Apelin Is a Prototype of Novel Drugs for the Treatment of Acute Myocardial Infarction and Adverse Myocardial Remodeling
Abstract
:1. Introduction
2. Discovery of the Apelin Receptor and Apelins
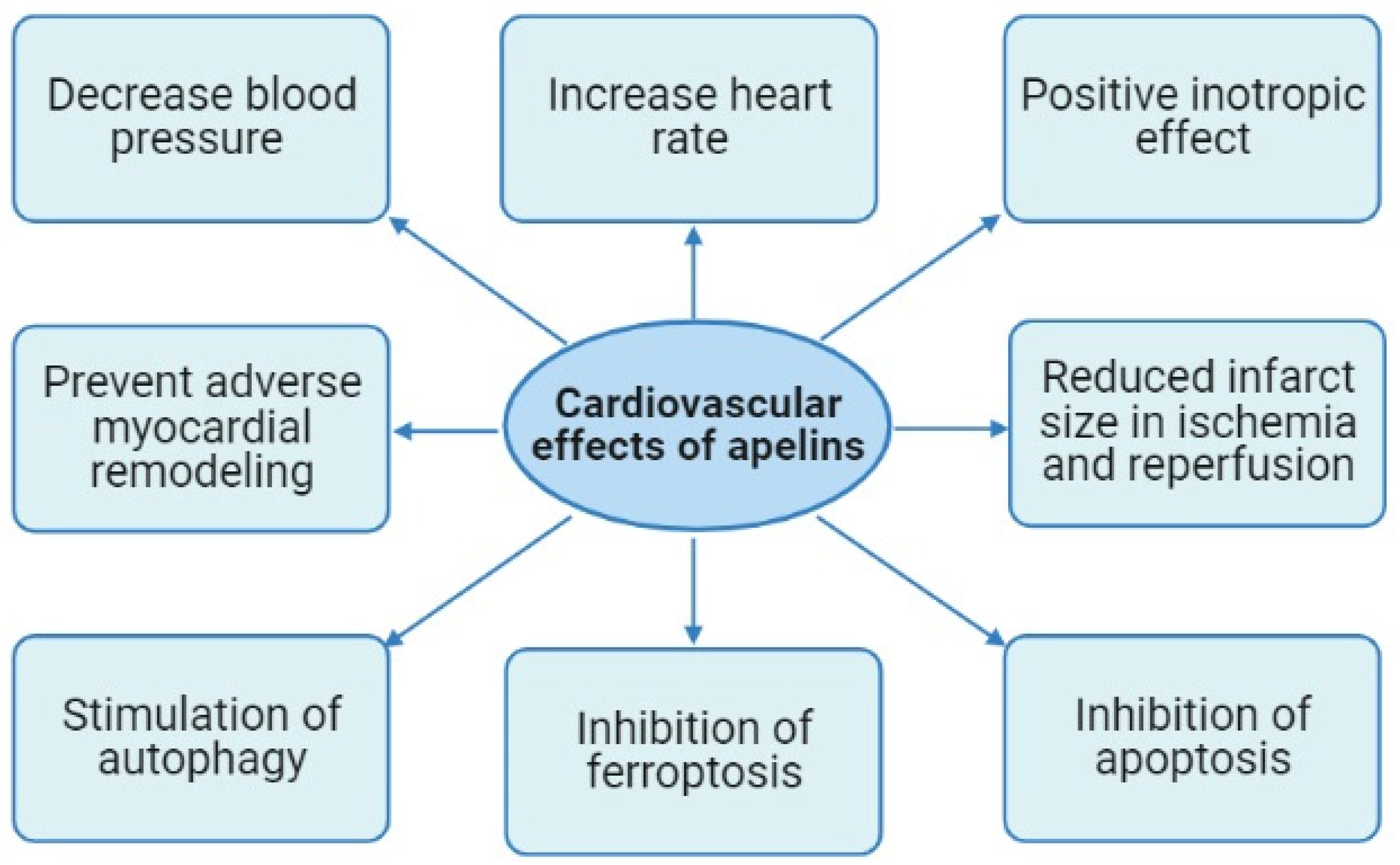
3. Cardiovascular Effects of Apelins
3.1. The Expression of Apelins and Their Receptor in Rats and Humans
3.2. The Effects of Apelins on Blood Pressure and Heart Rate
3.3. The Effect of Apelins on the Contractile Function of the Heart
3.4. The Cardioprotective Effect of Apelins in Ischemia and Reperfusion of the Heart
3.5. Apelins Prevent Adverse Myocardial Remodeling
3.6. The Effects of Apelins on Regulated Cell Death
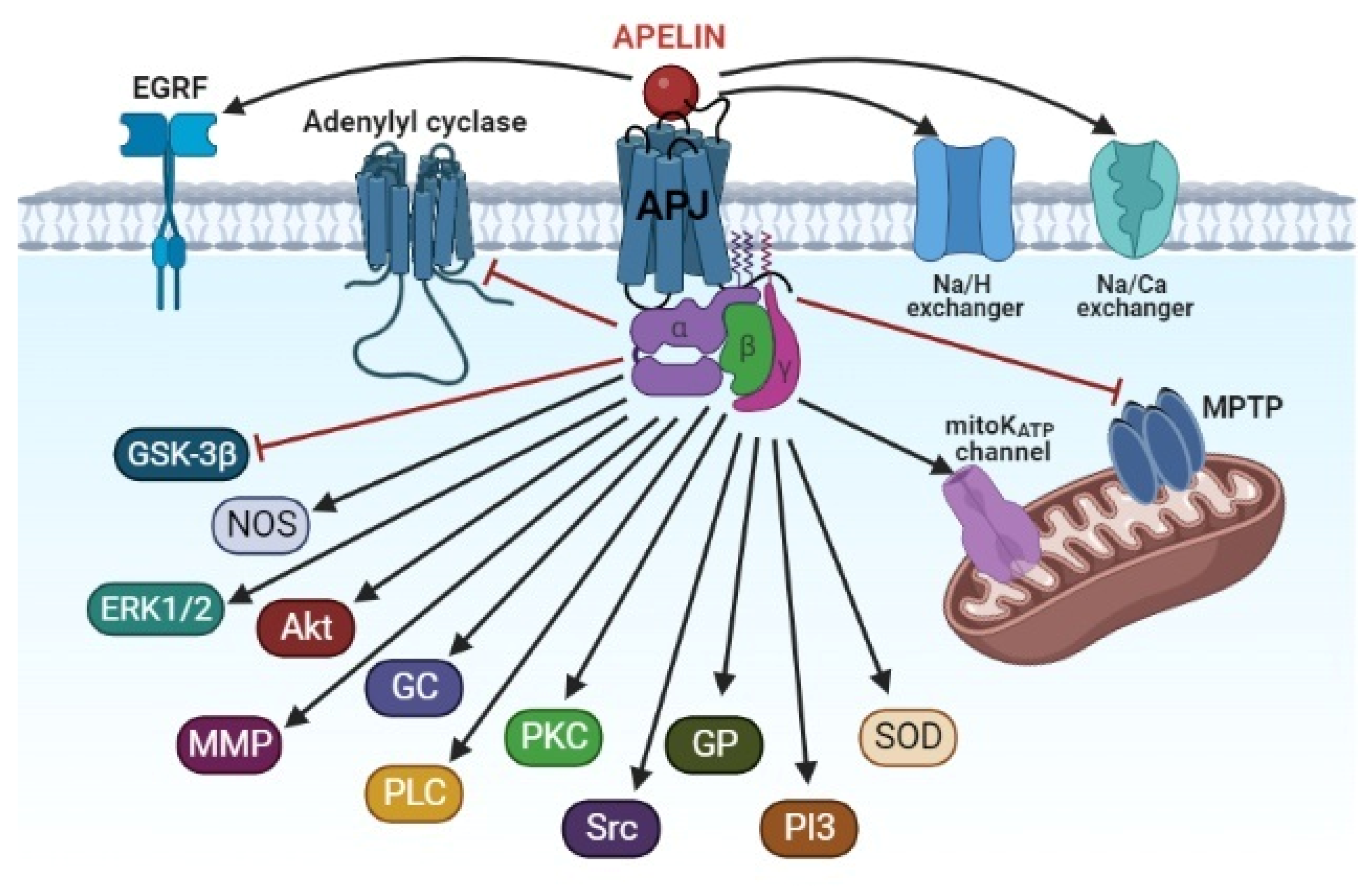
4. The Signaling Mechanism of the Cardioprotective Effect of Apelins
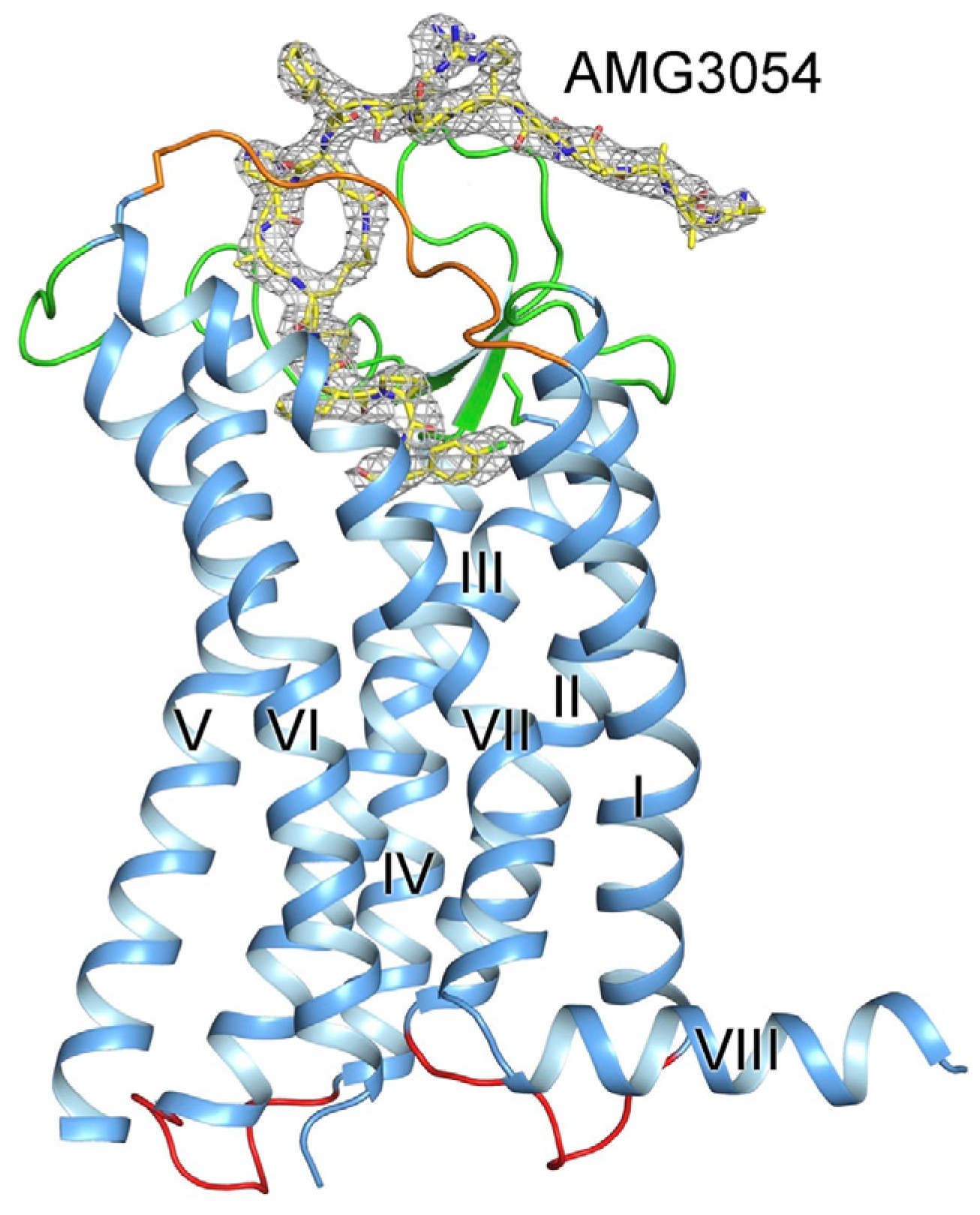
5. The Synthetic Analogues of Apelins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Welch, T.D.; Yang, E.H.; Reeder, G.S.; Gersh, B.J. Modern management of acute myocardial infarction. Curr. Probl. Cardiol. 2012, 37, 237–310. [Google Scholar] [CrossRef] [PubMed]
- Megaly, M.; Pershad, A.; Glogoza, M.; Elbadawi, A.; Omer, M.; Saad, M.; Mentias, A.; Elgendy, I.; Burke, M.N.; Capodanno, D.; et al. Use of intravascular imaging in patients with ST-segment elevation acute myocardial infarction. Cardiovasc. Revasc. Med. 2021, 30, 59–64. [Google Scholar] [CrossRef]
- Ya’qoub, L.; Gad, M.; Saad, A.M.; Elgendy, I.Y.; Mahmoud, A.N. National trends of utilization and readmission rates with intravascular ultrasound use for ST-elevation myocardial infarction. Catheter. Cardiovasc. Interv. 2021, 98, 1–9. [Google Scholar] [CrossRef]
- Garcia, S.; Schmidt, C.W.; Garberich, R.; Henry, T.D.; Bradley, S.M.; Brilakis, E.S.; Burke, N.; Chavez, I.J.; Eckman, P.; Gössl, M.; et al. Temporal changes in patient characteristics and outcomes in ST-segment elevation myocardial infarction 2003–2018. Catheter. Cardiovasc. Interv. 2021, 97, 1109–1117. [Google Scholar] [CrossRef]
- Basir, M.B.; Lemor, A.; Gorgis, S.; Taylor, A.M.; Tehrani, B.; Truesdell, A.G.; Bharadwaj, A.; Kolski, B.; Patel, K.; Gelormini, J.; et al. National Cardiogenic Shock Initiative Investigators. Vasopressors independently associated with mortality in acute myocardial infarction and cardiogenic shock. Catheter. Cardiovasc. Interv. 2022, 99, 650–657. [Google Scholar] [CrossRef]
- Sambola, A.; Elola, F.J.; Buera, I.; Fernández, C.; Bernal, J.L.; Ariza, A.; Brindis, R.; Bueno, H.; Rodríguez-Padial, L.; Marín, F.; et al. Sex bias in admission to tertiary-care centres for acute myocardial infarction and cardiogenic shock. Eur. J. Clin. Investig. 2021, 51, e13526. [Google Scholar] [CrossRef]
- Liakopoulos, O.J.; Schlachtenberger, G.; Wendt, D.; Choi, Y.H.; Slottosch, I.; Welp, H.; Schiller, W.; Martens, S.; Welz, A.; Neuhäuser, M.; et al. Early clinical outcomes of surgical myocardial revascularization for acute coronary syndromes complicated by cardiogenic shock: A Report from the North-Rhine-Westphalia Surgical Myocardial Infarction Registry. J. Am. Heart Assoc. 2019, 8, e012049. [Google Scholar] [CrossRef]
- Braile-Sternieri, M.C.V.B.; Mustafa, E.M.; Ferreira, V.R.R.; Braile Sabino, S.; Braile Sternieri, G.; Buffulin de Faria, L.A.; Sbardellini, B.C.; Vianna Queiroz, C.O.; Braile, D.M.; Zotarelli Filho, I.J. Main considerations of cardiogenic shock and its predictors: Systematic review. Cardiol. Res. 2018, 9, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslov, L.N.; Popov, S.V.; Mukhomedzyanov, A.V.; Naryzhnaya, N.V.; Voronkov, N.S.; Ryabov, V.V.; Boshchenko, A.A.; Khaliulin, I.; Prasad, N.R.; Fu, F.; et al. Reperfusion cardiac injury: Receptors and the signaling mechanisms. Curr. Cardiol. Rev. 2022, 18, 63–79. