Formation of Hydrophilic Nanofibers from Nanostructural Design in the Co-Encapsulation of Celecoxib through Electrospinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Celecoxib Hydrophilic Nanofibers
2.2.1. Nanostructural Design in Co-Encapsulation of Celecoxib
2.2.2. Electrospinning
2.3. Nanocarrier Size, Polydispersity, and Particle Charge
2.4. Shape and Morphology
2.5. Encapsulation Efficiency
2.6. Determination of Drug Encapsulation Form in Nanofiber Membrane
2.6.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.2. Differential Scanning Calorimetry (DSC)
2.6.3. X-ray Diffraction (XRD)
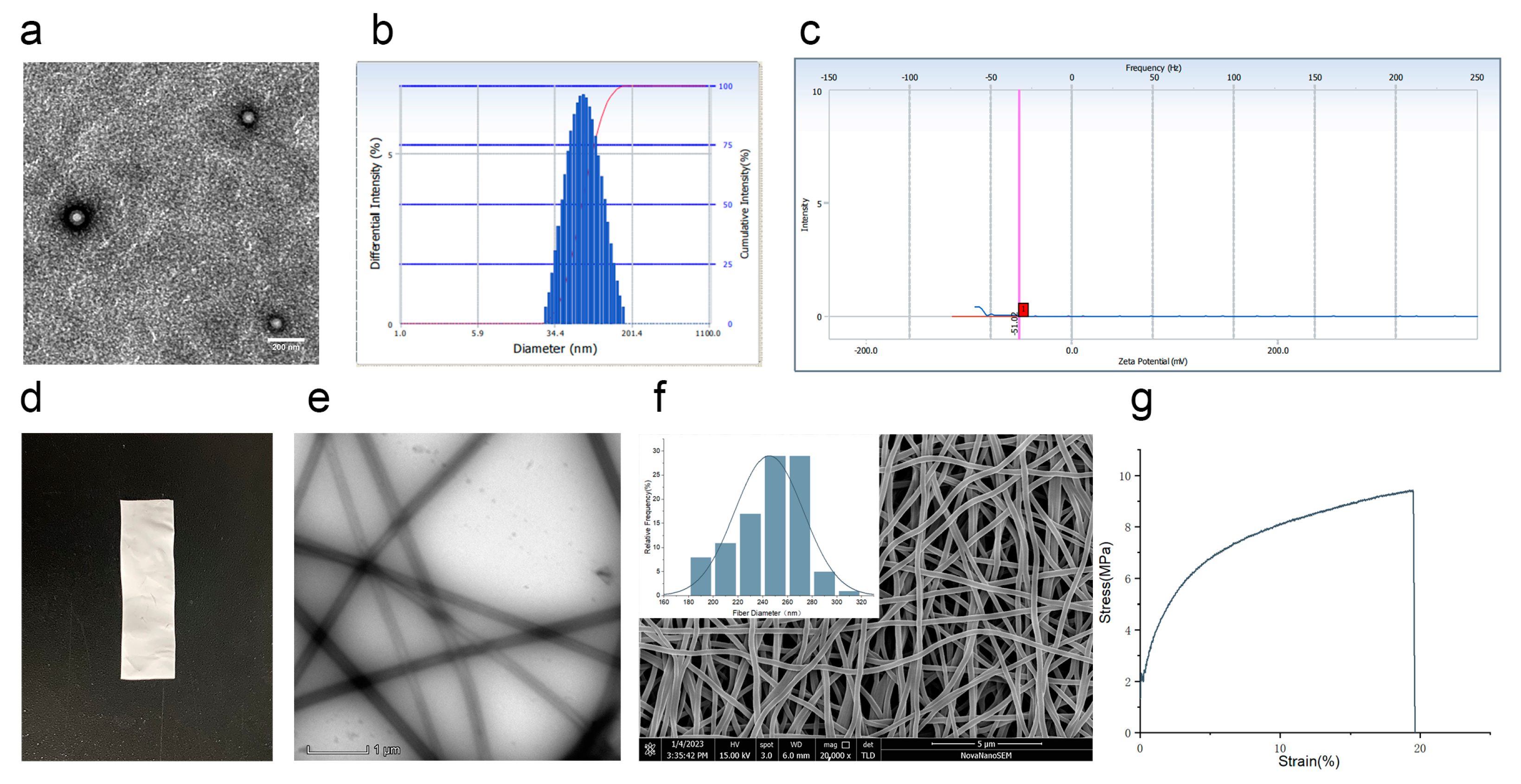
2.7. Performance Test of Nanofiber Membrane
2.8. In Vitro Celecoxib Release Study
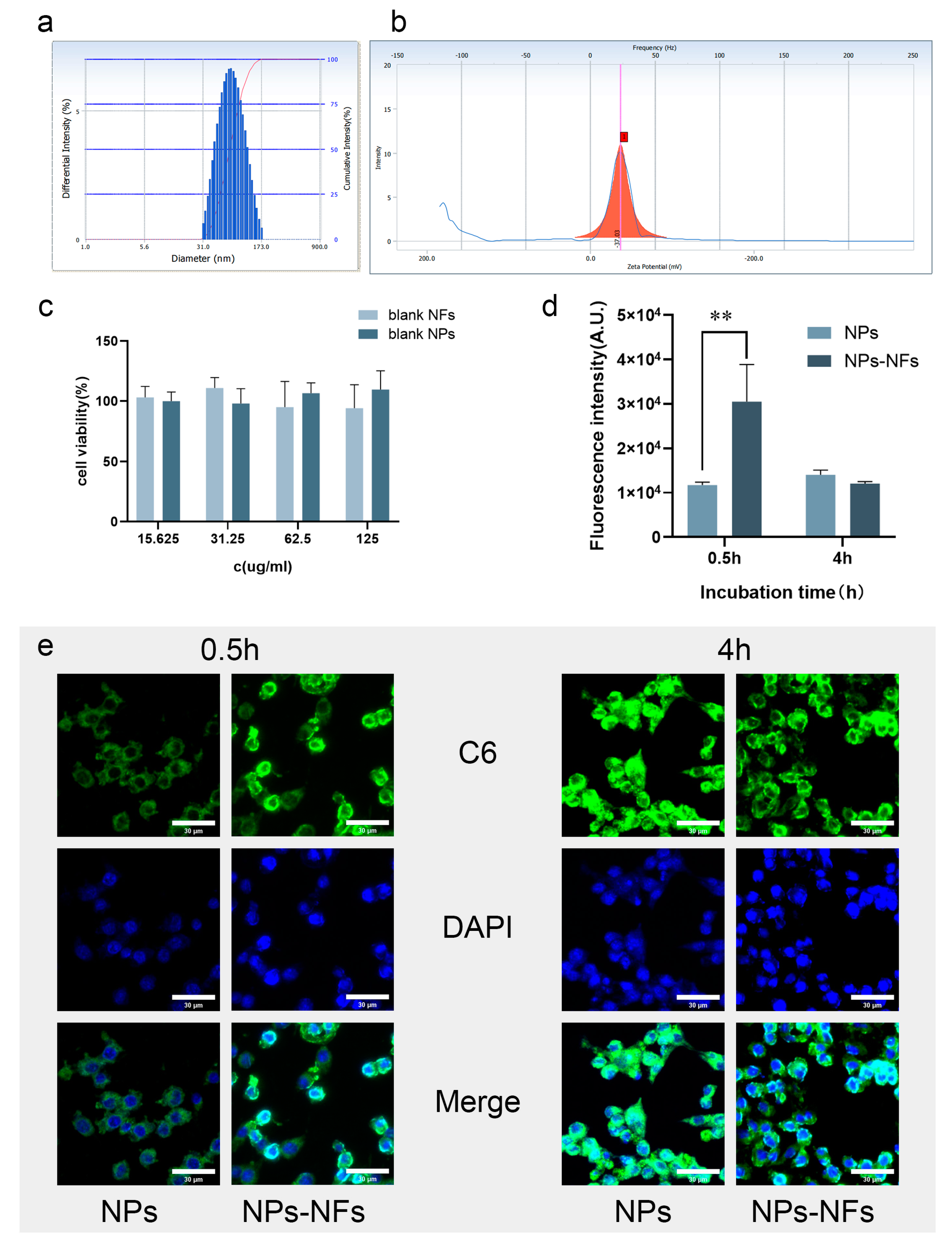
2.9. Evaluation of Cellular Uptake Efficiency
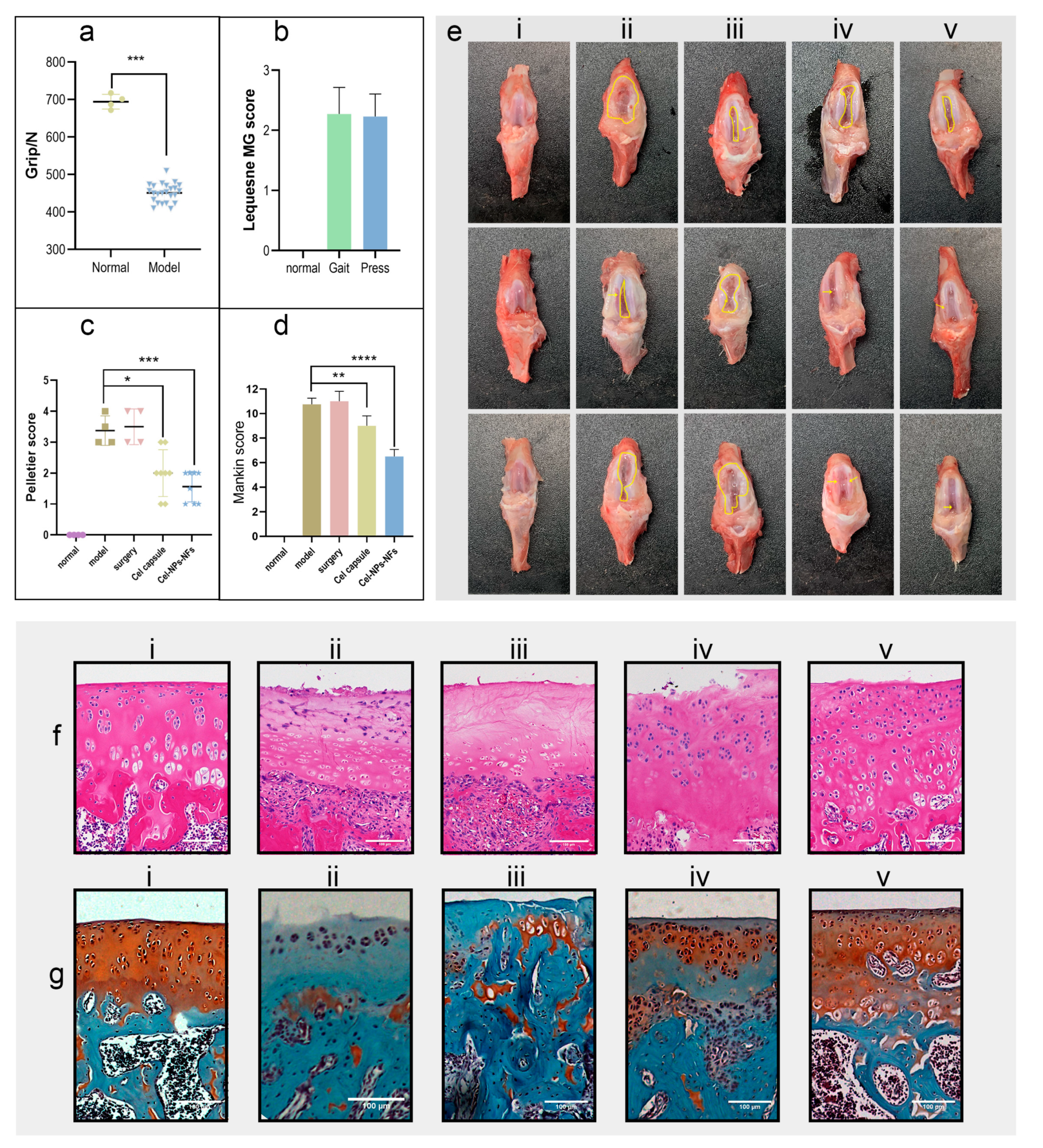
2.10. In Vivo Safety and Effect Study
2.11. Statistical Analysis
3. Results and Discussion
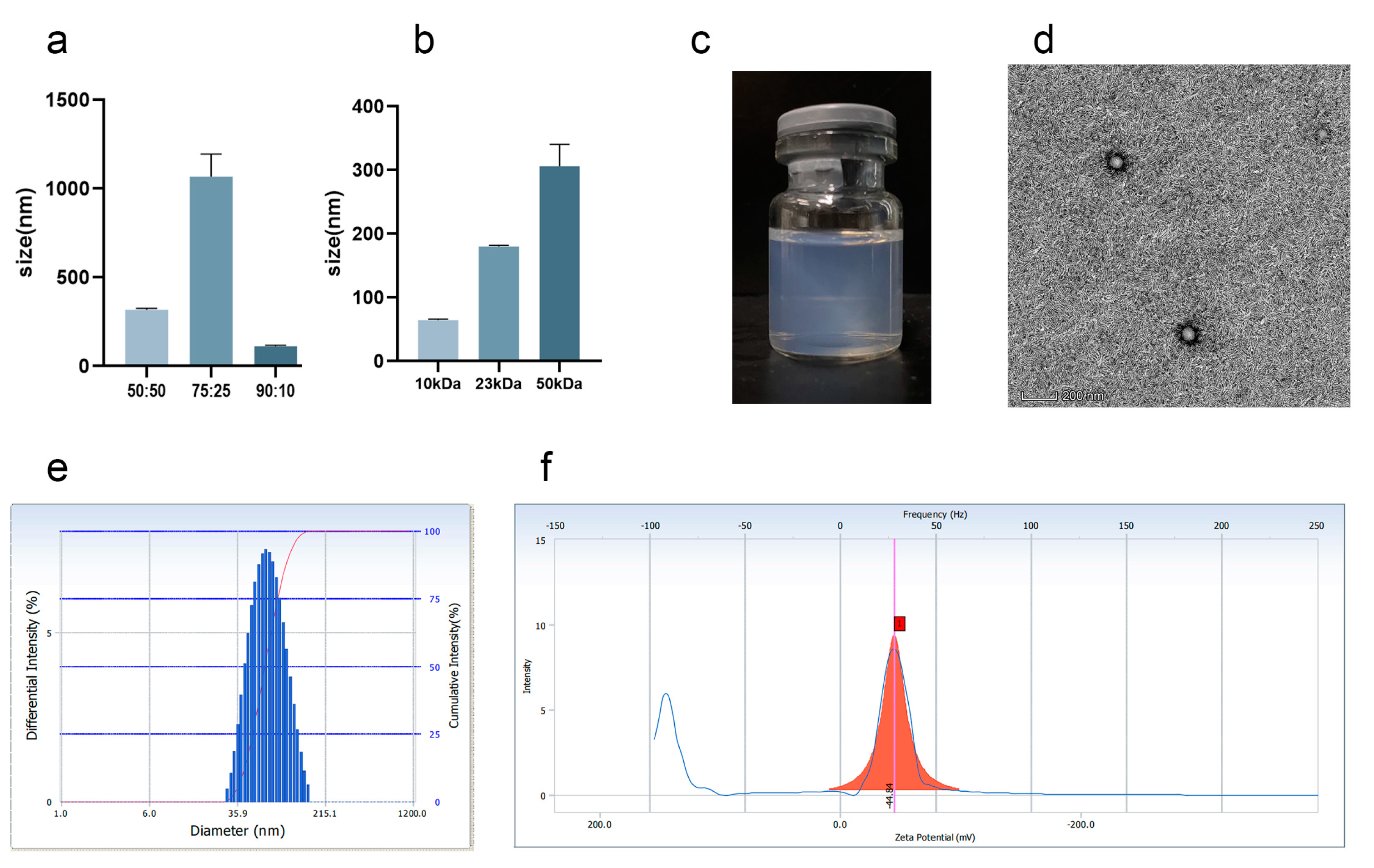
3.1. Preparation and Characterization of Cel-NPs
3.2. Characteristics of Co-Encapsulation Obtained by Nanoemulsion Structural Design
3.3. Analysis of Drug Distribution
3.4. Analysis of In Vitro Drug Release by Co-Encapsulation Cel-NPs-NFs
3.5. Analysis of Cellular Uptake Characteristics In Vitro
3.6. In Vivo Assessment of Cel-NPs-NFs Safety and Effectiveness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eaton, C.B.; Schaefer, L.F.; Duryea, J.; Driban, J.B.; Lo, G.H.; Roberts, M.B.; Haugen, I.K.; Lu, B.; Nevitt, M.C.; Hochberg, M.C.; et al. Prevalence, Incidence, and Progression of Radiographic and Symptomatic Hand Osteoarthritis: The Osteoarthritis Initiative. Arthritis Rheumatol. 2022, 74, 992–1000. [Google Scholar] [CrossRef]
