New Technological Approaches for Dental Caries Treatment: From Liquid Crystalline Systems to Nanocarriers
Abstract
1. Introduction
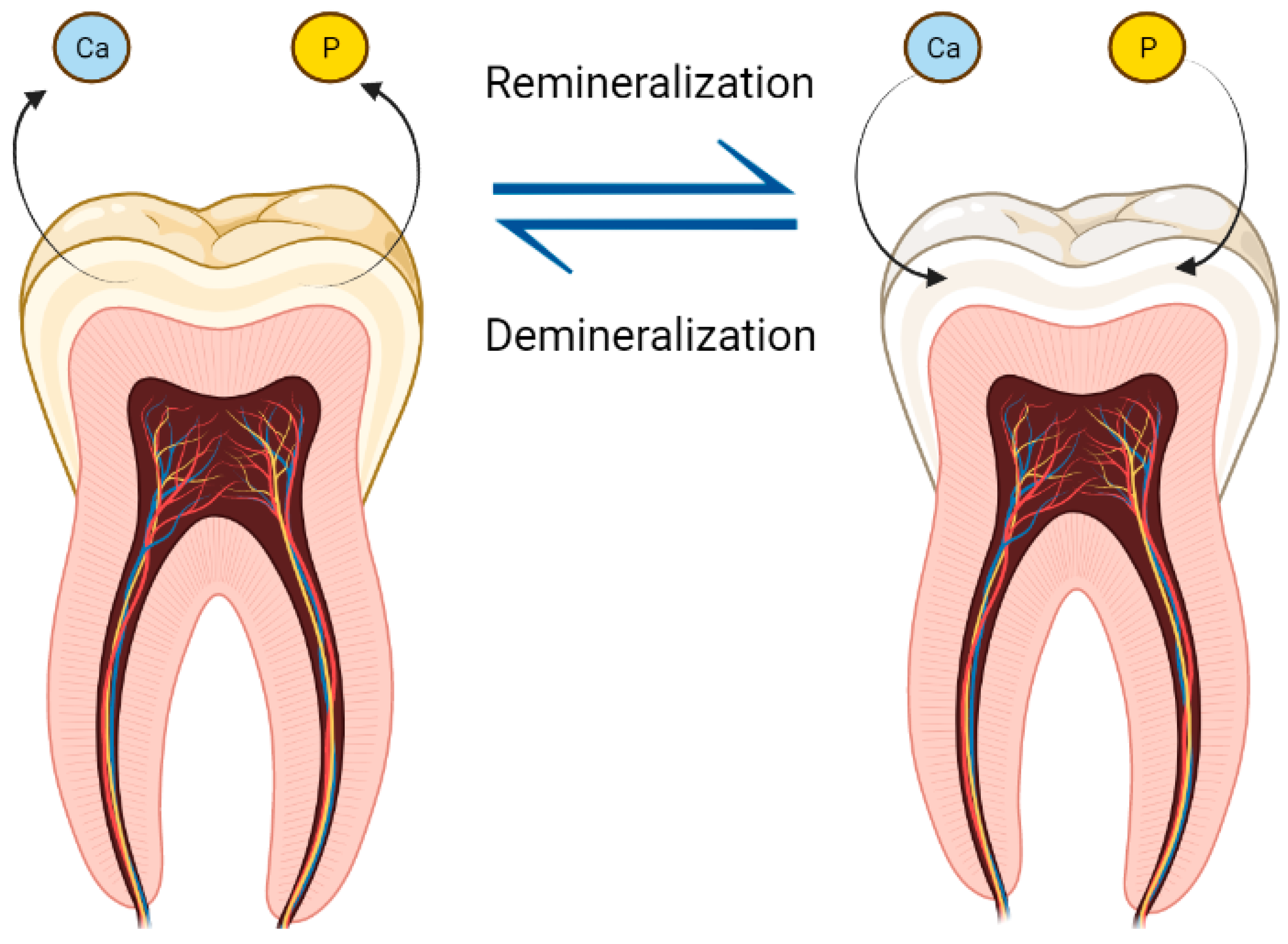
2. Physiopathology and the Formation of Biofilms
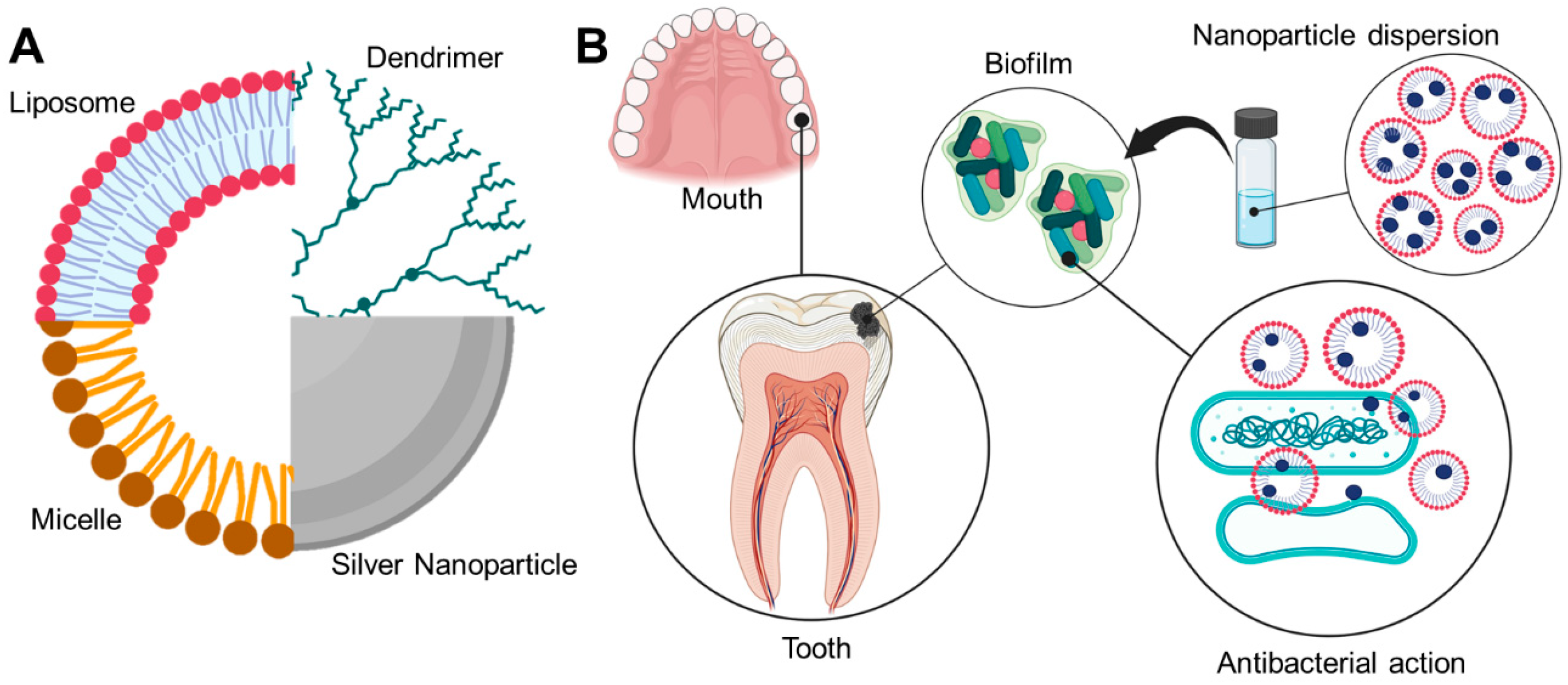
3. Drug Delivery Systems Used in the Prevention and Treatment of Caries
3.1. Organic Systems
3.1.1. Liquid Crystalline Systems
3.1.2. Liposomes
3.1.3. Nanoemulsion
3.1.4. Polymeric Nanoparticles
3.1.5. Hydrogels
3.1.6. Dendrimers
3.1.7. Micelles
3.2. Inorganic Nanoparticles
3.2.1. Silver Nanoparticles
3.2.2. Zinc Nanoparticles
3.2.3. Calcium Nanoparticles
3.2.4. Titanium Nanoparticles
4. Conclusions
Funding
Conflicts of Interest
References
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- World Health Organization. Dental Health. Available online: www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 12 July 2022).