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, B.F.; Heiber, M.; Chan, A.; Heng, H.H.; Tsui, L.C.; Kennedy, J.L.; Shi, X.; Petronis, A.; George, S.R.; Nguyen, T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993, 136, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Devic, E.; Paquereau, L.; Vernier, P.; Knibiehler, B.; Audigier, Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech. Dev. 1996, 59, 129–140. [Google Scholar] [CrossRef]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, Y.; Ma, Y.; Zhang, Q.; Zhou, Q.; Song, Y.; Shen, Y.; Li, X.; Ma, X.; Li, C.; et al. Structural Basis for Apelin Control of the Human Apelin Receptor. Structure 2017, 25, 858–866.e4. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Wang, Z.; Zhang, R.; Sun, W.; Chen, X. The role of apelin/apelin receptor in energy metabolism and water homeostasis: A comprehensive narrative review. Front. Physiol. 2021, 12, 632886. [Google Scholar] [CrossRef] [PubMed]
- Chng, S.C.; Ho, L.; Tian, J.; Reversade, B. ELABELA: A hormone essential for heart development signals via the apelin receptor. Dev. Cell. 2013, 27, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Pauli, A.; Norris, M.L.; Valen, E.; Chew, G.L.; Gagnon, J.A.; Zimmerman, S.; Mitchell, A.; Ma, J.; Dubrulle, J.; Reyon, D.; et al. Toddler: An embryonic signal that promotes cell movement via Apelin receptors. Science 2014, 343, 1248636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, D.; Ni, L.; Shi, L.; Xu, W.; Shi, M.; Chen, J.; Ai, Y.; Zhang, X. Serum elabela/toddler levels are associated with albuminuria in patients with type 2 diabetes. Cell Physiol. Biochem. 2018, 48, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Perjes, A.; Skoumal, R.; Tenhunen, O.; Kónyi, A.; Simon, M.; Horváth, I.G.; Kerkelä, R.; Ruskoaho, H.; Szokodi, I. Apelin increases cardiac contractility via protein kinase Cε- and extracellular signal-regulated kinase-dependent mechanisms. PLoS ONE 2014, 9, e93473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamata, Y.; Habata, Y.; Fukusumi, S.; Hosoya, M.; Fujii, R.; Hinuma, S.; Nishizawa, N.; Kitada, C.; Onda, H.; Nishimura, O.; et al. Molecular properties of apelin: Tissue distribution and receptor binding. Biochim. Biophys. Acta 2001, 1538, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef]
- Than, A.; He, H.L.; Chua, S.H.; Xu, D.; Sun, L.; Leow, M.K.; Chen, P. Apelin enhances brown adipogenesis and browning of white adipocytes. J. Biol. Chem. 2015, 290, 14679–14691. [Google Scholar] [CrossRef] [Green Version]
- Gargalovic, P.; Wong, P.; Onorato, J.; Finlay, H.; Wang, T.; Yan, M.; Crain, E.; St-Onge, S.; Héroux, M.; Bouvier, M.; et al. In vitro and in vivo evaluation of a small-molecule APJ (apelin receptor) agonist, BMS-986224, as a potential treatment for heart failure. Circ. Heart Fail. 2021, 14, e007351. [Google Scholar] [CrossRef]
- Chen, M.M.; Ashley, E.A.; Deng, D.X.; Tsalenko, A.; Deng, A.; Tabibiazar, R.; Ben-Dor, A.; Fenster, B.; Yang, E.; King, J.Y.; et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 2003, 108, 1432–1439. [Google Scholar] [CrossRef]
- Ustunel, I.; Acar, N.; Gemici, B.; Ozbey, O.; Edizer, I.; Soylu, H.; Tepekoy, F.; Izgut-Uysal, V.N. The effects of water immersion and restraint stress on the expressions of apelin, apelin receptor (APJR) and apoptosis rate in the rat heart. Acta Histochem. 2014, 116, 675–681. [Google Scholar] [CrossRef]