- Gao, J.; Xia, Z.; Mary, H.B.; Joseph, J.; Luo, J.N.; Joshi, N. Overcoming barriers for intra-articular delivery of disease-modifying osteoarthritis drugs. Trends Pharmacol. Sci. 2022, 43, 171–187. [Google Scholar] [CrossRef]
- Kavanaugh, T.E.; Werfel, T.A.; Cho, H.; Hasty, K.A.; Duvall, C.L. Particle-based technologies for osteoarthritis detection and therapy. Drug Deliv. Transl. Res. 2016, 6, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, S.; Gowler, P.R.W.; Woodhams, S.G.; Turnbull, J.; Hathway, G.; Chapman, V. The challenges of treating osteoarthritis pain and opportunities for novel peripherally directed therapeutic strategies. Neuropharmacology 2022, 213, 109075. [Google Scholar] [CrossRef]
- Gerwin, N.; Hops, C.; Lucke, A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 226–242. [Google Scholar] [CrossRef]
- Larsen, C.; Ostergaard, J.; Larsen, S.W.; Jensen, H.; Jacobsen, S.; Lindegaard, C.; Andersen, P.H. Intra-articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders. J. Pharm. Sci. 2008, 97, 4622–4654. [Google Scholar] [CrossRef]
- Wallis, W.J.; Simkin, P.A.; Nelp, W.B. Protein traffic in human synovial effusions. Arthritis Rheum. 1987, 30, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef]
