Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications
Abstract
:1. Introduction
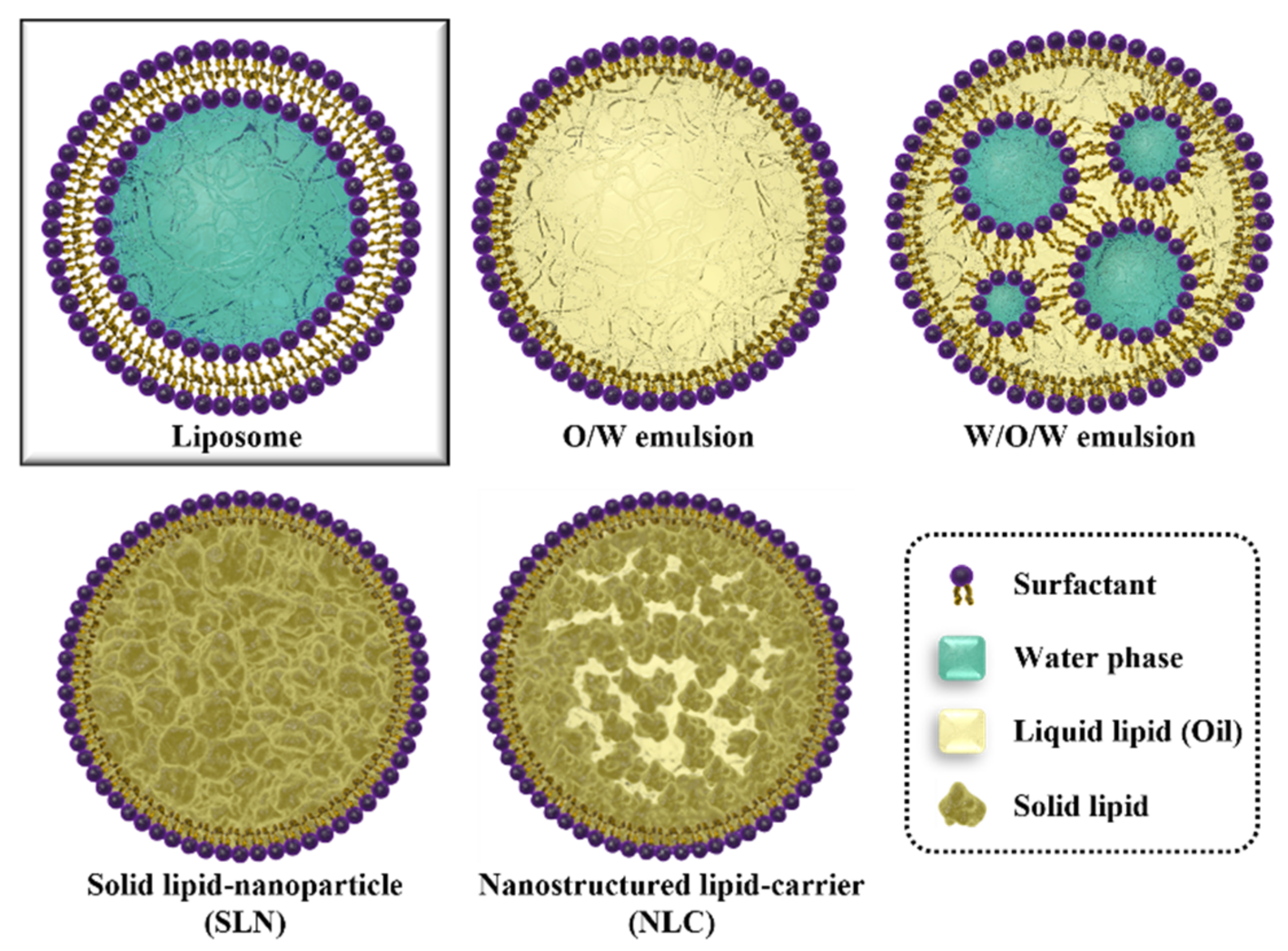
2. Various Types of Lipid Nanoparticles
2.1. Liquid Lipid-Core LNP
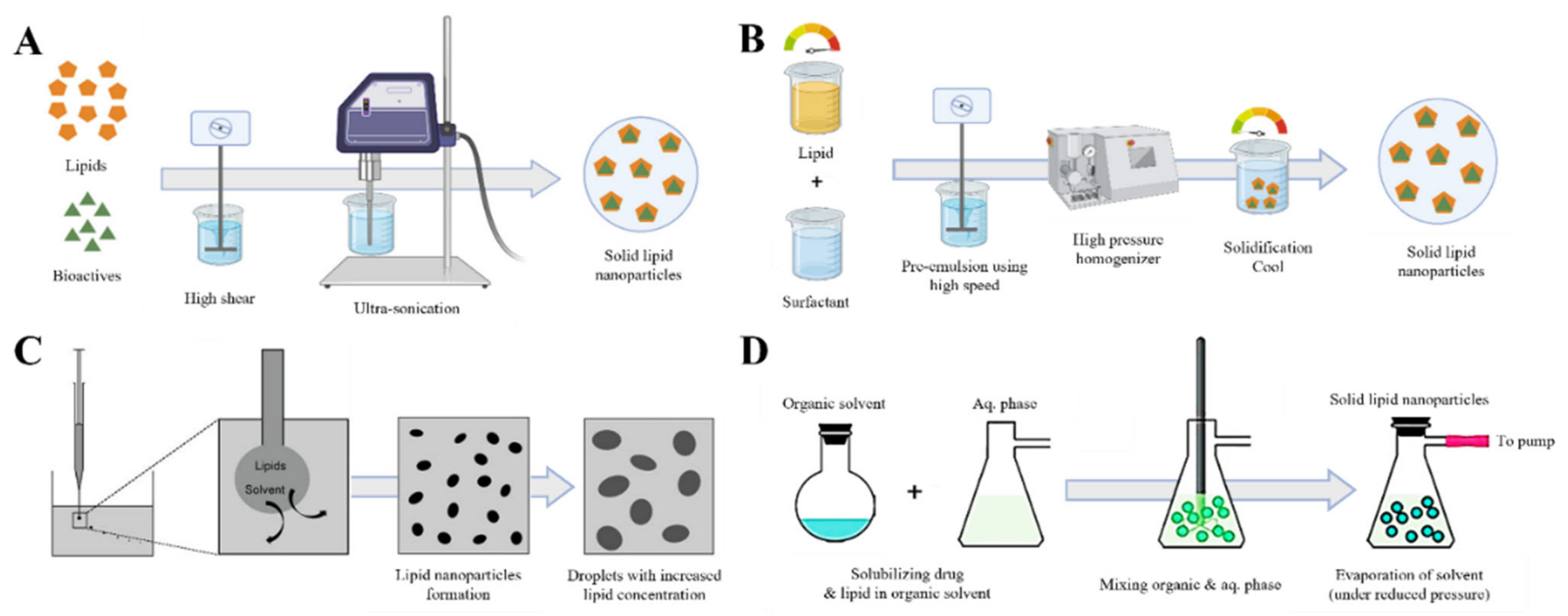
2.2. Solid Lipid-Core LNP (Solid Lipid Nanoparticle)
2.3. Nanostructured Lipid Carrier (NLC)
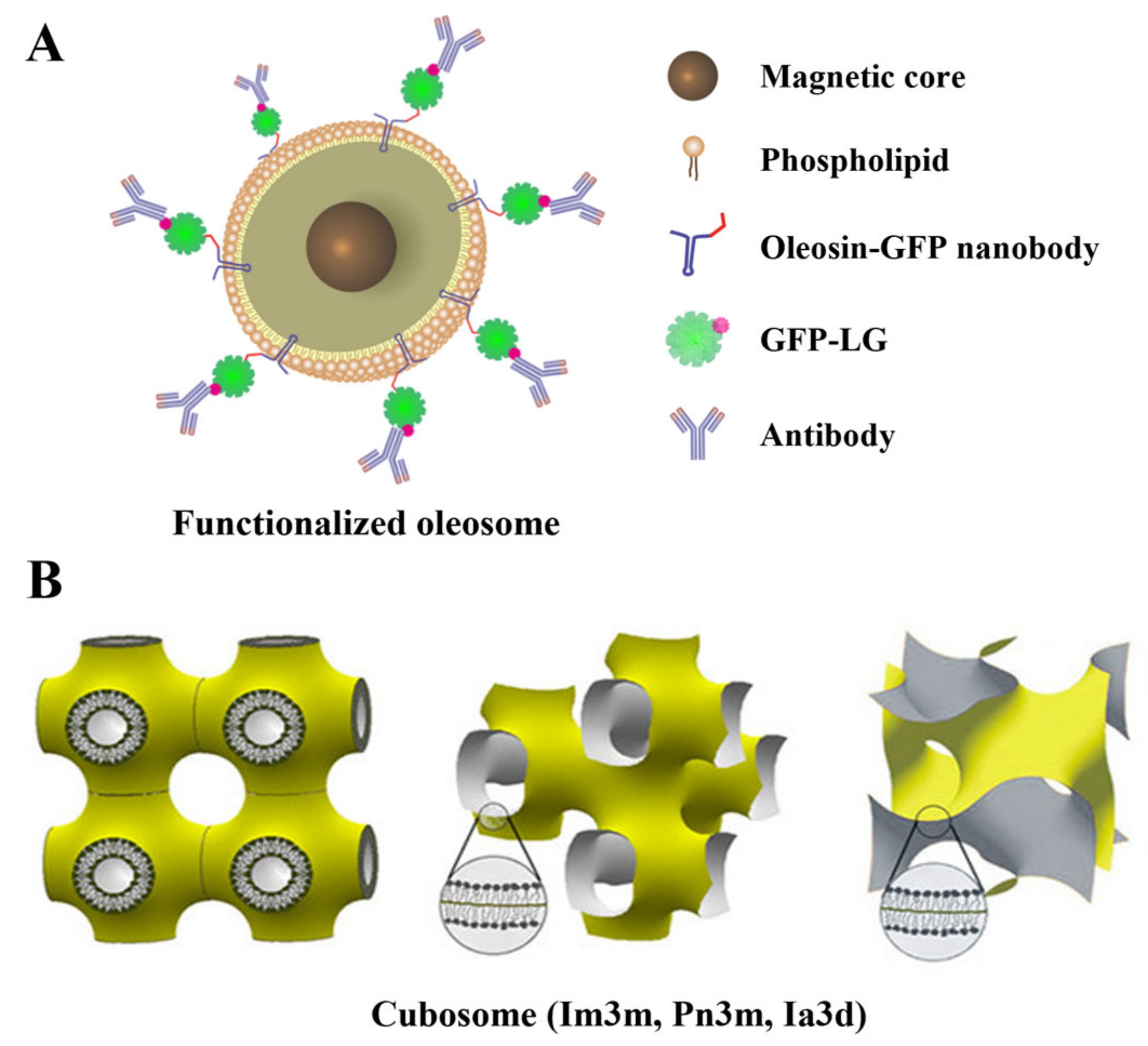
2.4. Hollow LNPs
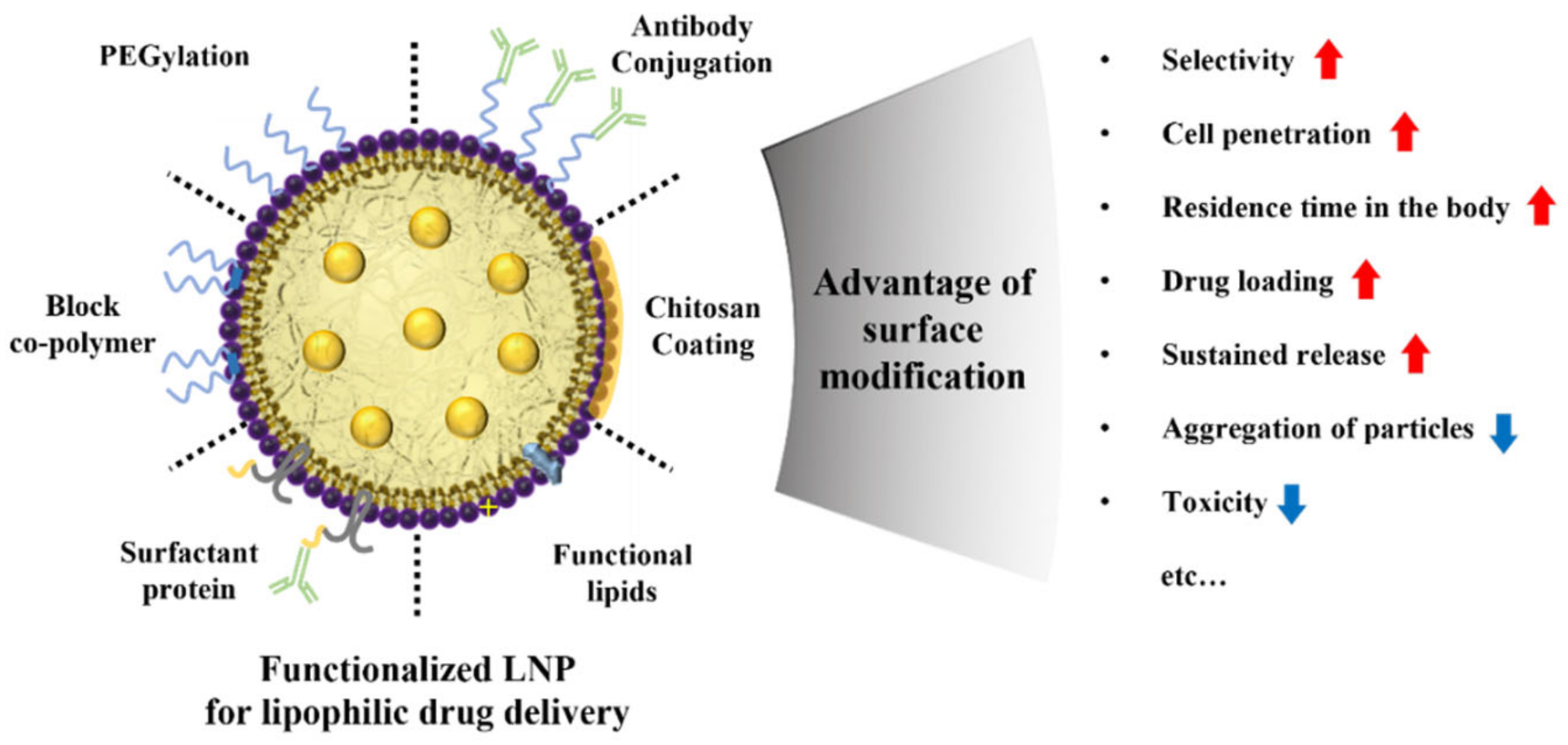
3. Surface Modifications of Lipid Nanoparticles
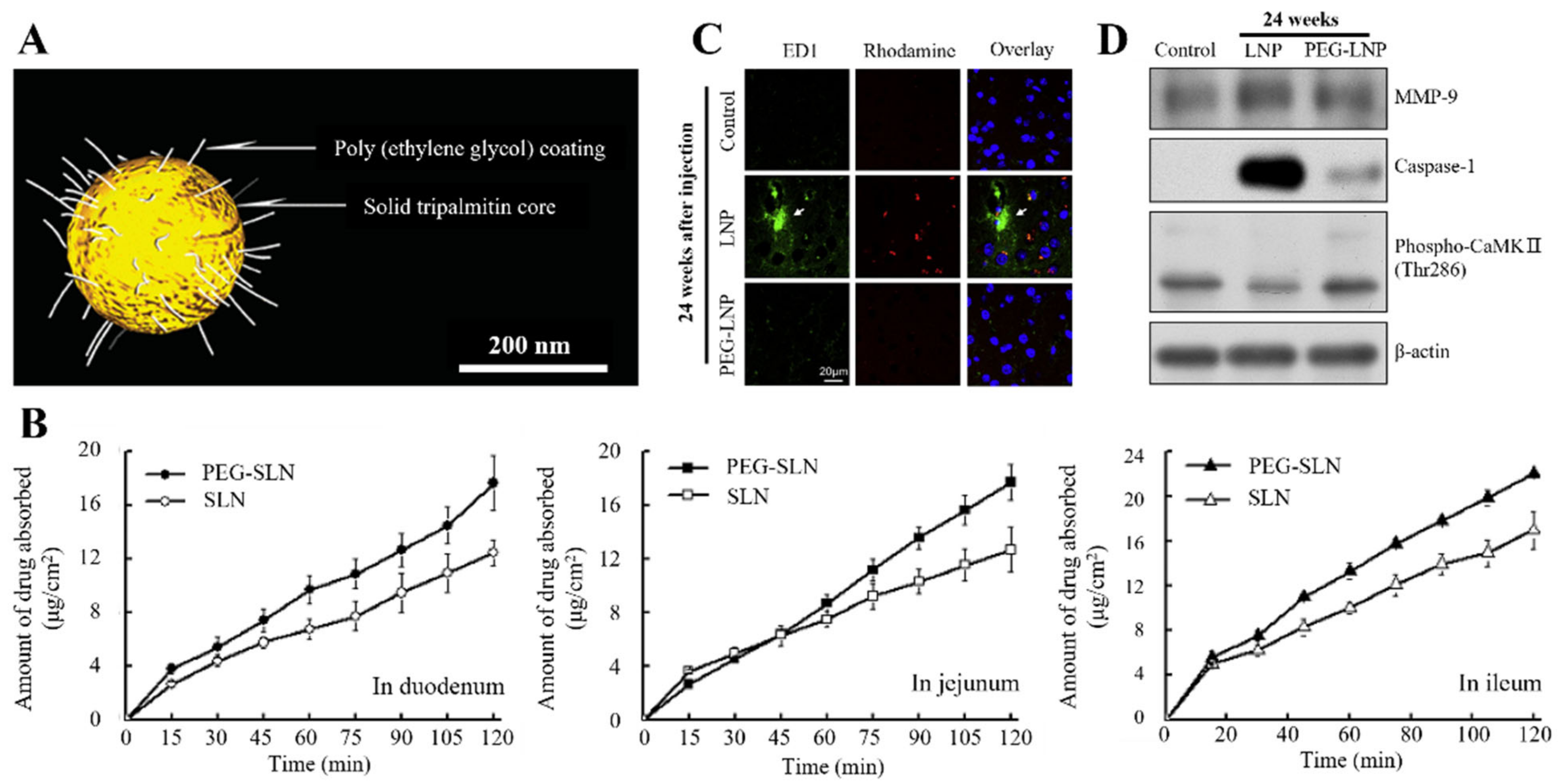
3.1. Polymer
3.2. Chitosan Coating
3.3. Functional Lipid
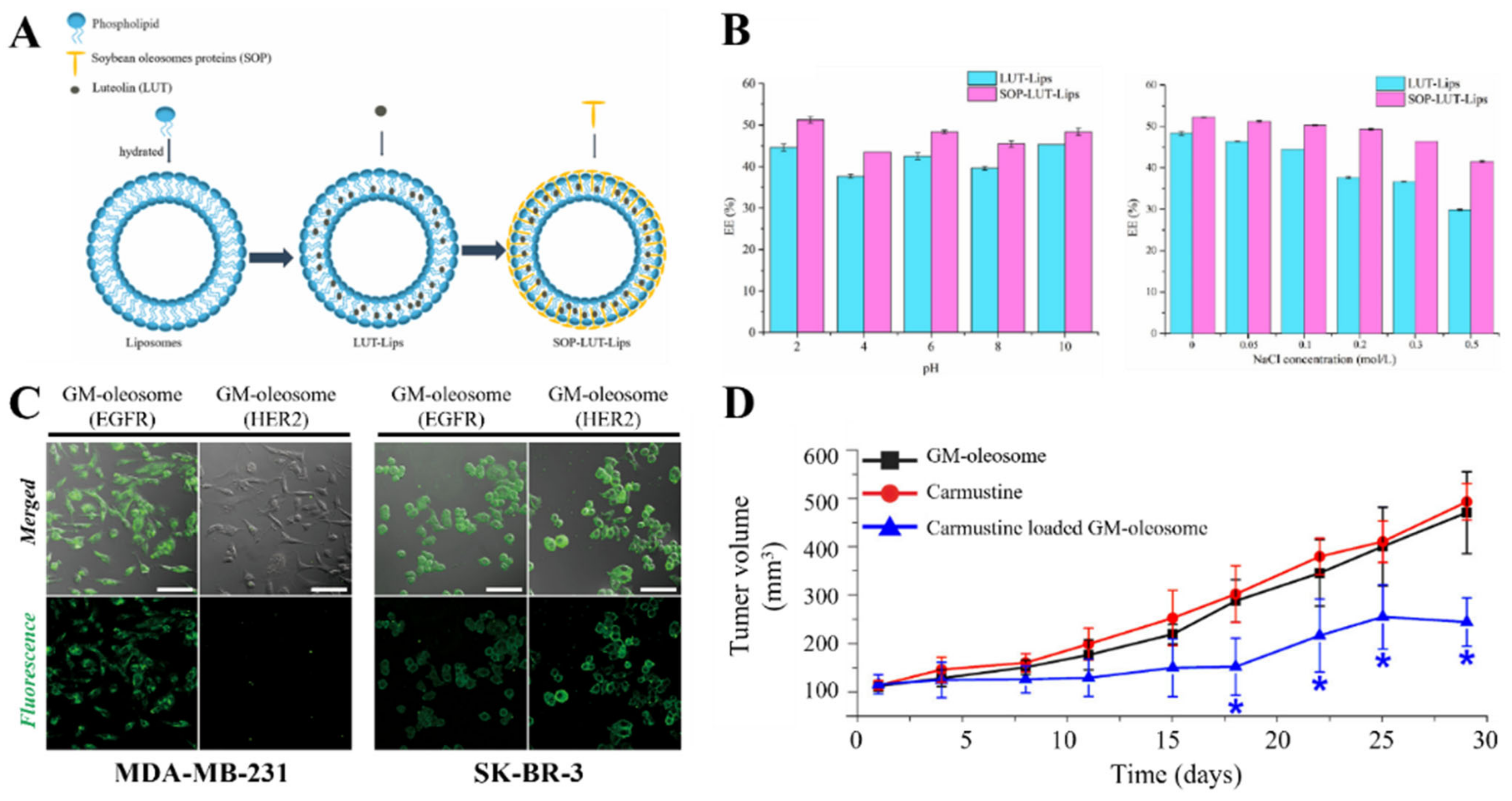
3.4. Surfactant Protein
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef] [PubMed]
- Sahbaz, Y.; Williams, H.D.; Nguyen, T.-H.; Saunders, J.; Ford, L.; Charman, S.A.; Scammells, P.J.; Porter, C.J.H. Transformation of Poorly Water-Soluble Drugs into Lipophilic Ionic Liquids Enhances Oral Drug Exposure from Lipid Based Formulations. Mol. Pharm. 