- Sekerci, R.; Acar, N.; Tepekoy, F.; Ustunel, I.; Keles-Celik, N. Apelin/APJ expression in the heart and kidneys of hypertensive rats. Acta Histochem. 2018, 120, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Danton, M.H.; Lang, A.D.; Lyall, F. Apelin receptor (APJ) expression during cardiopulmonary bypass in children undergoing surgical repair. PLoS ONE 2014, 9, e106262. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; Sun, C.; O’Rourke, S.T. Activation of large conductance, calcium-activated potassium channels by nitric oxide mediates apelin-induced relaxation of isolated rat coronary arteries. J. Pharmacol. Exp. Ther. 2018, 366, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.K.; Cheng, R.; Nguyen, T.; Fan, T.; Kariyawasam, A.P.; Liu, Y.; Osmond, D.H.; George, S.R.; O’Dowd, B.F. Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 2000, 74, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Katovich, M.J.; Mecca, A.; Rowland, N.E. Effects of central and peripheral injections of apelin on fluid intake and cardiovascular parameters in rats. Physiol. Behav. 2006, 89, 221–225. [Google Scholar] [CrossRef]
- Pisarenko, O.I.; Serebriakova, L.I.; Pelogeĭkina, I.A.; Studneva, I.M.; Kkhatri, D.N.; Tskitishvili, O.V.; Bespalova, Z.hD.; Az’muko, A.A.; Sidorova, M.V.; Pal’keeva, M.E.; et al. Involvement of NO-dependent mechanisms of apelin action in myocardial protection against ischemia/reperfusion damage. Kardiologiia 2012, 52, 52–58. [Google Scholar]
- Azizi, Y.; Faghihi, M.; Imani, A.; Roghani, M.; Nazari, A. Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the rat model of myocardial infarction. Peptides 2013, 46, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; McKinnie, S.M.; Farhan, M.; Paul, M.; McDonald, T.; McLean, B.; Llorens-Cortes, C.; Hazra, S.; Murray, A.G.; Vederas, J.C.; et al. Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-Apelin-13 and Apelin-17: Physiological effects in the cardiovascular system. Hypertension 2016, 68, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, K.X.; Fischer, C.; Vu, J.; Gheblawi, M.; Wang, W.; Gottschalk, S.; Iturrioz, X.; Llorens-Cortés, C.; Oudit, G.Y.; Vederas, J.C. Metabolically stable apelin-analogues, incorporating cyclohexylalanine and homoarginine, as potent apelin receptor activators. RSC Med. Chem. 2021, 12, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Pisarenko, O.I.; Serebryakova, L.I.; Pelogeykina, Y.A.; Studneva, I.M.; Khatri, D.N.; Tskitishvili, O.V.; Bespalova, Z.D.; Az’muko, A.A.; Sidorova, M.V.; Pal’keeva, M.E. In vivo reduction of reperfusion injury to the heart with apelin-12 peptide in rats. Bull. Exp. Biol. Med. 2011, 152, 79–82. [Google Scholar] [CrossRef]
- Japp, A.G.; Cruden, N.L.; Barnes, G.; van Gemeren, N.; Mathews, J.; Adamson, J.; Johnston, N.R.; Denvir, M.A.; Megson, I.L.; Flapan, A.D.; et al. Acute cardiovascular effects of apelin in humans: Potential role in patients with chronic heart failure. Circulation 2010, 121, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.J.; Kleinz, M.J.; Pitkin, S.L.; Davenport, A.P. [Pyr1]Apelin-13 identified as the predominant apelin isoform in the human heart: Vasoactive mechanisms and inotropic action in disease. Hypertension 2009, 54, 598–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szokodi, I.; Tavi, P.; Foldes, G.; Voutilainen-Myllyla, S.; Ilves, M.; Tokola, H.; Pikkarainen, S.; Piuhola, J.; Rysa, J.; Toth, M.; et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 2002, 91, 434–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Du, J.F.; Wu, F.; Wang, H.C. Apelin decreases the SR Ca2+ content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2540–H2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostamzadeh, F.; Najafipour, H.; Yeganeh-Hajahmadi, M.; Esmaeili-Mahani, S.; Joukar, S.; Iranpour, M. Heterodimerization of apelin and opioid receptors and cardiac inotropic and lusitropic effects of apelin in 2K1C hypertension: Role of pERK1/2 and PKC. Life Sci. 2017, 191, 24–33. [Google Scholar] [CrossRef]