- Hongxu, Q.; Ping, H.; Jun, X.; Aijun, W. Encapsulation of Drug Reservoirs in Fibers by Emulsion Electrospinning: Morphology Characterization and Preliminary Release Assessment. Biomacromolecules 2006, 7, 2327–2330. [Google Scholar]
- Wang, Y.; Qiao, W.; Wang, B.; Zhang, Y.; Shao, P.; Yin, T. Electrospun composite nanofibers containing nanoparticles for the programmable release of dual drugs. Polym. J. 2011, 43, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Siddique, R.; Muhammad, F.; Aslam, B.; Faisal, M.N. Characterization and in vivo evaluation of nanoformulations in FCA induced rheumatoid arthritis in rats. Pak. J. Pharm. Sci. 2021, 34, 787–793. [Google Scholar]
- Duarte Junior, A.P.; Tavares, E.J.M.; Alves, T.V.G.; Moura, M.R.; Costa, C.E.F.; Silva Júnior, J.O.C.; Ribeiro Costa, R.M. Chitosan nanoparticles as a modified diclofenac drug release system. J. Nanoparticle Res. 2017, 19, 274. [Google Scholar] [CrossRef] [Green Version]
- Van Hees, S.; Elbrink, K.; De Schryver, M.; Delputte, P.L.; Kiekens, F. Improving cellular uptake and cytotoxicity of chitosan-coated poly(lactic-co-glycolic acid) nanoparticles in macrophages. Nanomedicine 2020, 15, 2671–2688. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Khattab, M.A.; Abd-Allah, H. Intra-articular multifunctional celecoxib loaded hyaluronan nanocapsules for the suppression of inflammation in an osteoarthritic rat model. Int. J. Pharm. 2020, 583, 119378. [Google Scholar] [CrossRef]
