The Pharmaceutical Formulation Plays a Pivotal Role in Hydroxytyrosol Pharmacokinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
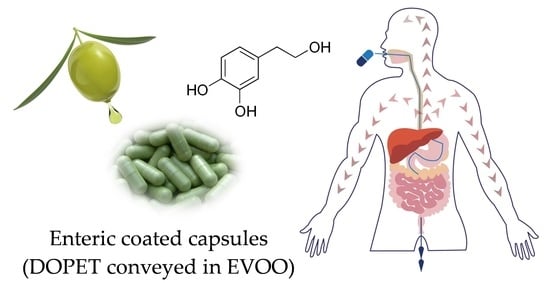
2.2. Study Design
2.3. Sample Preparation
2.4. Quali-Quantitative Determination of DOPET and Metabolites by LC-DAD-ESI-MS/MS
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urquiaga, I.; Echeverría, G.; Dussaillant, C.; Rigotti, A. Origin, components and mechanisms of action of the Mediterranean diet. Rev. Med. Chil. 2017, 145, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Olive Tree Derivatives and Hydroxytyrosol: Their Potential Effects on Human Health and Its Use as Functional Ingredient in Meat. Foods 2021, 10, 2611. [Google Scholar] [CrossRef]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Torre, R.; Covas, M.I.; Pujadas, M.A.; Fitó, M.; Farré, M. Is dopamine behind the health benefits of red wine? Eur. J. Nutr. 2006, 45, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa de Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell. Longev. 2017, 2017, 2473495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobili, V.; Alisi, A.; Mosca, A.; Crudele, A.; Zaffina, S.; Denaro, M.; Smeriglio, A.; Trombetta, D. The Antioxidant Effects of Hydroxytyrosol and Vitamin E on Pediatric Nonalcoholic Fatty Liver Disease, in a Clinical Trial: A New Treatment? Antioxid. Redox Signal. 2019, 31, 127–133. [Google Scholar] [CrossRef]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig. Liver Dis. 2021, 53, 1154–1158. [Google Scholar] [CrossRef]
- Panera, N.; Braghini, M.R.; Crudele, A.; Smeriglio, A.; Bianchi, M.; Condorelli, A.G.; Nobili, R.; Conti, L.A.; De Stefanis, C.; Lioci, G.; et al. Combination Treatment with Hydroxytyrosol and Vitamin E Improves NAFLD-Related Fibrosis. Nutrients 2022, 14, 3791. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Mastracci, L.; Grillo, F.; Cornara, L.; Shirooie, S.; Nabavi, S.M.; Trombetta, D. Safety and efficacy of hydroxytyrosol-based formulation on skin inflammation: In vitro evaluation on reconstructed human epidermis model. Daru 2019, 27, 283–293. [Google Scholar] [CrossRef]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Crudele, A.; Smeriglio, A.; Ingegneri, M.; Panera, N.; Bianchi, M.; Braghini, M.R.; Pastore, A.; Tocco, V.; Carsetti, R.; Zaffina, S.; et al. Hydroxytyrosol Recovers SARS-CoV-2-PLpro-Dependent Impairment of Interferon Related Genes in Polarized Human Airway, Intestinal and Liver Epithelial Cells. Antioxidants 2022, 11, 1466. [Google Scholar] [CrossRef] [PubMed]
- López de las Hazas, M.C.; Piñol, C.; Macià, A.; Romero, M.P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Nimmagadda, D.; Cherala, G.; Ghatta, S. Cytosolic sulfotransferases. Indian J. Exp. Biol. 2006, 44, 171–182. [Google Scholar] [PubMed]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Tzounis, X.; Dessì, M.A.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radical Res. 2006, 40, 647–658. [Google Scholar] [CrossRef]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human Absorption of a Supplement Containing Purified Hydroxytyrosol, a Natural Antioxidant from Olive Oil, and Evidence for Its Transient Association with Low-Density Lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef]