- Khattak, O.; Iqbal, A.; Nadeem Baig, M.; Magar, S.P.; Magar, S.S.; Mehmood Khan, A.; Osamah Molla, F.; Alhabib, S.; Alruwaili, S. Prevalence of Caries in Anterior Teeth in Adults; An Epidemiology Study. Pak. J. Med. Health Sci. 2021, 15, 3421–3423. [Google Scholar] [CrossRef]
- Frencken, J. Caries Epidemiology and Its Challenges. Monogr. Oral Sci. 2018, 27, 11–23. [Google Scholar] [CrossRef]
- Bashir, N.Z. Update on the prevalence of untreated caries in the US adult population, 2017–2020. J. Am. Dent. Assoc. 2022, 153, 300–308. [Google Scholar] [CrossRef]
- Nguyen, S.; Hiorth, M. Advanced drug delivery systems for local treatment of the oral cavity. Ther. Deliv. 2015, 6, 197–210. [Google Scholar] [CrossRef]
- Sun, C.; Xie, Y.; Hu, X.; Fu, J.; Zhou, J.; Wu, L. Relationship between Clinical Symptoms and the Microbiota in Advanced Caries. J. Endod. 2020, 46, 763–770. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Garcia, M.H.; Cilli, E.M.; Chiavacci, L.A.; Chorilli, M. Design and characterization of a novel p1025 peptide-loaded liquid crystalline system for the treatment of dental caries. Molecules 2016, 21, 158. [Google Scholar] [CrossRef]
- Liang, J.; Peng, X.; Zhou, X.; Zou, J.; Cheng, L. Emerging applications of drug delivery systems in oral infectious diseases prevention and treatment. Molecules 2020, 25, 516. [Google Scholar] [CrossRef]
- Ahmadian, E.; Shahi, S.; Yazdani, J.; Maleki Dizaj, S.; Sharifi, S. Local treatment of the dental caries using nanomaterials. Biomed. Pharmacother. 2018, 108, 443–447. [Google Scholar] [CrossRef]
- Amissah, F.; Andey, T.; Ahlschwede, K.M. Nanotechnology-based therapies for the prevention and treatment of Streptococcus mutans-derived dental caries. J. Oral Biosci. 2021, 63, 327–336. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Victorelli, F.D.; Dovigo, L.N.; Chorilli, M. Polyethyleneimine and Chitosan Polymer-Based Mucoadhesive Liquid Crystalline Systems Intended for Buccal Drug Delivery. AAPS PharmSciTech 2018, 19, 820–836. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Gui, S. Cubic and hexagonal liquid crystals as drug delivery systems. Biomed Res. Int. 2014, 2014, 815981. [Google Scholar] [CrossRef]
- Bernegossi, J.; Maria, G.; Calixto, F.; Ricardo, P.; Fontana, C.R.; Cilli, E.M.; Garrido, S.S. Peptide KSL-W-Loaded Mucoadhesive Liquid Crystalline Vehicle as an Alternative Treatment for Multispecies Oral Biofilm. Molecules 2016, 21, 37. [Google Scholar] [CrossRef]
- Aida, K.L.; Kreling, P.F.; Caiaffa, K.S.; Santos-, N.A.; Duque, C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomed. 2018, 13, 3081–3091. [Google Scholar] [CrossRef]
- Cintra, A.B.; Delboni, L.A.; Lara, M.G. Influence of additives on swelling and mucoadhesion properties of glyceryl monooleate liquid crystals. Braz. J. Pharm. Sci. 2022, 58. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pispas, S.; Tseti, I.K.; Demetzos, C. Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals 2022, 15, 429. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Bonifácio, B.V.; Baub, T.M.; Gremião, M.P.D.; Chorilli, M. In-situ gelling liquid crystal mucoadhesive vehicle for curcumin buccal administration and its potential application in the treatment of oral candidiasis. J. Biomed. Nanotechnol. 2019, 16, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and nanotechnology in drug development: Focus on ocular targets. Int. J. Nanomed. 2013, 8, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Hiorth, M.; Rykke, M.; Smistad, G. The potential of liposomes as dental drug delivery systems. Eur. J. Pharm. Biopharm. 2011, 77, 75–83. [Google Scholar] [CrossRef]
- Upadhyay, T.; Ansari, V.A.; Ahmad, U.; Sultana, N.; Akhtar, J. Exploring Nanoemulsion for Liver Cancer Therapy. Curr. Cancer Ther. Rev. 2020, 16, 260–268. [Google Scholar] [CrossRef]
- Sahu, P.; Das, D.; Mishra, V.K.; Kashaw, V.; Kashaw, S.K. Nanoemulsion: A Novel Eon in Cancer Chemotherapy. Mini-Rev. Med. Chem. 2016, 17, 1778–1792. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Dharmadhikari, S.; Chaubal, T.V.; Amin, M.C.I.M.; Bapat, P.; Gorain, B.; Choudhury, H.; Vincent, C.; Kesharwani, P. The potential of dendrimer in delivery of therapeutics for dentistry. Heliyon 2019, 5, e02544. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; Da Cruz, A.D.; Poiate, E.; Poiate, I.A.V.P. Silver nanoparticles in dental biomaterials. Int. J. Biomater. 2015, 2015, 485275. [Google Scholar] [CrossRef]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Carrouel, F.; Viennot, S.; Ottolenghi, L.; Gaillard, C.; Bourgeois, D. Nanoparticles as Anti-Microbial, Anti-Inflammatory, and Remineralizing Agents in Oral Care Cosmetics: A Review of the Current Situation. Nanomaterials 2020, 10, 140. [Google Scholar] [CrossRef]
- Al-Wrafy, F.A.; Al-Gheethi, A.A.; Ponnusamy, S.K.; Noman, E.A.; Fattah, S.A. Nanoparticles approach to eradicate bacterial biofilm-related infections: A critical review. Chemosphere 2022, 288, 132603. [Google Scholar] [CrossRef]
- Keerthana, S.; Kumar, A. Potential risks and benefits of zinc oxide nanoparticles: A systematic review. Crit. Rev. Toxicol. 2020, 50, 47–71. [Google Scholar] [CrossRef]
- Cai, Y.; Tang, R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787. [Google Scholar] [CrossRef]
- d’Amora, M.; Liendo, F.; Deorsola, F.A.; Bensaid, S.; Giordani, S. Toxicological profile of calcium carbonate nanoparticles for industrial applications. Colloids Surfaces B Biointerfaces 2020, 190, 110947. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Akhoundi, M.S.A.; Bahador, A.; Jalali, Y.F.; Behzadi, Z.; Elhaminejad, F.; Mirhashemi, A.H. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in orthodontics. Dental Press J. Orthod. 2017, 22, 67–74. [Google Scholar] [CrossRef]