- Saudi, S.; Bhattarai, S.R.; Adhikari, U.; Khanal, S.; Sankar, J.; Aravamudhan, S.; Bhattarai, N. Nanonet-nano fiber electrospun mesh of PCL-chitosan for controlled and extended release of diclofenac sodium. Nanoscale 2020, 12, 23556–23569. [Google Scholar] [CrossRef]
- Lou, L.; Subbiah, S.; Smith, E.; Kendall, R.J.; Ramkumar, S.S. Functional PVA/VB2/TiO2 Nanofiber Webs for Controlled Drug Delivery. Appl. Bio Mater. 2019, 2, 5916–5929. [Google Scholar] [CrossRef]
- Marxen, I.; Schneider, J. Biochemische Synoviaanalyse und Bestimmung des Synoviavolumens an distalen Gelenken der Vordergliedmaße von Pferden. Tierärztl. Prax. Ausg. G Großtiere/Nutztiere 2002, 31, 52–56. [Google Scholar] [CrossRef]
- Song, J.; Fan, X.; Shen, Q. Daidzein-loaded nanostructured lipid carriers-PLGA nanofibers for transdermal delivery. Int. J. Pharm. 2016, 501, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeei, S.; Berenjian, K.; Khazaei, R. Preparation of the Potential Ocular Inserts by Electrospinning Method to Achieve the Prolong Release Profile of Triamcinolone Acetonide. Adv. Pharm. Bull. 2018, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Huang, Z.; Xing, R.; Zhang, N.; Jun, M.; Wang, P. Effect of Yiceng on the Synovial Fibrosis in KOA Rat Based on HMGB1. J. Nan Jing Univ. Tradit. Chin. Med. 2019, 35, 547–551. [Google Scholar]
- Yanxing, K.; Jiayi, G.; Feng, L.; Yiming, F.; Yuan, L.; Zhenya, W.; Yanxing, G. Research Advances in Animal Modeling of Knee Osteoarthritis. J. Liaoning Univ. Tcm 2020, 22, 205–208. [Google Scholar]
