Exploration of the Safety and Solubilization, Dissolution, Analgesic Effects of Common Basic Excipients on the NSAID Drug Ketoprofen
Abstract
:1. Introduction
2. Materials and Methods
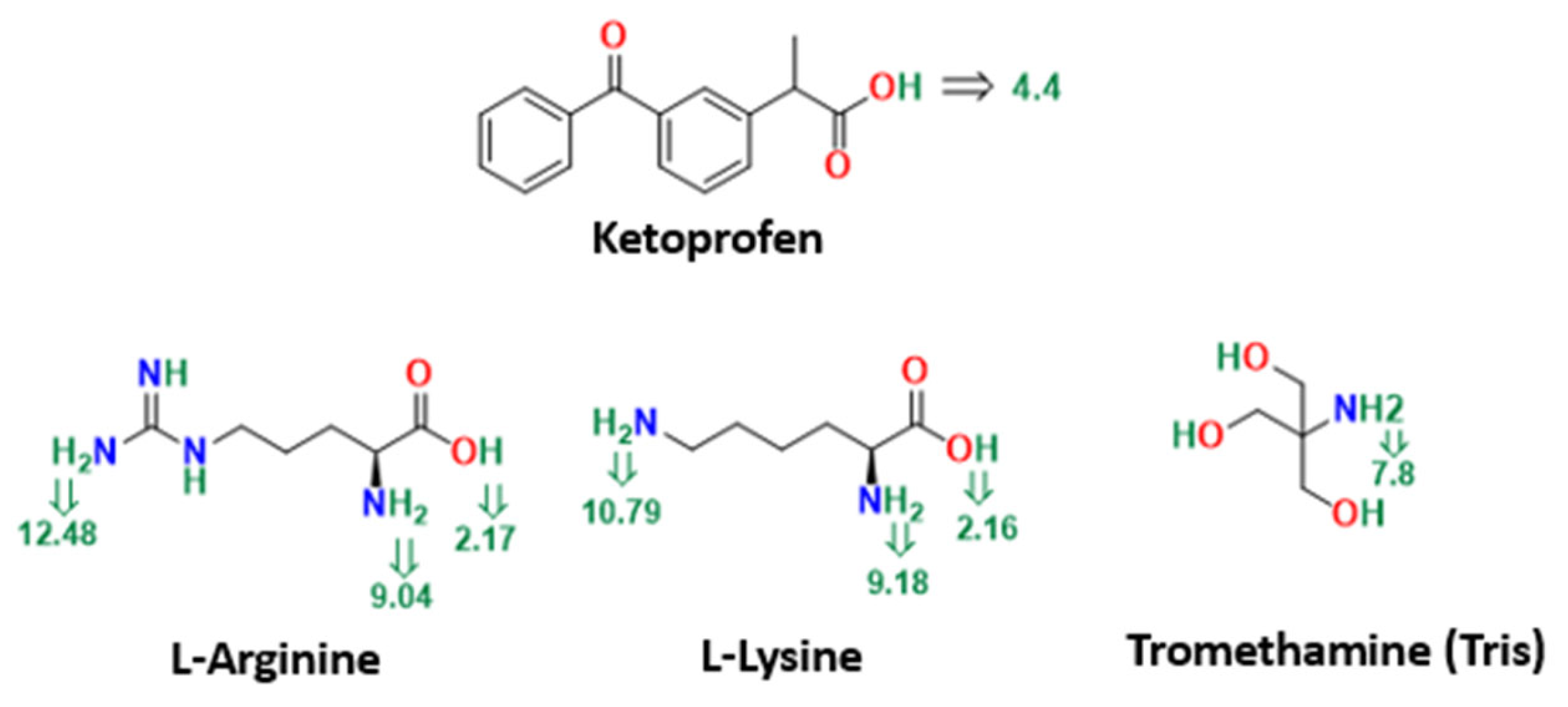
2.1. Materials
2.2. Preparation of Ketoprofen-Excipients Physical and Dispersed Mixtures
2.2.1. Physical Mixtures
2.2.2. Coprecipitated Mixtures of Ketoprofen:L-lysine, Ketoprofen:L-arginine, and Ketoprofen:tris
2.3. Equilibrium Solubility Studies
2.4. Differential Scanning Calorimetry (DSC) and Fourier Transfer Infrared Spectroscopy (FTIR)
2.5. In Vitro Dissolution
2.6. Molecular Docking
2.7. In Vivo Studies
2.7.1. Writhing Assay
2.7.2. Indomethacin-Induced Ulcer
2.8. Histopathological Documentation
3. Results and Discussion
3.1. Solubility Studies
3.2. FTIR and DSC Studies
3.3. Dissolution Studies
3.4. Molecular Docking
3.5. In Vivo Studies
3.5.1. Writhing Assay
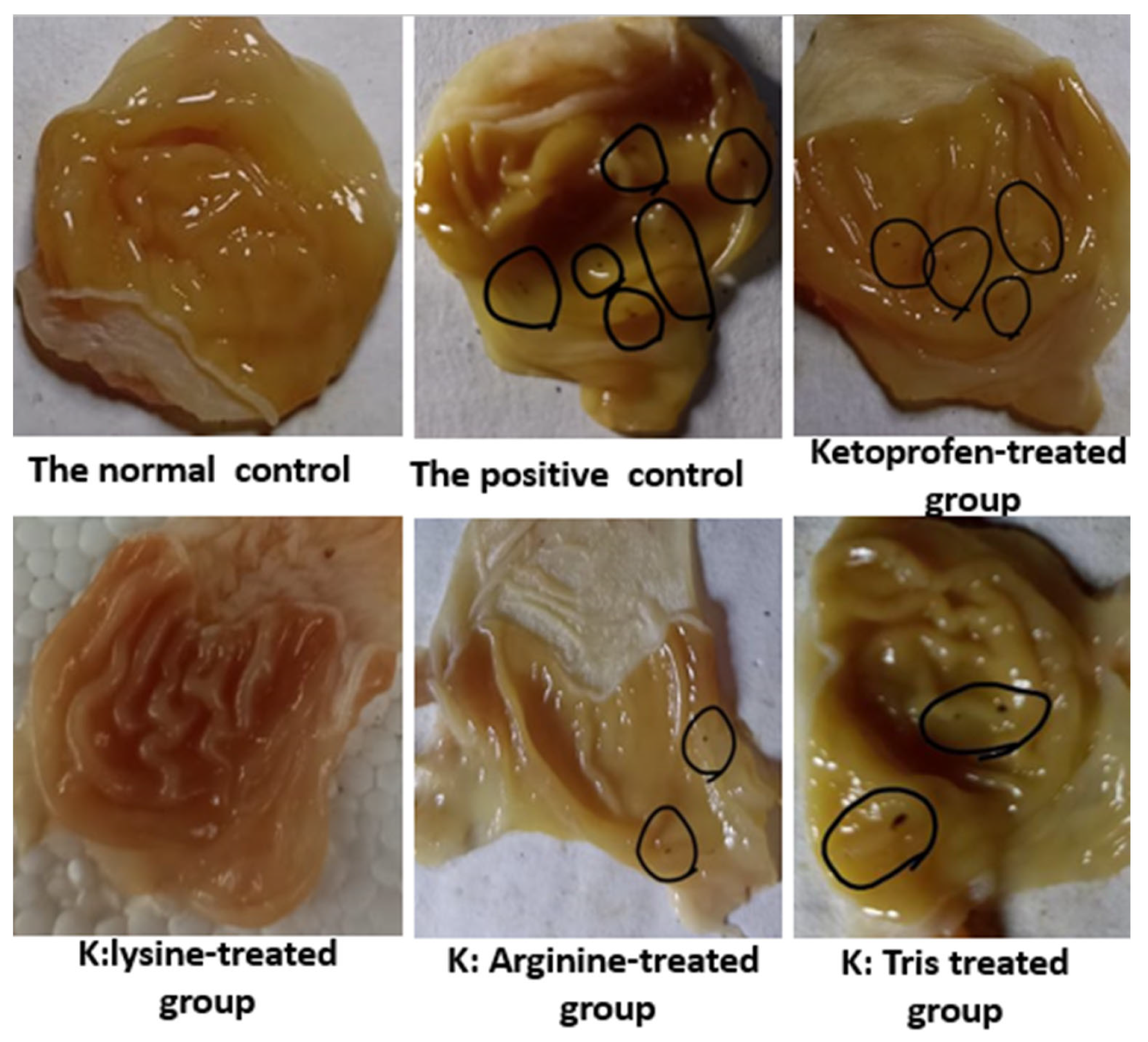
3.5.2. Indomethacin-Induced Ulcer
3.5.3. Histopathological Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelkader, H.; Fathalla, Z. Investigation into the emerging role of the basic Amino acid L-lysine in enhancing solubility and permeability of BCS Class II and BCS Class IV drugs. Pharm. Res. 2018, 35, 160–178. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin B. 2015, 15, 442–453. [Google Scholar] [CrossRef] [Green Version]
- Abou-Taleb, H.; Fathalla, Z.; Abdelkader, H. Comparative studies of the effects of novel excipients amino acids with cyclodextrins on enhancement of dissolution and oral bioavailability of the non-ionizable drug carbamazepine. Eur. J. Pharm. Sci. 2020, 155, 105562. [Google Scholar] [CrossRef] [PubMed]
- Bongioanni, A.; Bueno, M.; Mezzano, B.; Longhi, M.; Garnero, C. Amino acids and its pharmaceutical applications: A mini review. Int. J. Pharm. 2022, 613, 121375. [Google Scholar] [CrossRef]
- Kuczynska, J.; Nieradko-Iwanicka, B. Future prospects of ketoprofen in improving the safety of the gastric mucosa. Biomed. Pharmacother. 2021, 139, 111608. [Google Scholar]
- Granero, G.E.; Ramachandran, C.; Amidon, G.L. Rapid in vivo dissolution of ketoprofen: Implications on the biopharmaceutics classification system. Pharmazie 2006, 61, 673–676. [Google Scholar]
- Yousif, N.; Abdulrasool, A.; Mowafaq, G.; Hussain, S. Solubility and dissolution improvement of ketoprofen by solid dispersion in polymer and surfactant using solvent evaporation method. Int. J. Pharm. Pharm. Sci. 2011, 3, 431–435. [Google Scholar]