- Kantovitz, K.R.; Fernandes, F.P.; Feitosa, I.V.; Lazzarini, M.O.; Denucci, G.C.; Gomes, O.P.; Giovani, P.A.; Moreira, K.M.S.; Pecorari, V.G.A.; Borges, A.F.S.; et al. TiO2 nanotubes improve physico-mechanical properties of glass ionomer cement. Dent. Mater. 2020, 36, e85–e92. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontology 2000 2006, 42, 47–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms:difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Saini, S.; Sharma, S. Biofilm: A dental microbial infection. J. Nat. Sci. Biol. Med. 2011, 2, 71–75. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Hojo, K.; Nagaoka, S.; Ohshima, T.; Maeda, N. Bacterial Interactions in Dental Biofilm Development. J. Dent. Res. 2009, 88, 982–990. [Google Scholar] [CrossRef]
- Paster, B.J.; Olsen, I.; Aas, J.A.; Dewhirst, F.E. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology 2000 2006, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Joiner, A. The Teeth and Their Environment: Physical, Chemical and Biochemical Influences. In Monographs in Oral Science; Karger: Basel, Switzerland, 2006; Volume 19, pp. 29–64. [Google Scholar]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M. Transmission electron microscopy of early plaque formation on dental materials in vivo. Eur. J. Oral Sci. 1999, 107, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Hannig, C. The pellicle and erosion. Monogr. Oral Sci. 2014, 25, 206–214. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Heller, D.; Helmerhorst, E.J.; Gower, A.C.; Siqueira, W.L.; Paster, B.J.; Oppenheim, F.G. Microbial diversity in the early in vivo-formed dental biofilm. Appl. Environ. Microbiol. 2016, 82, 1881–1888. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Koo, H.; Falsetta, M.L.; Klein, M.I. The Exopolysaccharide Matrix: A Virulence Determinant of Cariogenic Biofilm. J. Dent. Res. 2013, 92, 1065–1073. [Google Scholar] [CrossRef]
- Phelan, V.V.; Liu, W.T.; Pogliano, K.; Dorrestein, P.C. Microbial metabolic exchangeg-the chemotype-to-phenotype link. Nat. Chem. Biol. 2012, 8, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Wozniak, D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Klein, M.I.; Falsetta, M.L.; Lu, B.; Delahunty, C.M.; Yates, J.R.; Heydorn, A.; Koo, H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012, 8, e1002623. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.; Guggenheim, B. A novel TEM contrasting technique for extracellular polysaccharides in in vitro biofilms. Microsc. Res. Tech. 2007, 70, 816–822. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef]
- Hayacibara, M.F.; Koo, H.; Vacca Smith, A.M.; Kopec, L.K.; Scott-Anne, K.; Cury, J.A.; Bowen, W.H. The influence of mutanase and dextranase on the production and structure of glucans synthesized by streptococcal glucosyltransferases. Carbohydr. Res. 2004, 339, 2127–2137. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental Caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nalone, L.; Marques, C.; Costa, S.; Souto, E.B.; Severino, P. Liquid crystalline drug delivery systems. In Drug Delivery Trends; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–149. [Google Scholar]
- Howard University. Chapter 11.8: Liquid Crystals-Chemistry LibreTexts. In General Chemistry: An Atoms First Approach; Howard University: Washington, DC, USA, 2021. [Google Scholar]
- Calixto, G.M.F.; Duque, C.; Aida, K.L.; dos Santos, V.R.; Massunari, L.; Chorilli, M. Development and characterization of p1025-loaded bioadhesive liquid-crystalline system for the prevention of Streptococcus mutans biofilms. Int. J. Nanomed. 2017, 13, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Reina, B.; Santezi, C.; Malheiros, S.; Calixto, G.; Rodero, C.; Victorelli, F.; Chorilli, M.; Dovigo, L.N. Liquid Crystal Precursor System as a Vehicle for Curcumin-Mediated Photodynamic Inactivation of Oral Biofilms. J. Biophotonics 2022, 16, e202200040. [Google Scholar] [CrossRef] [PubMed]
- Yamakami, K.; Tsumori, H.; Shimizu, Y.; Sakurai, Y.; Nagatoshi, K.; Sonomoto, K. Cationic Lipid Content in Liposome-Encapsulated Nisin Improves Sustainable Bactericidal Activity against Streptococcus mutans. Open Dent. J. 2016, 10, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hu, F.; Hu, S.; Kong, M.; Feng, C.; Liu, Y.; Cheng, X.; Ji, Q.; Chen, X. PH-Activated nanoparticles with targeting for the treatment of oral plaque biofilm. J. Mater. Chem. B 2018, 6, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Melling, G.E.; Colombo, J.S.; Avery, S.J.; Ayre, W.N.; Evans, S.L.; Waddington, R.J.; Sloan, A.J. Liposomal delivery of demineralized dentin matrix for dental tissue regeneration. Tissue Eng. Part A 2018, 24, 1057–1065. [Google Scholar] [CrossRef]
- Ramalingam, K.; Amaechi, B.T.; Ralph, R.H.; Lee, V.A. Antimicrobial activity of nanoemulsion on cariogenic planktonic and biofilm organisms. Arch. Oral Biol. 2012, 57, 15–22. [Google Scholar] [CrossRef]
- Li, Y.F.; Sun, H.W.; Gao, R.; Liu, K.Y.; Zhang, H.Q.; Fu, Q.H.; Qing, S.L.; Guo, G.; Zou, Q.M. Inhibited biofilm formation and improved antibacterial activity of a novel nanoemulsion against cariogenic Streptococcus mutans in vitro and in vivo. Int. J. Nanomed. 2015, 10, 447–462. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, H.E.; Han, S.J.; Choi, J.S. Antibacterial and antibiofilm activities of cinnamon essential oil nanoemulsion against multi-species oral biofilms. Sci. Rep. 2021, 11, 5911. [Google Scholar] [CrossRef]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Aliasghari, A.; Khorasgani, M.R.; Vaezifar, S.; Rahimi, F. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran J. Microbiol. 2016, 8, 93–100. [Google Scholar] [PubMed]
- Cavalcante, L.L.R.; Tedesco, A.C.; Takahashi, L.A.U.; Curylofo-Zotti, F.A.; Souza-Gabriel, A.E.; Corona, S.A.M. Conjugate of chitosan nanoparticles with chloroaluminium phthalocyanine: Synthesis, characterization and photoinactivation of Streptococcus mutans biofilm. Photodiagnosis Photodyn. Ther. 2020, 30, 101709. [Google Scholar] [CrossRef]
- Ren, Q.; Ding, L.; Li, Z.; Wang, X.; Wang, K.; Han, S.; Li, W. Archives of Oral Biology Chitosan hydrogel containing amelogenin-derived peptide: Inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesions. Arch. Oral Biol. 2019, 100, 42–48. [Google Scholar] [CrossRef]