- Simpkin, J.C.; Yellon, D.M.; Davidson, S.M.; Lim, S.Y.; Wynne, A.M.; Smith, C.C. Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia-reperfusion injury. Basic Res. Cardiol. 2007, 102, 518–528. [Google Scholar] [CrossRef]
- Kleinz, M.J.; Baxter, G.F. Apelin reduces myocardial reperfusion injury independently of PI3K/Akt and P70S6 kinase. Regul. Pept. 2008, 146, 271–277. [Google Scholar] [CrossRef]
- Zeng, X.J.; Zhang, L.K.; Wang, H.X.; Lu, L.Q.; Ma, L.Q.; Tang, C.S. Apelin protects the heart against ischemia/reperfusion injury in rat. Peptides 2009, 30, 1144–1152. [Google Scholar] [CrossRef]
- Pisarenko, O.I.; Shulzhenko, V.S.; Pelogeĭkina, I.A.; Studneva, I.M.; Kkhatri, D.N.; Bespalova, Z.D.; Az’muko, A.A.; Sidorova, M.V.; Pal’keeva, M.E. Effects of exogenous apelin-12 on functional and metabolic recovery of isolated rat heart after ischemia. Kardiologiia 2010, 50, 44–49. [Google Scholar]
- Pisarenko, O.I.; Pelogeĭkina, I.A.; Shul’zhenko, V.S.; Studneva, I.M.; Bespalova, Z.D.; Az’muko, A.A.; Sidorova, M.V.; Pal’keeva, M.E. The influence of inhibiting no formation on metabolic recovery of ischemic rat heart by apelin-12. Biomed. Khim. 2012, 58, 702–711. [Google Scholar] [CrossRef]
- Rastaldo, R.; Cappello, S.; Folino, A.; Berta, G.N.; Sprio, A.E.; Losano, G.; Samaja, M.; Pagliaro, P. Apelin-13 limits infarct size and improves cardiac postischemic mechanical recovery only if given after ischemia. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2308–H2315. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Zhu, W.; Li, Y.; Xin, P.; Li, J.; Liu, M.; Li, J.; Redington, A.N.; Wei, M. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1471–H1486. [Google Scholar] [CrossRef]
- Pisarenko, O.I.; Bespalova, Z.D.; Lankin, V.Z.; Timoshin, A.A.; Serebriakova, L.I.; Shul’zhenko, V.S.; Pelogeĭkina, I.A.; Studneva, I.M.; Tskitishvili, O.V.; Az’muko, A.A.; et al. Antioxidant properties of apelin-12 and its structural analogue in experimental ischemia and reperfusion. Kardiologiia 2013, 53, 61–67. [Google Scholar] [PubMed]
- Wang, W.; McKinnie, S.M.; Patel, V.B.; Haddad, G.; Wang, Z.; Zhabyeyev, P.; Das, S.K.; Basu, R.; McLean, B.; Kandalam, V.; et al. Loss of apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: Therapeutic potential of synthetic apelin analogues. J. Am. Heart Assoc. 2013, 2, e000249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Liu, N.; Luan, R.; Li, Y.; Wang, D.; Zou, W.; Xing, Y.; Tao, L.; Cao, F.; Wang, H. Apelin protects sarcoplasmic reticulum function and cardiac performance in ischaemia-reperfusion by attenuating oxidation of sarcoplasmic reticulum Ca2+ -ATPase and ryanodine receptor. Cardiovasc. Res. 2013, 100, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boal, F.; Roumegoux, J.; Alfarano, C.; Timotin, A.; Calise, D.; Anesia, R.; Drougard, A.; Knauf, C.; Lagente, C.; Roncalli, J.; et al. Apelin regulates FoxO3 translocation to mediate cardioprotective responses to myocardial injury and obesity. Sci. Rep. 2015, 5, 16104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisarenko, O.I.; Shulzhenko, V.S.; Pelogeykina, Y.A.; Studneva, I.M. Enhancement of crystalloid cardioplegic protection by structural analogs of apelin-12. J. Surg. Res. 2015, 194, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Pisarenko, O.; Shulzhenko, V.; Studneva, I.; Pelogeykina, Y.; Timoshin, A.; Anesia, R.; Valet, P.; Parini, A.; Kunduzova, O. Structural apelin analogues: Mitochondrial ROS inhibition and cardiometabolic protection in myocardial ischaemia reperfusion injury. Br. J. Pharmacol. 2015, 172, 2933–2945. [Google Scholar] [CrossRef] [Green Version]
