vaccine Development. Pharmaceutics, 15(2), 654. https://doi.org/10.3390/pharmaceutics15020654