- Kountouri, A.M.; Mylona, A.; Kaliora, A.C.; Andrikopoulos, N.K. Bioavailability of the phenolic compounds of the fruits (drupes) of Olea europaea (olives): Impact on plasma antioxidant status in humans. Phytomedicine 2007, 14, 659–667. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Covas, M.I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [Green Version]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human Absorption and Metabolism of Oleuropein and Hydroxytyrosol Ingested as Olive (Olea europaea L.) Leaf Extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef]
- Visioli, F.; Bernardini, E. Extra virgin olive oil’s polyphenols: Biological activities. Curr. Pharm. Des. 2011, 17, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Valls, R.M.; Romero, M.P.; Macià, A.; Fernández, S.; Giralt, M.; Solà, R.; Motilva, M.J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011, 106, 1691–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubió, L.; Valls, R.M.; MacIà, A.; Pedret, A.; Giralt, M.; Romero, M.P.; De La Torre, R.; Covas, M.I.; Solà, R.; Motilva, M.J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Carrasco-Pancorbo, A.; Nevedomskaya, E.; Mayboroda, O.A.; Deelder, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Exploratory Analysis of Human Urine by LC-ESI-TOF MS after High Intake of Olive Oil: Understanding the Metabolism of Polyphenols. Anal. Bioanal. Chem. 2010, 398, 463–475. [Google Scholar] [CrossRef]
- Kendall, M.; Batterham, M.; Callahan, D.L.; Jardine, D.; Prenzler, P.D.; Robards, K.; Ryan, D. Randomized Controlled Study of the Urinary Excretion of Biophenols Following Acute and Chronic Intake of Olive Leaf Supplements. Food Chem. 2012, 130, 651–659. [Google Scholar] [CrossRef]
- Kotronoulas, A.; Pizarro, N.; Serra, A.; Robledo, P.; Joglar, J.; Rubió, L.; Hernaéz, Á.; Tormos, C.; Motilva, M.J.; Fitó, M.; et al. Dose-dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacol. Res. 2013, 77, 47–56. [Google Scholar] [CrossRef]
- Motilva, M.J.; Serra, A.; Rubió, L. Nutrikinetic studies of food bioactive compounds: From in vitro to in vivo approaches. Int. J. Food Sci. Nutr. 2015, 66, S41–S52. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Farré, M.; Pujadas, M.; Ortiz, E.; Joglar, J.; Covas, M.I.; De La Torre, R. Direct Analysis of Glucuronidated Metabolites of Main Olive Oil Phenols in Human Urine after Dietary Consumption of Virgin Olive Oil. Food Chem. 2011, 126, 306–314. [Google Scholar] [CrossRef]
- Khymenets, O.; Crespo, M.C.; Dangles, O.; Rakotomanomana, N.; Andres-Lacueva, C.; Visioli, F. Human Hydroxytyrosol’s Absorption and Excretion from a Nutraceutical. J. Funct. Foods 2016, 23, 278–282. [Google Scholar] [CrossRef]





| Parameters | Values |
|---|---|
| Participants | 20 |
| Weight (kg) | 65.1 ± 2.4 |
| Height (cm) | 169.2 ± 4.0 |
| BMI (kg/m2) | 22.8 ± 1.0 |
| Age (years) | 49.6 ± 5.9 |
| Sex (M/F) | 9/11 |
| Analyte | RT (min) | ESI Mode | [M-H]−/[M-H]+ (m/z) | MS/MS (m/z) | λmax (nm) |
|---|---|---|---|---|---|
| DOPAC | 3.263 | Positive | 169/ | 123 | 280 |
| DOPET | 4.042 | Negative | 153/ | 123 | 280 |
| HVA | 5.054 | Positive | /183 | 137 | 280 |
| MOPET | 5.431 | Positive | /169 | 151 | 280 |