- Khoder, M.; Abdelkader, H.; Elshaer, A.; Karam, A.; Najlah, M.; Alany, R. The use of albumin solid dispersion to enhance the solubility of unionisable drugs. Pharm. Dev. Technol. 2018, 23, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Drug solubilization by complexation. Int. J. Pharm. 2017, 531, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, H.; Abdallah, O.Y.; Salem, H.; Alani, A.; Alany, R. Eutectic, monotectic and immiscibility systems of nimesulide with water-soluble carriers: Phase equilibria, solid-state characterisation and in-vivo/pharmacodynamic evaluation. J. Pharm. Pharmacol. 2014, 66, 439–450. [Google Scholar] [CrossRef]
- ElShaer, A.; Hanson, P.; Mohammed, A. A novel concentration dependent amino acid ion pair strategy to mediate drug permeation using indomethacin as a model insoluble drug. Eur. J. Pharm. Sci. 2014, 62, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Aramini, A.; Bianchini, G.; Lillini, S.; Bordignon, S.; Tomassetti, M.; Novelli, R.; Mattioli, S.; Lvova, L.; Paolesse, R.; Chierotti, M.R.; et al. Unexpected Salt/Cocrystal Polymorphism of the Ketoprofen–Lysine System: Discovery of a New Ketoprofen–L-Lysine Salt Polymorph with Different Physicochemical and Pharmacokinetic Properties. Pharmaceuticals 2021, 14, 555. [Google Scholar] [CrossRef]
- Fitriani, L.; Firdaus, W.; Sidadang, W.; Rosaini, H.; Putra, O.; Oyama, H.; Uekusa, H.; Zaini, E. Improved solubility and dissolution rate of ketoprofen by the formation of multicomponent crystals with tromethamine. Crystals 2022, 12, 275. [Google Scholar] [CrossRef]
- Abdelkader, H.; Abdallah, O.Y.; Salem, H. Comparison of the effect of tromethamine and polyvinylpyrrolidone on dissolution properties and analgesic effect of nimesulide. AAPS PharmSciTech 2007, 8, E1–E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Moselhy, M.A.; Abdel-Hamid, N.M.; Abdel-Raheim, S.R. Gastroprotective effect of nicorandil in indomethacin and alcohol-induced acute Ulcers. Appl. Biochem. Biotechnol. 2009, 152, 449–459. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Labib, M.B.; Ali, W.A.M.; Kamel, G.; Azouz, A.A.; EL-Nahass, E.S. Design, synthesis, analgesic, anti-inflammatory activity of novel pyrazolones possessing aminosulfonyl pharmacophore as inhibitors of COX-2/5-LOX enzymes: Histopathological and docking studies. Bioorg. Chem. 2018, 78, 103–114. [Google Scholar] [CrossRef]