- Folino, A.; Accomasso, L.; Giachino, C.; Montarolo, P.G.; Losano, G.; Pagliaro, P.; Rastaldo, R. Apelin-induced cardioprotection against ischaemia/reperfusion injury: Roles of epidermal growth factor and Src. Acta Physiol. 2018, 222, e12924. [Google Scholar] [CrossRef] [PubMed]
- Abbasloo, E.; Najafipour, H.; Vakili, A. Chronic treatment with apelin, losartan and their combination reduces myocardial infarct size and improves cardiac mechanical function. Clin. Exp. Pharmacol. Physiol. 2020, 47, 393–402. [Google Scholar] [CrossRef]
- Li, L.; Zeng, H.; Chen, J.X. Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H605–H618. [Google Scholar] [CrossRef]
- Pchejetski, D.; Foussal, C.; Alfarano, C.; Lairez, O.; Calise, D.; Guilbeau-Frugier, C.; Schaak, S.; Seguelas, M.H.; Wanecq, E.; Valet, P.; et al. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur. Heart J. 2012, 33, 2360–2369. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Zhang, P.; Li, C.; Si, J.; Wang, Y.; Zhang, X.; Zhang, D.; Zhang, H.; Lin, C. Apelin 13 promotes cell proliferation in the H9c2 cardiomyoblast cell line by triggering extracellular signal regulated kinase 1/2 and protein kinase B phosphorylation. Mol. Med. Rep. 2018, 17, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.H.; Guo, C.X.; Wang, H.X.; Lu, L.Q.; Wang, Y.J.; Zhang, L.K.; Du, F.H.; Zeng, X.J. Cardioprotective effects of adipokine apelin on myocardial infarction. Heart Vessels 2014, 29, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, J.M.; Pfeffer, M.A.; Fletcher, P.J.; Braunwald, E. Progressive ventricular remodeling in rat with myocardial infarction. Am. J. Physiol. 1991, 260, H1406–H1414. [Google Scholar] [CrossRef]
- Azizi, Y.; Faghihi, M.; Imani, A.; Roghani, M.; Zekri, A.; Mobasheri, M.B.; Rastgar, T.; Moghimian, M. Post-infarct treatment with [Pyr1]apelin-13 improves myocardial function by increasing neovascularization and overexpression of angiogenic growth factors in rats. Eur. J. Pharmacol. 2015, 761, 101–108. [Google Scholar] [CrossRef]
- Azizi, Y.; Imani, A.; Fanaei, H.; Khamse, S.; Parvizi, M.R.; Faghihi, M. Post-infarct treatment with [Pyr1]apelin-13 exerts anti-remodelling and anti-apoptotic effects in rats’ hearts. Kardiol. Pol. 2017, 75, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Guo, H.; Wang, H.; Xing, D.; Lu, T.; Yang, J.; Wang, C. Apelin-13 alleviated cardiac fibrosis via inhibiting the PI3K/Akt pathway to attenuate oxidative stress in rats with myocardial infarction-induced heart failure. Biosci. Rep. 2020, 40, BSR20200040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Li, Q.; Yan, H.; Huang, J.; Wang, Z. Apela improves cardiac and renal function in mice with acute myocardial infarction. J. Cell Mol. Med. 2020, 24, 10382–10390. [Google Scholar] [CrossRef]
- Valls-Lacalle, L.; Consegal, M.; Ruiz-Meana, M.; Benito, B.; Inserte, J.; Barba, I.; Ferreira-González, I.; Rodríguez-Sinovas, A. Connexin 43 deficiency is associated with reduced myocardial scar size and attenuated TGFβ1 signaling after transient coronary occlusion in conditional knock-out mice. Biomolecules 2020, 10, 651. [Google Scholar] [CrossRef] [Green Version]
- Kuba, K.; Zhang, L.; Imai, Y.; Arab, S.; Chen, M.; Maekawa, Y.; Leschnik, M.; Leibbrandt, A.; Markovic, M.; Schwaighofer, J.; et al. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ. Res. 2007, 101, e32–e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Tang, J.; Song, J.; Xie, M.; Liu, Y.; Dong, Z.; Liu, X.; Li, X.; Zhang, M.; Chen, Y.; et al. Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling. Free Radic. Biol. Med. 2022, 181, 130–142. [Google Scholar] [CrossRef]