- Ashra, B.; Rashidipour, M.; Marzban, A.; Soroush, S. Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Sosnowska, K.; Pleszczyńska, M.; Strawa, J.; Wiater, A.; Grochowski, D.M.; Tomczykowa, M.; Winnicka, K. Hydrogel containing an extract of tormentillae rhizoma for the treatment of biofilm-related oral diseases. Nat. Prod. Commun. 2017, 12, 417–421. [Google Scholar] [CrossRef]
- Santos, Z.; Jr, S.; Huang, Y.; De Freitas, L.F.; Mesquita-ferrari, R.A.; Porta, K.; Fernandes, S.; Deana, A.; Raquel, C.; Leal, L.; et al. Papain gel containing methylene blue for simultaneous caries removal and antimicrobial photoinactivation against Streptococcus mutans biofilms. Nat. Publ. Gr. 2016, 6, 33270. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Lin, Z.; Li, J.; Liang, K.; Yuan, H.; Li, S.; Li, J. Triclosan-loaded poly(amido amine) dendrimer for simultaneous treatment and remineralization of human dentine. Colloids Surfaces B Biointerfaces 2014, 115, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yang, X.; Liao, L.; Yang, J.; Liang, K.; Zeng, S.; Zhou, J.; Zhang, M.; Li, J. A novel anticaries agent, honokiol-loaded poly(amido amine) dendrimer, for simultaneous long-term antibacterial treatment and remineralization of demineralized enamel. Dent. Mater. 2021, 37, 1337–1349. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.; Xu, X.; Li, J.; Ding, C.; Zhao, C.; Li, J. One-step phosphorylated poly(amide-amine) dendrimer loaded with apigenin for simultaneous remineralization and antibacterial of dentine. Colloids Surfaces B Biointerfaces 2018, 172, 760–768. [Google Scholar] [CrossRef]
- Backlund, C.J.; Worley, B.V.; Schoenfisch, M.H. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly(amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 2016, 29, 198–205. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, L.; Chen, L.; Lin, Y.; Luo, Z.; Chen, Z.; Li, T.; Wu, J.; Zhong, Z. Farnesal-loaded pH-sensitive polymeric micelles provided effective prevention and treatment on dental caries. J. Nanobiotechnol. 2020, 18, 89. [Google Scholar] [CrossRef]
- Chen, F.; Rice, K.C.; Liu, X.-M.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. Triclosan-Loaded Tooth-Binding Micelles for Prevention and Treatment of Dental Biofilm. Pharm. Res. 2010, 27, 2356–2364. [Google Scholar] [CrossRef]
- Xu, Y.; You, Y.; Yi, L.; Wu, X.; Zhao, Y.; Yu, J.; Liu, H.; Shen, Y.; Guo, J.; Huang, C. Dental plaque-inspired versatile nanosystem for caries prevention and tooth restoration. Bioact. Mater. 2023, 20, 418–433. [Google Scholar] [CrossRef]
- Souza, C.; Watanabe, E.; Borgheti-Cardoso, L.N.; De Fantini, M.C.A.; Lara, M.G. Mucoadhesive system formed by liquid crystals for buccal administration of poly(hexamethylene biguanide) hydrochloride. J. Pharm. Sci. 2014, 103, 3914–3923. [Google Scholar] [CrossRef]
- Foong, L.K.; Foroughi, M.M.; Mirhosseini, A.F.; Safaei, M.; Jahani, S.; Mostafavi, M.; Abrahimpoor, N.; Sharifi, M.; Varma, R.S.; Khatami, M. Applications of nano-materials in diverse dentistry. RSC Adv. 2020, 10, 15430–15460. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Lamberti, G. Lipid Delivery Systems for Nucleic-Acid-Based-Drugs: From Production to Clinical Applications. Pharmaceutics 2019, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodriguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Gomez, A.G.; Hosseinidoust, Z. Liposomes For Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef]
- Steinberg, D.; Friedman, M. Sustained-release drug delivery of antimicrobials in controlling of supragingival oral biofilms. Expert Opin. Drug Deliv. 2017, 14, 571–581. [Google Scholar] [CrossRef]
- Harper, R.A.; Saleh, M.M.; Carpenter, G.; Abbate, V.; Proctor, G.; Harvey, R.D.; Gambogi, R.J.; Geonnotti, A.; Hider, R.; Jones, S.A. Soft, adhesive (+) alpha tocopherol phosphate planar bilayers that control oral biofilm growth through a substantive antimicrobial effect. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2307–2316. [Google Scholar] [CrossRef]
- Sugano, M.; Morisaki, H.; Negishi, Y.; Endo-Takahashi, Y.; Kuwata, H.; Miyazaki, T.; Yamamoto, M. Potential effect of cationic liposomes on interactions with oral bacterial cells and biofilms. J. Liposome Res. 2016, 26, 156–162. [Google Scholar] [CrossRef]
- Chong, J.R.; Le, D.L.; Sato, H.; Sou, K. Nanocapsule pH Regulator: Sustained Continuous Alkali Release from Thermosensitive Liposomes Reduces Acid Erosion. ACS Appl. Mater. Interfaces 2020, 12, 21463–21469. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Quan, H.; Yang, H.C. Effects of phosphatidylserine-containing liposomes on odontogenic differentiation of human dental pulp cells. Dent. Mater. J. 2017, 36, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Pistone, S.; Rykke, M.; Smistad, G.; Hiorth, M. Polysaccharide-coated liposomal formulations for dental targeting. Int. J. Pharm. 2017, 516, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Jarai, B.M.; Kolewe, E.L.; Stillman, Z.S.; Raman, N.; Fromen, C.A. Polymeric Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128166628. [Google Scholar]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Kleine-Brueggeney, H.; Zorzi, G.K.; Fecker, T.; El Gueddari, N.E.; Moerschbacher, B.M.; Goycoolea, F.M. A rational approach towards the design of chitosan-based nanoparticles obtained by ionotropic gelation. Colloids Surfaces B Biointerfaces 2015, 135, 99–108. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef]
- Gharieh, A.; Khoee, S.; Mahdavian, A.R. Emulsion and miniemulsion techniques in preparation of polymer nanoparticles with versatile characteristics. Adv. Colloid Interface Sci. 2019, 269, 152–186. [Google Scholar] [CrossRef]
- Sutar, Y.B.; Telvekar, V.N. Chitosan based copolymer-drug conjugate and its protein targeted polyelectrolyte complex nanoparticles to enhance the efficiency and specificity of low potency anticancer agent. Mater. Sci. Eng. C 2018, 92, 393–406. [Google Scholar] [CrossRef]
- Maghsoudi, A.; Yazdian, F.; Shahmoradi, S.; Ghaderi, L.; Hemati, M.; Amoabediny, G. Curcumin-loaded polysaccharide nanoparticles: Optimization and anticariogenic activity against Streptococcus mutans. Mater. Sci. Eng. C 2017, 75, 1259–1267. [Google Scholar] [CrossRef]