- Abdellatif, K.; Abdelall, E.; Elshemy, H.; Philoppes, J.; Hassanein, E.; Kahk, N. Optimization of pyrazole-based compounds with 1,2,4-triazole-3-thiol moiety as selective COX-2 inhibitors cardioprotective drug candidates: Design, synthesis, cyclooxygenase inhibition, anti-inflammatory, ulcerogenicity, cardiovascular evaluation, and molecular modeling studies. Bioorg. Chem. 2021, 114, 105122. [Google Scholar] [PubMed]
- Harakeh, S.; Saber, S.; Akefe, I.; Shaker, S.; Hussain, M.; Almasaudi, A.; Saleh, S.; Almasaudi, S. Saudi honey alleviates indomethacin-induced gastric ulcer via improving antioxidant and anti-inflammatory responses in male albino rats. Saudi J. Biol. Sci. 2022, 29, 3040–3050. [Google Scholar] [CrossRef]
- Al Fatease, A.; Shoman, M.; Abourehab, M.; Abou-Taleb, H.; Abdelkader, H. A Novel Curcumin Arginine Salt: A Solution for Poor Solubility and Potential Anticancer Activities. Molecules 2023, 28, 262. [Google Scholar] [CrossRef]
- Ishihara, S.; Hattori, Y.; Otsuka, M.; Sasaki, T. Cocrystal formation through solid-state reaction between ibuprofen and nicotinamide revealed using THz and IR spectroscopy with multivariate analysis. Crystals 2020, 10, 760. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of solubility and permeability class membership: Provisional BCS classification of the world’s top oral drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saers, E.; Nyström, C.; Aldén, M. Physicochemical aspects of drug release. XVI. The effect of storage on drug dissolution from solid dispersions and the influence of cooling rate and incorporation of surfactant. Int. J. Pharm. 1993, 90, 105–118. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Tape and layer structures in cocrystals of some di- and tricarboxylic acids with 4,4’- bipyridines and isonicotinamide. Cryst. Eng. Comm. 2005, 7, 551–562. [Google Scholar] [CrossRef]
- Delori, A.; Suresh, E.; Pedireddi, V.R. pKa-directed host- guest assemblies: Rational analysis of molecular adducts of 2,4-diamino-6-methyl-1,3,5-triazine with various aliphatic di- carboxylic acids. Chem. Eur. J 2008, 14, 6967–6977. [Google Scholar] [CrossRef]
- Mohamed, S.; Tocher, D.A.; Vickers, M.; Karamertzanis, P.G.; Price, S.L. Salts or cocrystals? A new series of crystal structures formed from simple pyridines and carboxylic acids. Cryst. Growth Des. 2009, 9, 2881–2889. [Google Scholar] [CrossRef]