2015, 12, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooque, F.; Wasi, M.; Mughees, M.M. Liposomes as Drug Delivery System: An Updated Review. J. Drug Deliv. Ther. 2021, 11, 149–158. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Wilson, B.; Geetha, K.M. Lipid Nanoparticles in the Development of mRNA Vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef]
- Anand, P.; Stahel, V.P. The Safety of COVID-19 mRNA Vaccines: A Review. Patient Saf. Surg. 2021, 15, 1–9. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Malayala, S. V Side Effects of BNT162b2 mRNA COVID-19 Vaccine: A Randomized, Cross-Sectional Study with Detailed Self-Reported Symptoms from Healthcare Workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef]
- Long, J.; Yu, C.; Zhang, H.; Cao, Y.; Sang, Y.; Lu, H.; Zhang, Z.; Wang, X.; Wang, H.; Song, G. Novel Ionizable Lipid Nanoparticles for SARS-CoV-2 Omicron mRNA Delivery. Adv. Healthc. Mater. 2023, 2202590. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 MRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Igyártó, B.Z.; Jacobsen, S.; Ndeupen, S. Future Considerations for the mRNA-Lipid Nanoparticle Vaccine Platform. Curr. Opin. Virol. 2021, 48, 65–72. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. Iscience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, Y.; Yamada, Y.; Abd Elwakil, M.M.; Kimura, S.; Younis, M.A.; Harashima, H. Extrahepatic Targeting of Lipid Nanoparticles in Vivo with Intracellular Targeting for Future Nanomedicines. Adv. Drug Deliv. Rev. 2022, 188, 114417. [Google Scholar] [CrossRef]
- Risma, K.A.; Edwards, K.M.; Hummell, D.S.; Little, F.F.; Norton, A.E.; Stallings, A.; Wood, R.A.; Milner, J.D. Potential Mechanisms of Anaphylaxis to COVID-19 mRNA Vaccines. J. Allergy Clin. Immunol. 2021, 147, 2075–2082. [Google Scholar] [CrossRef]