- Feksa, L.R.; Troian, E.A.; Muller, C.D.; Viegas, F.; Machado, A.B.; Rech, V.C. Hydrogels for Biomedical Applications. In Nanostructures for the Engineering of Cells, Tissues and Organs: From Design to Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128136669. [Google Scholar]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S. A review on biomacromolecular hydrogel classification and its applications. Int. J. Biol. Macromol. 2020, 162, 737–747. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system. Drug Deliv. Transl. Res. 2022, 13, 135–163. [Google Scholar] [CrossRef]
- Limsitthichaikoon, S.; Soontaranon, S.; Hanpramukkun, N.; Thumanu, K.; Priprem, A. Polymeric Micelles Enhance Mucosal Contact Time and Deposition of Fluocinolone Acetonide. Polymers 2022, 14, 2247. [Google Scholar] [CrossRef]
- Koopaie, M. Nanoparticulate systems for dental drug delivery. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–559. [Google Scholar]
- Schestakow, A.; Hannig, M. Effects of Experimental Agents Containing Tannic Acid or Chitosan on the Bacterial Biofilm Formation in Situ. Biomolecules 2020, 10, 1315. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gopinath, K.; Zhang, K.; Kang, E.-T.; Xu, L.; Yu, Y. Recent progress in tannic acid-driven antibacterial/antifouling surface coating strategies. J. Mater. Chem. B 2022, 10, 2296–2315. [Google Scholar] [CrossRef]
- Dantas Lopes dos Santos, D.; Besegato, J.F.; Melo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; de Souza Rastelli, A.N. Curcumin-loaded Pluronic® F-127 Micelles as a Drug Delivery System for Curcumin-mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. 2021, 97, 1072–1088. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.A.; Aqlima, S. Synthesis, characterization and biomedical applications of silver nanoparticles. Materials 2022, 15, 427–470. [Google Scholar] [CrossRef]
- Jiménez-Ramírez, A.J.; Martínez-Martínez, R.E.; Ayala-Herrera, J.L.; Zaragoza-Contreras, E.A.; Domínguez-Pérez, R.A.; Reyes-López, S.Y.; Donohue-Cornejo, A.; Cuevas-González, J.C.; Silva-Benítez, E.L.; Espinosa-Cristóbal, L.F. Antimicrobial Activity of Silver Nanoparticles against Clinical Biofilms from Patients with and without Dental Caries. J. Nanomater. 2021, 2021, 5587455. [Google Scholar] [CrossRef]
- Butrón Téllez Girón, C.; Hernández Sierra, J.F.; Dealba-Montero, I.; Urbano Peña, M.d.l.A.; Ruiz, F. Therapeutic Use of Silver Nanoparticles in the Prevention and Arrest of Dental Caries. Bioinorg. Chem. Appl. 2020, 2020, 8882930. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Oves, M.; Khan, A.U. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE 2017, 12, e0181363. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Li, Q.L.; Tang, J.; Lo, E.C.M.; Chu, C.H. Inhibition of dentine caries using fluoride solution with silver nanoparticles: An in vitro study. J. Dent. 2020, 103, 103512. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cristóbal, L.F.; Holguín-Meráz, C.; Zaragoza-Contreras, E.A.; Martínez-Martínez, R.E.; Donohue-Cornejo, A.; Loyola-Rodríguez, J.P.; Cuevas-González, J.C.; Reyes-López, S.Y. Antimicrobial and Substantivity Properties of Silver Nanoparticles against Oral Microbiomes Clinically Isolated from Young and Young-Adult Patients. J. Nanomater. 2019, 2019, 3205971. [Google Scholar] [CrossRef]
- Shakerimoghaddam, A.; Safardoust-Hojaghan, H.; Amiri, O.; Salavati-Niasari, M.; Khorshidi, A.; Khaledi, A. Ca19Zn2(PO4)14 Nanoparticles: Synthesis, characterization and its effect on the colonization of Streptococcus mutans on tooth surface. J. Mol. Liq. 2022, 350, 118507. [Google Scholar] [CrossRef]
- Mohd Bakhori, S.K.; Mahmud, S.; Ling, C.A.; Sirelkhatim, A.H.; Hasan, H.; Mohamad, D.; Masudi, S.M.; Seeni, A.; Abd Rahman, R. In-vitro efficacy of different morphology zinc oxide nanopowders on Streptococcus sobrinus and Streptococcus mutans. Mater. Sci. Eng. C 2017, 78, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.; Safwat, E.; Soliman, Z.; Salama, H.; El-Sayed, H.; Anwar, M. Antibacterial and Remineralization Efficacy of Casein Phosphopeptide, Glycomacropeptide Nanocomplex, and Probiotics in Experimental Toothpastes: An in Vitro Comparative Study. Eur. J. Dent. 2019, 13, 391–398. [Google Scholar] [CrossRef]
- Kensche, A.; Holder, C.; Basche, S.; Tahan, N.; Hannig, C.; Hannig, M. Efficacy of a mouthrinse based on hydroxyapatite to reduce initial bacterial colonisation in situ. Arch. Oral Biol. 2017, 80, 18–26. [Google Scholar] [CrossRef]
- Viana, Í.E.L.; Lopes, R.M.; Silva, F.R.O.; Lima, N.B.; Aranha, A.C.C.; Feitosa, S.; Scaramucci, T. Novel fluoride and stannous -functionalized β-tricalcium phosphate nanoparticles for the management of dental erosion. J. Dent. 2020, 92, 103263. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Degli Esposti, L.; Ramírez-Rodríguez, G.B.; Carella, F.; Gómez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-López, J.M. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef]
- Mansoor, A.; Khan, M.T.; Mehmood, M.; Khurshid, Z.; Ali, M.I.; Jamal, A. Synthesis and Characterization of Titanium Oxide Nanoparticles with a Novel Biogenic Process for Dental Application. Nanomaterials 2022, 12, 1078. [Google Scholar] [CrossRef]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical properties and microstructures of glass-ionomer cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef]
