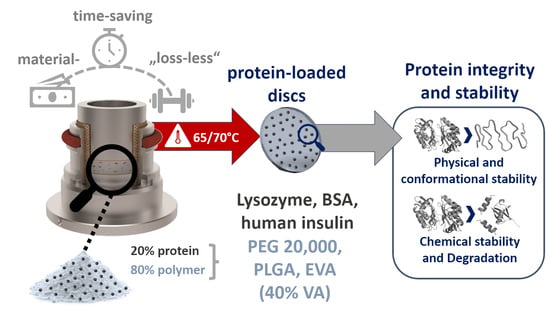
Micro-Scale Vacuum Compression Molding as a Predictive Screening Tool of Protein Integrity for Potential Hot-Melt Extrusion Processes
Abstract
Share and Cite
Dauer, K.; Wagner, K.G. Micro-Scale Vacuum Compression Molding as a Predictive Screening Tool of Protein Integrity for Potential Hot-Melt Extrusion Processes. Pharmaceutics 2023, 15, 723. https://doi.org/10.3390/pharmaceutics15030723
Dauer K, Wagner KG. Micro-Scale Vacuum Compression Molding as a Predictive Screening Tool of Protein Integrity for Potential Hot-Melt Extrusion Processes. Pharmaceutics. 2023; 15(3):723. https://doi.org/10.3390/pharmaceutics15030723
Chicago/Turabian StyleDauer, Katharina, and Karl G. Wagner. 2023. "Micro-Scale Vacuum Compression Molding as a Predictive Screening Tool of Protein Integrity for Potential Hot-Melt Extrusion Processes" Pharmaceutics 15, no. 3: 723. https://doi.org/10.3390/pharmaceutics15030723
APA StyleDauer, K., & Wagner, K. G. (2023). Micro-Scale Vacuum Compression Molding as a Predictive Screening Tool of Protein Integrity for Potential Hot-Melt Extrusion Processes. Pharmaceutics, 15(3), 723. https://doi.org/10.3390/pharmaceutics15030723