- Bahari, L.A.S.; Hamishehkar, H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; a Comparative Literature Review. Adv. Pharm. Bull. 2016, 6, 143. [Google Scholar] [CrossRef]
- De Melo Barbosa, R.; Severino, P.; Finkler, C.L.L.; de Paula, E. Lipid-Based Colloidal Carriers for Transdermal Administration of Bioactives. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–397. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Böttger, R.; Pauli, G.; Chao, P.-H.; Fayez, N.A.L.; Hohenwarter, L.; Li, S.-D. Lipid-Based Nanoparticle Technologies for Liver Targeting. Adv. Drug Deliv. Rev. 2020, 154, 79–101. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Joshi, M.D.; Müller, R.H. Lipid Nanoparticles for Parenteral Delivery of Actives. Eur. J. Pharm. Biopharm. 2009, 71, 161–172. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, C.; Matosevic, S. Pharmaceutical Liposomal Drug Delivery: A Review of New Delivery Systems and a Look at the Regulatory Landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─ from Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Hamidi, M. Passive and Active Targeting in Cancer Therapy by Liposomes and Lipid Nanoparticles. Drug Metab. Pers. Ther. 2019, 34. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, M.; Assi, S.; Fatokun, A.A.; Khan, I. The Effects of Solid and Liquid Lipids on the Physicochemical Properties of Nanostructured Lipid Carriers. J. Pharm. Sci. 2021, 110, 2859–2872. [Google Scholar] [CrossRef]
- Ban, C.; Lim, S.; Chang, P.-S.; Choi, Y.J. Enhancing the Stability of Lipid Nanoparticle Systems by Sonication during the Cooling Step and Controlling the Liquid Oil Content. J. Agric. Food Chem. 2014, 62, 11557–11567. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Martín-Banderas, L.; Lopes, R.; Gonçalves, L.M.D.; Fernández-Arévalo, M.; Almeida, A.J. Lipid Nanoparticles as an Emerging Platform for Cannabinoid Delivery: Physicochemical Optimization and Biocompatibility. Drug Dev. Ind. Pharm. 2016, 42, 190–198. [Google Scholar] [CrossRef]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef]