- Mastbergen, S.C.; Lafeber, F.P.; Bijlsma, J.W. Selective COX-2 inhibition prevents proinflammatory cytokine-induced cartilage damage. Rheumatology 2002, 41, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Faucher, M.; Poiraudeau, S.; Lefevre-Colau, M.M.; Rannou, F.; Fermanian, J.; Revel, M. Algo-functional assessment of knee osteoarthritis: Comparison of the test-retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarthr. Cartil. 2002, 10, 602–610. [Google Scholar] [CrossRef] [Green Version]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E.; Fuller, C.J.; Hurtig, M.; Cruz, A. The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr. Cartil. 2010, 18, S93–S105. [Google Scholar] [CrossRef] [Green Version]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Horisawa, E.; Kubota, K.; Tuboi, I.; Sato, K.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm. Res. 2002, 19, 132–139. [Google Scholar] [CrossRef]
- Jia, T.; Tian, D.; Zhang, Z.; Chai, Z.; Lu, C.; Li, H. Synthesis and Spectral Properties of Celecoxib. J. Synth. Cryst. 2016, 45, 2160–2166. [Google Scholar]
- Jiru, J.; Chaoxia, W.; Yunjie, Y. Preparation of indomethacin-loaded nanoparticle/PVA nanofiber film by electrospinning. New Chem. Mater. 2018, 46, 88–94. [Google Scholar]
- Mengyao, W.; Yaxiu, Z.; Jiaxin, L.; Yiran, K.; Hui, F.; Xiaoxuan, W.; Yuan, R.; Hongwei, Y. Middle Infrared Spectroscopy Study od Ployving Akohol. J. Textile Sci. Eng. 2021, 38, 48–52. [Google Scholar]