- Araújo, I.J.D.S.; Ricardo, M.G.; Gomes, O.P.; Giovani, P.A.; Puppin-Rontani, J.; Pecorari, V.A.; Martinez, E.F.; Napimoga, M.H.; Junior, F.H.N.; Puppin-Rontani, R.M.; et al. Titanium dioxide nanotubes added to glass ionomer cements affect S. mutans viability and mechanisms of virulence. Braz. Oral Res. 2021, 35, e062. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhao, I.S.; Mei, M.L.; Li, Q.; Yu, O.Y.; Chu, C.H. Use of silver nanomaterials for caries prevention: A concise review. Int. J. Nanomed. 2020, 15, 3181–3191. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Al-Dahmash, N.D.; Ranjitsingh, A.J.A. Synthesis of silver nanoparticles using gum Arabic: Evaluation of its inhibitory action on Streptococcus mutans causing dental caries and endocarditis. J. Infect. Public Health 2021, 14, 324–330. [Google Scholar] [CrossRef]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Sawada, T.; Yamaguchi, R. Silver nanoparticles in orthodontics, a new alternative in bacterial inhibition: In vitro study. Prog. Orthod. 2020, 21, 24. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; López-Ruiz, N.; Cabada-Tarín, D.; Reyes-López, S.Y.; Zaragoza-Contreras, A.; Constandse-Cortéz, D.; Donohué-Cornejo, A.; Tovar-Carrillo, K.; Cuevas-González, J.C.; Kobayashi, T. Antiadherence and antimicrobial properties of silver nanoparticles against Streptococcus mutans on brackets and wires used for orthodontic treatments. J. Nanomater. 2018, 2018, 9248527. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Loyola-Rodríguez, J.P.; Niño-Martínez, N.; Ruiz, F.; Zavala-Alonso, N.V.; Lara, R.H.; Reyes-López, S.Y. Bovine serum albumin and chitosan coated silver nanoparticles and its antimicrobial activity against oral and nonoral bacteria. J. Nanomater. 2015, 2015, 420853. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C 2020, 108, 110319. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Xu, V.W.; Yin, I.X.; Yu, O.Y.; Chu, C.H. Metal and metal oxide nanoparticles in caries prevention: A review. Nanomaterials 2021, 11, 3446. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Bin Emran, T.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Moradpoor, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Imani, M.M.; Golshah, A.; Bashardoust, N. An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC Adv. 2021, 11, 21189–21206. [Google Scholar] [CrossRef]
- Mirhosseini, F.; Amiri, M.; Daneshkazemi, A.; Zandi, H.; Javadi, Z.S. Antimicrobial effect of different sizes of nano zinc oxide on oral microorganisms. Front. Dent. 2019, 16, 105–112. [Google Scholar] [CrossRef]
- Biradar, S.; Ravichandran, P.; Gopikrishnan, R.; Goornavar, V.; Hall, J.C.; Ramesh, V.; Baluchamy, S.; Jeffers, R.B.; Ramesh, G.T. Calcium carbonate nanoparticles: Synthesis, characterization and biocompatibility. J. Nanosci. Nanotechnol. 2011, 11, 6868–6874. [Google Scholar] [CrossRef]
- Mostafa, F.A.; Gad, A.N.; Gaber, A.A.M.; Abdel-Wahab, A.M.A. Preparation, Characterization and Application of Calcium Oxide Nanoparticles from Waste Carbonation Mud in Clarification of Raw Sugar Melt. Sugar Tech 2022, 25, 331–338. [Google Scholar] [CrossRef]
- Schwiertz, J.; Wiehe, A.; Gräfe, S.; Gitter, B.; Epple, M. Calcium phosphate nanoparticles as efficient carriers for photodynamic therapy against cells and bacteria. Biomaterials 2009, 30, 3324–3331. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of calcium phosphate nanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef]
- Urch, H.; Vallet-Regi, M.; Ruiz, L.; Gonzalez-Calbet, J.M.; Epple, M. Calcium phosphate nanoparticles with adjustable dispersability and crystallinity. J. Mater. Chem. 2009, 19, 2166–2171. [Google Scholar] [CrossRef]
- Sun, L.; Chow, L.C.; Frukhtbeyn, S.A.; Bonevich, J.E. Preparation and properties of nanoparticles of Calcium Phosphates with Various Ca/P Ratios. J. Res. Nac. Inst. Stand. Technol. 2010, 115, 243–255. [Google Scholar] [CrossRef]
- Pakravanan, K.; Rezaee Roknabadi, M.; Farzanegan, F.; Hashemzadeh, A.; Darroudi, M. Amorphous calcium phosphate nanoparticles-based mouthwash: Preparation, characterization, and anti-bacterial effects. Green Chem. Lett. Rev. 2019, 12, 278–285. [Google Scholar] [CrossRef]
- Rao, S.K.; Bhat, G.S.; Aradhya, S.; Devi, A.; Bhat, M. Study of the efficacy of toothpaste containing casein phosphopeptide in the prevention of dental caries: A randomized controlled trial in 12- to 15-year-old high caries risk children in Bangalore, India. Caries Res. 2009, 43, 430–435. [Google Scholar] [CrossRef]
- Hedge, M.; Moany, A. Remineralization of enamel subsurface lesions with casein phosphopeptide-amorphous calcium phosphate: A quantitative energy dispersive X-ray analysis using scanning electron microscopy: An in vitro study. J. Conserv. Dent. 2012, 15, 61–67. [Google Scholar] [CrossRef]
- White, A.J.; Gracia, L.H.; Barbour, M.E. Inhibition of dental erosion by casein and casein-derived proteins. Caries Res. 2011, 45, 13–20. [Google Scholar] [CrossRef]
- Hilbrig, F.; Freitag, R. Hydroxyapatite in Bioprocessing. In Biopharmaceutical Production Technology, 1 & 2; Wiley-VCH: New York, NY, USA, 2012; Volume 1, pp. 283–331. [Google Scholar] [CrossRef]
- Jardim, R.N.; Rocha, A.A.; Rossi, A.M.; de Almeida Neves, A.; Portela, M.B.; Lopes, R.T.; Pires dos Santos, T.M.; Xing, Y.; Moreira da Silva, E. Fabrication and characterization of remineralizing dental composites containing hydroxyapatite nanoparticles. J. Mech. Behav. Biomed. Mater. 2020, 109, 103817. [Google Scholar] [CrossRef]
- Bahadur, J.; Agrawal, S.; Panwar, V.; Parveen, A.; Pal, K. Antibacterial properties of silver doped TiO2 nanoparticles synthesized via sol-gel technique. Macromol. Res. 2016, 24, 488–493. [Google Scholar] [CrossRef]
- Cai, Y.; Strømme, M.; Welch, K. Photocatalytic Antibacterial Effects Are Maintained on Resin-Based TiO2 Nanocomposites after Cessation of UV Irradiation. PLoS ONE 2013, 8, e75929. [Google Scholar] [CrossRef]
- Sun, J.; Forster, A.M.; Johnson, P.M.; Eidelman, N.; Quinn, G.; Schumacher, G.; Zhang, X.; Wu, W.L. Improving performance of dental resins by adding titanium dioxide nanoparticles. Dent. Mater. 2011, 27, 972–982. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Yu, M.; Li, J.; Liu, J.; Xie, Z.; Gao, F.; Liu, Y. Applications of Titanium Dioxide Nanostructure in Stomatology. Molecules 2022, 27, 3881. [Google Scholar] [CrossRef]
| Drugs Delivery System | Advantages | Limitations | Ref. |
|---|---|---|---|
| Liquid crystalline system |
|
| [16,17,18] |
| Liposomes |
|
| [19,20] |
| Nanoemulsion |
|
| [21,22] |
| Polymeric nanoparticles |
|
| [23,24] |
| Hydrogel |
|
| [25,26] |
| Dendrimer |
|
| [27] |
| Polymeric micelles |
|
| [27] |
| Silver nanoparticles |
|
| [28,29] |