- An, S.; Wang, X.; Shi, H.; Zhang, X.; Meng, H.; Li, W.; Chen, D.; Ge, J. Apelin protects against ischemia-reperfusion injury in diabetic myocardium via inhibiting apoptosis and oxidative stress through PI3K and p38-MAPK signaling pathways. Aging 2020, 12, 25120–25137. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Ma, S.; Dai, X.; Cao, F. Elabela alleviates myocardial ischemia reperfusion-induced apoptosis, fibrosis and mitochondrial dysfunction through PI3K/AKT signaling. Am. J. Transl. Res. 2020, 12, 4467–4477. [Google Scholar]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Klionsky, D.J. Autophagy and human disease. Cell Cycle 2007, 6, 1837–1849. [Google Scholar] [CrossRef] [Green Version]
- Sala-Mercado. J.A.; Wider, J.; Undyala, V.V.; Jahania, S.; Yoo, W.; Mentzer, R.M., Jr.; Gottlieb, R.A.; Przyklenk, K. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation 2010, 122, S179–S184. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Hill, J.A.; Diwan, A. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 2012, 125, 3170–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giricz, Z.; Varga, Z.V.; Koncsos, G.; Nagy, C.T.; Görbe, A.; Mentzer, R.M.; Gottlieb, R.A.; Ferdinandy, P. Autophagosome formation is required for cardioprotection by chloramphenicol. Life Sci. 2017, 186, 11–16. [Google Scholar] [CrossRef]
- Xie, F.; Liu, W.; Feng, F.; Li, X.; He, L.; Lv, D.; Qin, X.; Li, L.; Li, L.; Chen, L. Apelin-13 promotes cardiomyocyte hypertrophy via PI3K-Akt-ERK1/2-p70S6K and PI3K-induced autophagy. Acta Biochim. Biophys. Sin. 2015, 47, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Zeng, H.; Tuo, Q.H.; Liao, D.F.; Chen, J.X. Apelin gene therapy increases autophagy via activation of Sirtuin 3 in diabetic heart. Diabetes Res. 2015, 1, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiao, X.; Zhang, L.; Li, X.; Liu, Q. Apelin-13 regulates angiotensin II-induced Cx43 downregulation and autophagy via the AMPK/mTOR signaling pathway in HL-1 cells. Physiol. Res. 2020, 69, 813–822. [Google Scholar] [CrossRef]
- Masri, B.; Morin, N.; Pedebernade, L.; Knibiehler, B.; Audigier, Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 2006, 281, 18317–18326. [Google Scholar] [CrossRef] [Green Version]
- Bai, B.; Cai, X.; Jiang, Y.; Karteris, E.; Chen, J. Heterodimerization of apelin receptor and neurotensin receptor 1 induces phosphorylation of ERK1/2 and cell proliferation via Gαq-mediated mechanism. J. Cell Mol. Med. 2014, 18, 2071–2081. [Google Scholar] [CrossRef]
- Chapman, N.A.; Dupré, D.J.; Rainey, J.K. The apelin receptor: Physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem. Cell Biol. 2014, 92, 431–440. [Google Scholar] [CrossRef] [Green Version]
- O’Harte, F.P.M.; Parthsarathy, V.; Hogg, C.; Flatt, P.R. Apelin-13 analogues show potent in vitro and in vivo insulinotropic and glucose lowering actions. Peptides 2018, 100, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.J.; Oh, D.Y.; Moon, J.S.; Kim, D.K.; Hwang, J.I.; Lee, J.Y.; Kim, J.I.; Cho, S.; Kwon, H.B.; Seong, J.Y. Cloning and activation of the bullfrog apelin receptor: Gi/o coupling and high affinity for [Pro1]apelin-13. Mol. Cell Endocrinol. 2007, 277, 51–60. [Google Scholar] [CrossRef]