- Cheney, M.L.; Weyna, D.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M. Coformer selection in pharmaceutical cocrystal development: A case study of a meloxicam aspirin cocrystal that exhibits enhanced solubility and pharmacokinetics. J. Pharm. Sci. 2011, 100, 2172–2181. [Google Scholar] [CrossRef]
- Shohin, I.; Kulinich, J.; Ramenskaya, G.; Abrahamsson, B.; Kopp, S.; Langguth, P.; Polli, J.; Shah, V.; Groot, D.; Barends, D.; et al. Biowaiver monographs for immediate release solid oral dosage forms: Ketoprofen. J. Pharm. Sci. 2012, 101, 3593–3603. [Google Scholar] [CrossRef]
- Cerciello, A.; Auriemma, G.; Del Gaudio, P.; Cantarini, M.; Aquino, R.P. Natural polysaccharides platforms for oral controlled release of ketoprofen lysine salt. Drug Dev. Ind. Pharm. 2016, 42, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, J.; Jee, U.; Rhyu, B. Ketoprofen lysinate. J. Korean Pharm. Sci. 1982, 12, 37–44. [Google Scholar]
- Adhage, N.A.; Vavia, P.R. β-Cyclodextrin Inclusion Complexation by Milling. Pharm. Pharmacol. Commun. 2000, 6, 13–17. [Google Scholar] [CrossRef]
- Irvin, R.T.; MacAlister, T.J.; Costerton, J.W. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J. Bacteriol. 1981, 145, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omachi, A.; Macey, R.; Waldeck, J.G. Permeability of cell membranes to amine buffers and their effects on electrolyte transport. Ann. N. Y. Acad. Sci. 1961, 92, 478–485. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.; Augustijns, P.; Brandl, M.; Brayden, D.; Brouwers, J.; Griffin, B.; Jacobsen, A.; Lennernäs, H.; Vinarov, Z.; O’Driscoll, C.M. Best practices in current models mimicking drug permeability in the gastrointestinal tract—An UNGAP review. Eur. J. Pharm. Sci. 2022, 170, 106098. [Google Scholar] [PubMed]
- Adinortey, M.B.; Ansah, A.; Galyuon, I.; Nyarko, A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Suleyman, H.; Abdulmecit, A.; Bilici, M.; Cadirci, E.; Halici, Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 2010, 33, 224–233. [Google Scholar] [CrossRef]
- Cimini, A.; Brandolini, L.; Gentile, R.; Cristiano, L.; Menghini, P.; Fidoamore, A.; Antonosante, A.; Benedetti, E.; Giordano, A.; Allegretti, M. Gastroprotective effects of L-lysine salification of ketoprofen in ethanol-injured gastric mucosa. J. Cell Physiol. 2015, 230, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T.; Konturek, S.; Sliwowski, Z.; Drozdowicz, D.; Zaczek, M.; Kedra, D. Role of L-arginine, a substrate for nitric oxide-synthase, in gastroprotection and ulcer healing. J. Gastroenterol. 1997, 32, 442–452. [Google Scholar] [CrossRef]