- Yuan, L.; Qunhua, Z.; Wenbo, Y.; Xiao, P.; Bing, D.; Pan, L.; Lanhua, X. Preparation and Properties of Sacha Inchi Oil/Polyvinyl Alcohol Nanofiber Membrane by Emulsion Electrospinning. J. Food Sci. 2021, 42, 233–240. [Google Scholar]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Hongze, L.; Min, Q.; Zhiyong, W.; Libo, L. Mathematical models for bulk erosion type drug delivery systems. J. Clin. Rehabilit. Tissue Eng. Res. 2009, 52, 10340–10344. [Google Scholar]
- Karishma, S.; Ayush, B.; Khushwant, S.Y. Nanoparticles incorporated in nanofibers using electrospinning: A novel nano-in-nano delivery system. J. Control. Release 2022, 350, 421–434. [Google Scholar]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87–100. [Google Scholar]
- Qaddoumi, M.G.; Gukasyan, H.J.; Davda, J.; Labhasetwar, V.; Kim, K.J.; Lee, V.H.L. Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: Role in PLGA nanoparticle endocytosis. Mol. Vision 2003, 9, 559–568. [Google Scholar]
- Herd, H.L.; Bartlett, K.T.; Gustafson, J.A.; McGill, L.D.; Ghandehari, H. Macrophage silica nanoparticle response is phenotypically dependent. Biomaterials 2015, 53, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Maghrebi, S.; Joyce, P.; Jambhrunkar, M.; Thomas, N.; Prestidge, C.A. Poly(lactic-co-glycolic) Acid-Lipid Hybrid Microparticles Enhance the Intracellular Uptake and Antibacterial Activity of Rifampicin. ACS Appl. Mater. Interfaces 2020, 12, 8030–8039. [Google Scholar] [CrossRef]



| Preparation | Drug Loading | Drug Loading Efficiency (%) | Encapsulation Efficiency of Nanoparticles (%) |
|---|---|---|---|
| Cel-NPs | 422.28 ± 16.90 µg/mL | 1.11 ± 0.04 | 88.23 ± 4.16 |