| Formulation | Subject | T1/2 (min) | Tmax (min) | Cmax (ng/mL) | AUC0–t (min*ng/mL) | AUC0–∞ (min*ng/mL) | AUCt–∞ (min*ng/mL) | AUCextrap_pred (%) | Clast (ng/mL) | Kel (1/min) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cps | 1 | 149.933 | 123.000 | 5.294 | 712.679 | 892.913 | 157.173 | 19.306 | 0.761 | 0.005 |
| 2 | 150.601 | 123.000 | 5.339 | 723.268 | 890.490 | 140.285 | 18.416 | 0.758 | 0.005 | |
| 3 | 148.323 | 123.000 | 5.478 | 695.670 | 880.506 | 149.079 | 17.557 | 0.732 | 0.005 | |
| 4 | 147.841 | 123.000 | 5.455 | 747.088 | 884.379 | 153.415 | 17.838 | 0.741 | 0.004 | |
| 5 | 149.380 | 123.000 | 5.398 | 706.174 | 902.077 | 154.248 | 17.937 | 0.744 | 0.005 | |
| 6 | 148.145 | 123.000 | 5.488 | 723.391 | 881.723 | 186.829 | 17.663 | 0.766 | 0.005 | |
| 7 | 151.791 | 123.000 | 5.610 | 776.967 | 902.792 | 158.029 | 17.517 | 0.730 | 0.005 | |
| 8 | 149.106 | 123.000 | 5.636 | 721.224 | 883.552 | 169.367 | 17.964 | 0.750 | 0.004 | |
| 9 | 151.339 | 123.000 | 5.547 | 744.207 | 877.017 | 157.389 | 17.496 | 0.761 | 0.005 | |
| 10 | 151.423 | 123.000 | 5.437 | 693.288 | 928.467 | 155.505 | 18.052 | 0.713 | 0.005 | |
| 11 | 150.435 | 123.000 | 5.492 | 693.742 | 889.982 | 173.295 | 18.456 | 0.734 | 0.005 | |
| 12 | 147.188 | 123.000 | 5.319 | 687.641 | 900.942 | 149.048 | 17.905 | 0.780 | 0.005 | |
| 13 | 145.501 | 123.000 | 5.540 | 730.696 | 869.484 | 124.409 | 18.387 | 0.731 | 0.005 | |
| 14 | 151.564 | 123.000 | 5.388 | 785.410 | 906.364 | 147.764 | 18.551 | 0.693 | 0.005 | |
| 15 | 147.207 | 123.000 | 5.650 | 723.804 | 892.232 | 181.822 | 18.185 | 0.751 | 0.005 | |
| 16 | 150.346 | 123.000 | 5.640 | 714.127 | 930.630 | 159.252 | 19.351 | 0.769 | 0.005 | |
| 17 | 147.919 | 123.000 | 5.502 | 716.517 | 907.057 | 180.365 | 18.262 | 0.735 | 0.005 | |
| 18 | 150.308 | 123.000 | 5.560 | 702.227 | 908.825 | 163.785 | 18.656 | 0.712 | 0.005 | |
| 19 | 152.516 | 123.000 | 5.548 | 728.582 | 881.112 | 155.566 | 18.511 | 0.748 | 0.005 | |
| 20 | 143.372 | 123.000 | 5.531 | 732.654 | 918.608 | 184.160 | 18.595 | 0.742 | 0.005 | |
| N | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| Mean | 149.212 | 123.000 | 5.493 | 722.968 | 896.457 | 160.039 | 18.230 | 0.743 | 0.005 | |
| SD | 2.299 | 0.000 | 0.106 | 25.857 | 16.814 | 15.649 | 0.528 | 0.021 | 0.000 | |
| CV% | 1.541 | 0.000 | 1.928 | 3.576 | 1.876 | 9.778 | 2.895 | 2.859 | 6.282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Renzo, L.; Smeriglio, A.; Ingegneri, M.; Gualtieri, P.; Trombetta, D. The Pharmaceutical Formulation Plays a Pivotal Role in Hydroxytyrosol Pharmacokinetics. Pharmaceutics 2023, 15, 743. https://doi.org/10.3390/pharmaceutics15030743
Di Renzo L, Smeriglio A, Ingegneri M, Gualtieri P, Trombetta D. The Pharmaceutical Formulation Plays a Pivotal Role in Hydroxytyrosol Pharmacokinetics. Pharmaceutics. 2023; 15(3):743. https://doi.org/10.3390/pharmaceutics15030743
Chicago/Turabian StyleDi Renzo, Laura, Antonella Smeriglio, Mariarosaria Ingegneri, Paola Gualtieri, and Domenico Trombetta. 2023. "The Pharmaceutical Formulation Plays a Pivotal Role in Hydroxytyrosol Pharmacokinetics" Pharmaceutics 15, no. 3: 743. https://doi.org/10.3390/pharmaceutics15030743
APA StyleDi Renzo, L., Smeriglio, A., Ingegneri, M., Gualtieri, P., & Trombetta, D. (2023). The Pharmaceutical Formulation Plays a Pivotal Role in Hydroxytyrosol Pharmacokinetics. Pharmaceutics, 15(3), 743. https://doi.org/10.3390/pharmaceutics15030743