| Group | Treatment |
|---|---|
| I | Solution of 0.25% CMC (Untreated) |
| II | Ketoprofen (K) suspended in 0.25% CMC |
| III | K:tris dispersed in 0.25% CMC |
| IV | K:L-lysine dispersed in 0.25% CMC |
| V | K:L-arginine dispersed in 0.25% CMC |
| Formulation | T50% (min) * | RDR60 ** | RDR300 *** |
|---|---|---|---|
| Ketoprofen powder | 240 | - | - |
| Ketofan capsule | 120 | 2 | 1.75 |
| K:tris PM | 180 | 1.28 | 1.26 |
| K:tris Coppt | 120 | 1.6 | 1.8 |
| K:lysine PM | 120 | 1.6 | 1.5 |
| K:lysine Coppt | 60 | 2.08 | 1.92 |
| K:arginine PM | 120 | 1.74 | 1.74 |
| K:arginine Coppt | 30 | 2.68 | 2.07 |
| Test Substance | Ulcer Number | Ulcer Index |
| Control | 0 ± 0.0 | 0 |
| Indomethacin | 8.66 ± 0.88 a | 7.8 a |
| Ketoprofen | 3.33 ± 0.66 a,b | 3.59 a,b |
| Ketoprofen:tris | 2.00 ± 0.0 b | 1.1 a,b,c |
| Ketoprofen:lysine | 1.0 ± 0.33 b,c | 0.55 a,b,c |
| Ketoprofen:arginine | 0.53.00 ± 0.17 b | 0.33 a,b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou-Taleb, H.A.; Shoman, M.E.; Makram, T.S.; Abdel-Aleem, J.A.; Abdelkader, H. Exploration of the Safety and Solubilization, Dissolution, Analgesic Effects of Common Basic Excipients on the NSAID Drug Ketoprofen. Pharmaceutics 2023, 15, 713. https://doi.org/10.3390/pharmaceutics15020713
Abou-Taleb HA, Shoman ME, Makram TS, Abdel-Aleem JA, Abdelkader H. Exploration of the Safety and Solubilization, Dissolution, Analgesic Effects of Common Basic Excipients on the NSAID Drug Ketoprofen. Pharmaceutics. 2023; 15(2):713. https://doi.org/10.3390/pharmaceutics15020713
Chicago/Turabian StyleAbou-Taleb, Heba A., Mai E. Shoman, Tarek Saad Makram, Jelan A. Abdel-Aleem, and Hamdy Abdelkader. 2023. "Exploration of the Safety and Solubilization, Dissolution, Analgesic Effects of Common Basic Excipients on the NSAID Drug Ketoprofen" Pharmaceutics 15, no. 2: 713. https://doi.org/10.3390/pharmaceutics15020713
APA StyleAbou-Taleb, H. A., Shoman, M. E., Makram, T. S., Abdel-Aleem, J. A., & Abdelkader, H. (2023). Exploration of the Safety and Solubilization, Dissolution, Analgesic Effects of Common Basic Excipients on the NSAID Drug Ketoprofen. Pharmaceutics, 15(2), 713. https://doi.org/10.3390/pharmaceutics15020713