| Zinc nanoparticles |
|
| [30,31,32] |
| Calcium phosphate nanoparticles |
|
| [33,34] |
| Titanium nanoparticles |
|
| [35,36,37] |
| Study Title | Identifier | Nanoparticle | Aim | Last Update |
|---|---|---|---|---|
| Clinical Evaluation of Silver Nanoparticles in Comparison to Silver Diamine Fluoride in Management of Deep Carious Lesions | NCT05231330 | Silver | To evaluate the effect of fluoride varnish with silver nanoparticles in comparison to silver diamine fluoride. | 9 February 2022 |
| Effect of the incorporation of copper and zinc nanoparticles into dental adhesives | NCT03635138 | Copper and Zinc | To study if the addition of copper or zinc nanoparticles to a dental adhesive confers antimicrobial and enzymatic degradation-resistant properties, retaining its adhesion mechanical properties and biocompatibility. | 17 August 2018 |
| Evaluation of the antibacterial effect of laser diode and zinc oxide nano particles in cavity disinfection | NCT03478150 | Zinc Oxide | To evaluate the antibacterial effect of laser diode and zinc oxide nano-particles when used as cavity disinfectants | 27 March 2018 |
| Nanosilver fluoride to prevent dental biofilms growth | NCT01950546 | Silver | To evaluate the effectiveness of nanosilver fluoride for controlling the growth of S. mutans present in dental plaque of children. | 10 June 2015 |
| Antibacterial effect of nano silver fluoride vs chlorhexidine on occlusal carious molars treated with partial caries removal technique | NCT03186261 | Silver | To evaluate the effect of silver nanoparticles in comparison with Chlorhexidine on Occlusal Carious Molars regarding the removal of bacterial plaques. | 16 September 2021 |
| Antibacterial effect and clinical performance of chitosan modified glass ionomer | NCT04365270 | Polymeric and Titanium dioxide | To assess the clinical success and the antibacterial effect on carious dentine of glass ionomer when modified with Chitosan and/or Titanium dioxide nano particles vs the control group of modification with Chlorhexidine as control when used in primary molars. | 12 January 2021 |
| Clinical performance and wear resistance of two nano ceramic resin composite in class I cavities | NCT04738604 | Ceramic resin | Tooth restorations. | 31 August 2021 |
| Remineralization of early carious lesion using natural agents versus bioadhesive polymers | NCT04390256 | Bioadhesive Polymers | Remineralization. | 15 May 2020 |
| Cariostatic and remineralizing effects of three different dental varnishes | NCT04887389 | Silver nanoparticles in varnishes | To evaluate the cariostatic and re-mineralizing effects of Nano silver fluoride, Nano Hydroxyapatite and sodium fluoride varnishes in caries prevention. | 8 June 2022 |
| P11-4 and nanosilver fluoride varnish in treatment of white spot carious lesions | NCT04929509 | Silver | To evaluate the biomimetic remineralization of initial carious lesions as a minimal invasive therapy using Self-Assembling Peptide P11-4 (Curodont Repair) which enhances remineralization of white spot lesions. | 18 June 2021 |
| Nanosystem | Drug | Composition | Study Model | Effect | Ref. |
|---|---|---|---|---|---|
| Liquid crystalline system | β-defensin-3 peptide fragment | Carbopol® 974P, Carbopol® 971P, polyoxypropylene-(5)- polyoxyethylene-(20)-cetyl alcohol, and oleic acid | In vitro | The developed formulation showed a cumulative effect against S. mutans. | [15] |
| Liquid crystalline system | p1025 peptide | Polyoxypropylene-(5)-polyoxyethylene-(20)-cetyl alcohol and tea tree oil | In vitro | The liquid crystalline systems showed shear thinning and thixotropy characteristics favorable for treatment of dental caries. | [8] |
| Liquid crystalline system | p1025 peptide | Polyoxypropylene-(5)-polyoxyethylene-(20)-cetyl alcohol, oleic acid, and Carbopol® 974P | In vitro | Reduced S. mutans biofilm formation with a limited cytotoxicity in human epithelial cells (HaCaT). | [64] |
| Liquid crystalline system | Curcumin | Polyoxypropylene-(5)-polyoxyethylene-(20)-cetyl alcohol, oleic acid, and Carbopol® 974P | In vitro | Reduced significantly the log10 when photodynamic therapy was applied. | [65] |
| Liposomes | Nisin | Dipalmitoylphosphatidylcholine and phytosphingosine | In vitro | Cationic nisin-loaded liposomes showed greater antimicrobial activity against S. mutans than neutral and anionic liposomes. | [66] |
| Liposomes | Doxycycline | Lecithin | In vitro | Doxycycline-loaded liposomes removed the biofilm from the hydroxyapatite surface. | [67] |
| Liposomes | Demineralized dentin matrix | Phosphatidylcholine, Phosphatidylserine, and cholesterol | In vitro | Activated the dental tissue repair in vitro. | [68] |
| Nanoemulsion | Cetylpyridinium chloride | Soybean oil and Triton X-100 | In vitro | Nanoemulsion showed greater inhibitory effect against microorganisms than chlorhexidine. | [69] |
| Nanoemulsion | Chlorhexidine acetate | Tween 80, propylene glycol, and isopropyl myristate | In vitro and in vivo | Nanoemulsion significantly reduced oral biofilm and inhibited biofilms formation in rats. | [70] |
| Nanoemulsion | Cinnamon essential oil | Cocamidopropyl betaine and cinnamon essential oil | In vitro | The developed formulation inhibited the maturation and growth of oral biofilms. | [71] |
| Polymeric Nanoparticle | - | Chitosan and tripolyphosphate | In vitro | Chitosan nanoparticles decreased the cell viability of S. mutans and C. albicans. | [72] |
| Polymeric Nanoparticle | - | Chitosan | In vitro | Decreased biofilm formation of S. mutans by 88.4%, S. salivarius by 93.4%, S. sobrinus by 78.9%, and S. sanguis by 72.6%. | [73] |
| Polymeric Nanoparticle | Chloroaluminum phthalocyanine | Chitosan | In vitro | The photodynamic mediated by the developed system significantly reduced S. mutans UFC | [74] |
| Hydrogel | QPs peptide | Chitosan | In vitro | Decreased biofilm formation of S. mutans by approximately 100%. | [75] |
| Hydrogel | Mentha piperita | Chitosan | In vitro | Decreased biofilm formation of S. mutans by 57%. | [76] |