| Cel-NPs-NFs | 74.73 ± 0.99 µg/cm2 | 0.374 ± 0.005 |
| Preparation | Release Model | Fitting Equation | R2 |
|---|---|---|---|
| Cel-NPs | Zero-order kinetics | Q = 0.2753t + 49.1036 | 0.6725 |
| First-order kinetics | Q = 77.8053(1 − ) | 0.7692 | |
| Higuchi | Q = 4.2911+ 37.3436 | 0.8504 | |
| Ritger-Peppas | Q = 34.8465 | 0.9491 | |
| Cel-NPs-NFs | Zero-order kinetics | Q = 0.3816t + 11.5753 | 0.9319 |
| First-order kinetics | Q = 69.3391(1 − ) | 0.9767 | |
| Higuchi | Q = 5.5293 − 1.8841 | 0.9929 | |
| Ritger-Peppas | Q = 4.8077 | 0.9937 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, K.; Zhu, Y.; Lu, G.; Huang, S.; Yang, C.; Zheng, J.; Chen, J.; Ban, J.; Jia, H.; Lu, Z. Formation of Hydrophilic Nanofibers from Nanostructural Design in the Co-Encapsulation of Celecoxib through Electrospinning. Pharmaceutics 2023, 15, 730. https://doi.org/10.3390/pharmaceutics15030730
Chu K, Zhu Y, Lu G, Huang S, Yang C, Zheng J, Chen J, Ban J, Jia H, Lu Z. Formation of Hydrophilic Nanofibers from Nanostructural Design in the Co-Encapsulation of Celecoxib through Electrospinning. Pharmaceutics. 2023; 15(3):730. https://doi.org/10.3390/pharmaceutics15030730
Chicago/Turabian StyleChu, Kedi, Yi Zhu, Geng Lu, Sa Huang, Chuangzan Yang, Juying Zheng, Junming Chen, Junfeng Ban, Huanhuan Jia, and Zhufen Lu. 2023. "Formation of Hydrophilic Nanofibers from Nanostructural Design in the Co-Encapsulation of Celecoxib through Electrospinning" Pharmaceutics 15, no. 3: 730. https://doi.org/10.3390/pharmaceutics15030730