- Deng, C.; Chen, H.; Yang, N.; Feng, Y.; Hsueh, A.J. Apela regulates fluid homeostasis by binding to the APJ receptor to activate Gi signaling. J. Biol. Chem. 2015, 290, 1826–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.C.; Mocanu, M.M.; Bowen, J.; Wynne, A.M.; Simpkin, J.C.; Dixon, R.A.; Cooper, M.B.; Yellon, D.M. Temporal changes in myocardial salvage kinases during reperfusion following ischemia: Studies involving the cardioprotective adipocytokine apelin. Cardiovasc. Drugs Ther. 2007, 21, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cai, Y.; Xia, E.; Shi, K.; Jin, Z.; Chen, H.; Xia, F.; Xia, Y.; Papadimos, T.J.; Xu, X.; et al. Apelin-13 reverses bupivacaine-induced cardiotoxicity via the adenosine monophosphate-activated protein kinase pathway. Anesth. Analg. 2021, 133, 1048–1059. [Google Scholar] [CrossRef]
- Pisarenko, O.I.; Lankin, V.Z.; Konovalova, G.G.; Serebryakova, L.I.; Shulzhenko, V.S.; Timoshin, A.A.; Tskitishvili, O.V.; Pelogeykina, Y.A.; Studneva, I.M. Apelin-12 and its structural analog enhance antioxidant defense in experimental myocardial ischemia and reperfusion. Mol. Cell Biochem. 2014, 391, 241–250. [Google Scholar] [CrossRef]
- Zhou, Q.; Cao, J.; Chen, L. Apelin/APJ system: A novel therapeutic target for oxidative stress-related inflammatory diseases (Review). Int. J. Mol. Med. 2016, 37, 1159–1169. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Li, H.; Tang, L.; Ge, G.; Ma, J.; Qiao, Z.; Liu, H.; Fang, W. Apelin-13 protects the heart against ischemia-reperfusion injury through the RISK-GSK-3β-mPTP pathway. Arch. Med. Sci. 2015, 11, 1065–1073. [Google Scholar] [CrossRef]
- Pisarenko, O.I.; Shulzhenko, V.S.; Studneva, I.M.; Serebryakova, L.I.; Pelogeykina, Y.A.; Veselova, O.M. Signaling pathways of a structural analogue of apelin-12 involved in myocardial protection against ischemia/reperfusion injury. Peptides 2015, 73, 67–76. [Google Scholar] [CrossRef]
- Reed, A.B.; Lanman, B.A.; Holder, J.R.; Yang, B.H.; Ma, J.; Humphreys, S.C.; Wang, Z.; Chan, J.C.Y.; Miranda, L.P.; Swaminath, G.; et al. Half-life extension of peptidic APJ agonists by N-terminal lipid conjugation. Bioorg. Med. Chem. Lett. 2020, 30, 127499. [Google Scholar] [CrossRef]
- Trân, K.; Murza, A.; Sainsily, X.; Coquerel, D.; Côté, J.; Belleville, K.; Haroune, L.; Longpré, J.M.; Dumaine, R.; Salvail, D.; et al. A systematic exploration of macrocyclization in apelin-13: Impact on binding, signaling, stability, and cardiovascular effects. J. Med. Chem. 2018, 61, 2266–2277. [Google Scholar] [CrossRef] [PubMed]
- O’Harte, F.P.M.; Parthsarathy, V.; Hogg, C.; Flatt, P.R. Long-term treatment with acylated analogues of apelin-13 amide ameliorates diabetes and improves lipid profile of high-fat fed mice. PLoS ONE 2018, 13, e0202350. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.; Sainsily, X.; Côté, J.; Coquerel, D.; Couvineau, P.; Saibi, S.; Haroune, L.; Besserer-Offroy, É.; Flynn-Robitaille, J.; Resua Rojas, M.; et al. Size-reduced macrocyclic analogues of [Pyr1]-apelin-13 showing negative Gα12 bias still produce prolonged cardiac effects. J. Med. Chem. 2022, 65, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Svendsgaard, D.J. U-shaped dose-response curves: Their occurrence and implications for risk assessment. J. Toxicol. Environ. Health 1990, 30, 71–83. [Google Scholar] [CrossRef] [PubMed]
| Peptide | Amino Acid Sequences |
|---|---|
| Preproapelin | MNLRLCVQALLLLWLSLTAVCGGSLMPLPDGNGLEDGNVRHLVQPRGSRNGPGPWQGGRRKFRRQRPRLSHKGPMPF |
| Apelin-55 | GSLMPLPDGNGLEDGNVRHLVQPRGSRNGPGPWQGGRRKFRRQRPRLSHKGPMPF |
| Apelin-36 | LVQPRGSRNGPGPWQGGRRKFRRQRPRLSHKGPMPF |
| Apelin-17 | KFRRQRPRLSHKGPMPF |
| Apelin-16 | FRRQRPRLSHKGPMPF |
| Apelin-13 | QRPRLSHKGPMPF |
| Pyr-apelin-13 | Pyr-RPRLSHKGPMPF |
| Apelin-12 | RPRLSHKGPMPF |
| Apelins | Model | Doses | Administration | Effect | References |