| Hydrogel | Tormentil | Carboxymethylcellulose | In vitro | Total S. mutans biofilm inhibition using 2 mg/mL of the tormentil. | [77] |
| Hydrogel | Methylene blue | Papain | In vitro | Methylene blue-loaded papain hydrogel showed a reduction in 2-log CFU | [78] |
| Dendrimer | Triclosan | Carboxyl-terminated PAMAM polymer | In vitro | In vitro remineralization of human dentine, adhesive properties, and sustained release. | [79] |
| Dendrimer | Honokiol | Carboxyl-terminated PAMAM polymer | In vitro and in vivo | In vitro sustained release and remineralization, adhesive properties, anti-biofilm action, and in vivo anti-cariogenic activity | [80] |
| Dendrimer | Apigenin | Phosphate ester-terminated PAMAM dendrimer | In vitro | In vitro sustained release, induced remineralization, antibiofilm activity, adhesive properties, biocompatible | [81] |
| Dendrimer | Nitric oxide | Octyl- and dodecyl-modified PAMAM | In vitro | Increased in vitro antibiofilm action and fast release, at acidic pH. More hydrophobic formulations showed increased dendrimer-bacteria interaction | [82] |
| Polymeric micelles | Farnesal | mPEG2000-PLA2000 | In vitro | Fast adhesion to hydroxyapatite and pH-triggered release in acidic pH, in vitro anti-demineralization, and in vivo anti-cariogenic properties | [83] |
| Polymeric micelles | Triclosan | ALN-modified Pluronic copolymers | In vitro | Fast and strong binding to hydroxyapatite in vitro, anti-biofilm, and strong killing effect against S. mutans, sustained release | [84] |
| Polymeric micelles | Tannic acid and NaF | 3-maleimidopropionic acid-poly(ethylene glycol)-block-poly(l-lysine)/phenylboronic acid (MAL-PEG-b-PLL/PBA) and SAP | In vitro and in vivo | pH triggered release, in vitro biocompatibility, strong enamel adhesion, anti-biofilm activity, and anti-demineralization activity. In vivo anti-cariogenic effect superior to conventional treatment and remineralization properties | [85] |
| Nanosystem | Composition | Study Model | Effect | Ref. |
|---|---|---|---|---|
| Silver nanoparticles | Silver nitrate and gallic acid | In vitro | Silver nanoparticles exhibited great antimicrobial activity against dental plaque. | [115] |
| Silver nanoparticles | Silver nitrate, epigallocatechin gallate, and chitosan | In vitro | The developed formulation can remineralize dentine caries | [118] |
| Silver nanoparticles | Silver nitrate and gallic acid | In vitro | Silver nanoparticles showed antimicrobial activity against microorganisms from oral biofilms, including S. mutans. | [119] |
| Zinc nanoparticles | Zinc acetate dihydrate | In vitro | Zinc nanoparticles reduced biofilm formation. | [120] |
| Zinc nanoparticles | Pure zinc block | In vitro | Rod-like shaped zinc nanoparticles exhibited greater inhibition on Streptococcus sobrinus and S. mutans compared with plate-like shaped nanoparticles. | [121] |
| Nanocomplex of calcium phosphate (nCPP), casein (nACP), probiotic and glycomacropeptide (nGMP) | nCPP, nACP, L. rhamnosus (109 CFU/g) e nGMP, and toothpaste base. | In vitro | Increased remineralization, antibacterial effect, increased deposition on enamel surface with a long-term protective effect | [122] |
| Hydroxyapatite nanocrystals | Hydroxyapatite microclusters in bidestilled water | In vitro and in situ | Reduced the viable bacteria and glucans on the surface of specimens and increased interactions of hydroxyapatite particles and bacterial fimbriae. | [123] |
| β-tricalcium phosphate Nanoparticles | Calcium hydroxide, magnesium hydroxide, fluoride and/or stannous ions. | In vitro | Reduced enamel and dentin surface loss, improved anti-erosive effect | [124] |
| Fluoride-doped amorphous calcium phosphate nanoparticles | Calcium chloride dihydrate, sodium citrate tribasic dihydrate, sodium phosphate dibasic dihydrate, sodium carbonate monohydrate, sodium fluoride | In vitro | Decreased conversion to the crystalline phase in water, increased occlusion of dentinal tubules and enamel remineralization. | [125] |
| TiO2 Nanoparticles | TiO2 nanoparticles and glass-ionomer cements | In vitro | Increase in compressive strength and decreased in porosity and micro-cracks increasing mechanical strength. | [126] |
| TiO2 Nanoparticles | TiO2 nanoparticles and glass-ionomer cements | In vitro | Increased flexural strength, compressive strength, and diametrical tensile strength. | [127] |
| TiO2 nanotubes | TiO2 nanotubes and glass-ionomer cements | In vitro | Increased antibacterial property against S. mutuans, change in morphology and organization of S. mutuans, reduction in covR expression, increase in anti-cariogenic properties of glass-ionomer cements | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luiz, M.T.; di Filippo, L.D.; Dutra, J.A.P.; Viegas, J.S.R.; Silvestre, A.L.P.; Anselmi, C.; Duarte, J.L.; Calixto, G.M.F.; Chorilli, M. New Technological Approaches for Dental Caries Treatment: From Liquid Crystalline Systems to Nanocarriers. Pharmaceutics 2023, 15, 762. https://doi.org/10.3390/pharmaceutics15030762
Luiz MT, di Filippo LD, Dutra JAP, Viegas JSR, Silvestre ALP, Anselmi C, Duarte JL, Calixto GMF, Chorilli M. New Technological Approaches for Dental Caries Treatment: From Liquid Crystalline Systems to Nanocarriers. Pharmaceutics. 2023; 15(3):762. https://doi.org/10.3390/pharmaceutics15030762
Chicago/Turabian StyleLuiz, Marcela Tavares, Leonardo Delello di Filippo, Jessyca Aparecida Paes Dutra, Juliana Santos Rosa Viegas, Amanda Letícia Polli Silvestre, Caroline Anselmi, Jonatas Lobato Duarte, Giovana Maria Fioramonti Calixto, and Marlus Chorilli. 2023. "New Technological Approaches for Dental Caries Treatment: From Liquid Crystalline Systems to Nanocarriers" Pharmaceutics 15, no. 3: 762. https://doi.org/10.3390/pharmaceutics15030762
APA StyleLuiz, M. T., di Filippo, L. D., Dutra, J. A. P., Viegas, J. S. R., Silvestre, A. L. P., Anselmi, C., Duarte, J. L., Calixto, G. M. F., & Chorilli, M. (2023). New Technological Approaches for Dental Caries Treatment: From Liquid Crystalline Systems to Nanocarriers. Pharmaceutics, 15(3), 762. https://doi.org/10.3390/pharmaceutics15030762