|---|---|---|---|---|---|
| Apelin-13 | Isolated mouse heart | 1 µmol | During reperfusion | Reduced infarct size | [22,40,45,49] |
| 30 pmol/L | During reperfusion | Improvement of contractile function | |||
| 30 pmol/L | During reperfusion | Reduced LDH and MDA | |||
| Apelin-13 | In vivo | 1 mg/kg | In reperfusion | Reduced infarct size | [40,46] |
| 1 µg/kg | 15 min before reperfusion | Prevention of necrosis and apoptosis | |||
| Apelin-12 | Isolated rat heart | 140 μmol/L | Prior to ischemia or at the onset of reperfusion | Improvement of contractile function after ischemia Increase in ATP Reduced lactate and LDH | [43,47] |
| Apelin-12 | In vivo | 0.5 mg/kg | Before reperfusion | Reduced infarct size | [34,47] |
| Pyr-apelin-13 | Isolated rat heart | 10 nmol/L | Prior to ischemia or after ischemia | Reduced infarct size after ischemia No effect before ischemia No improvement of contractile function | [41] |
| Apelins | Model | Doses | Mechanisms | References |
|---|---|---|---|---|
| Apelin-13 Apelin-36 | COS cells | 1 μmol | Stimulate Gi1 and Gi2 proteins, inhibit adenylyl cyclase, and cause the phosphorylation of ERK1/2 or Akt-kinase | [77] |
| Apelin-13 | HEK293 cells | 10 μmol | Activates Gq proteins and ERK1/2 | [78] |
| Isolated mouse heart | 1 μmol | Activates PI3-kinase, Akt, and ERK1/2-kinases | [40,83] | |
| In vivo | 1 μg/kg | Activates PI3 and ERK1/2-kinases, stimulates NOS | [46] | |
| Isolated rat heart | 1 μmol | Activates PKCε, PI3-kinases, and the mitoKATP-channel | [49] | |
| In vivo | 0.1 mg/kg | Activates PI3-kinase, Akt, ERK1/2, GSK-3β, and MPT pore closing | [87] | |
| Isolated rat heart | 0.5 µmol/L | Activates MMP, EGFR, Src kinase, the mitoKATP channel, and GC | [53] | |
| Apelin-12 | In vivo and in vitro | 140 μmol/L | Stimulates NOS Reduces the MDA level in myocardial tissue, increases SOD, GP, catalase, and DMPO Activates PLC, PKC, the Na+/H+ exchanger, the Na+/Ca2+ exchanger, ERK1/2, PI3-kinase, NOS, and the mitoKATP-channel | [43,44,47,85,88] |
| Pyr-apelin-13 | HEK293 cells | 80 nmol | Stimulates Gi/o and G12/13 proteins | [22] |
| In vivo | 10 nmol/kg | Reduces serum MDA, nitrite, and nitrate levels | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, S.V.; Maslov, L.N.; Mukhomedzyanov, A.V.; Kurbatov, B.K.; Gorbunov, A.S.; Kilin, M.; Azev, V.N.; Khlestkina, M.S.; Sufianova, G.Z. Apelin Is a Prototype of Novel Drugs for the Treatment of Acute Myocardial Infarction and Adverse Myocardial Remodeling. Pharmaceutics 2023, 15, 1029. https://doi.org/10.3390/pharmaceutics15031029
Popov SV, Maslov LN, Mukhomedzyanov AV, Kurbatov BK, Gorbunov AS, Kilin M, Azev VN, Khlestkina MS, Sufianova GZ. Apelin Is a Prototype of Novel Drugs for the Treatment of Acute Myocardial Infarction and Adverse Myocardial Remodeling. Pharmaceutics. 2023; 15(3):1029. https://doi.org/10.3390/pharmaceutics15031029
Chicago/Turabian StylePopov, Sergey V., Leonid N. Maslov, Alexandr V. Mukhomedzyanov, Boris K. Kurbatov, Alexandr S. Gorbunov, Michail Kilin, Viacheslav N. Azev, Maria S. Khlestkina, and Galina Z. Sufianova. 2023. "Apelin Is a Prototype of Novel Drugs for the Treatment of Acute Myocardial Infarction and Adverse Myocardial Remodeling" Pharmaceutics 15, no. 3: 1029. https://doi.org/10.3390/pharmaceutics15031029
APA StylePopov, S. V., Maslov, L. N., Mukhomedzyanov, A. V., Kurbatov, B. K., Gorbunov, A. S., Kilin, M., Azev, V. N., Khlestkina, M. S., & Sufianova, G. Z. (2023). Apelin Is a Prototype of Novel Drugs for the Treatment of Acute Myocardial Infarction and Adverse Myocardial Remodeling. Pharmaceutics, 15(3), 1029. https://doi.org/10.3390/pharmaceutics15031029