- McClements, D.J. Edible Lipid Nanoparticles: Digestion, Absorption, and Potential Toxicity. Prog. Lipid Res. 2013, 52, 409–423. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Jiang, Y.; Hu, Y.; Wang, Z.; Liu, J.; Feng, R.; Zhang, J.; Huang, G. Novel PEG-Grafted Nanostructured Lipid Carrier for Systematic Delivery of a Poorly Soluble Anti-Leukemia Agent Tamibarotene: Characterization and Evaluation. Drug Deliv. 2015, 22, 223–229. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith III, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Ovais, M.; Khan, A.; Raza, A. Docetaxel-loaded Solid Lipid Nanoparticles: A Novel Drug Delivery System. IET Nanobiotechnology 2017, 11, 621–629. [Google Scholar] [CrossRef]
- Lahkar, S.; Das, M.K. Brain-Targeted Drug Delivery with Surface-Modified Nanoparticles. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 277–310. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Shen, L.; Alrobaian, M.; Panda, S.K.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Ibrahim, I.A.A.; Singh, T. Paclitaxel and Naringenin-Loaded Solid Lipid Nanoparticles Surface Modified with Cyclic Peptides with Improved Tumor Targeting Ability in Glioblastoma Multiforme. Biomed. Pharmacother. 2021, 138, 111461. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Souto, E.B.; Nowak, I. Lipid Nanoparticles Loaded with Iridoid Glycosides: Development and Optimization Using Experimental Factorial Design. Molecules 2021, 26, 3161. [Google Scholar] [CrossRef]
- Ganesan, P.; Narayanasamy, D. Lipid Nanoparticles: Different Preparation Techniques, Characterization, Hurdles, and Strategies for the Production of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Oral Drug Delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Subramanian, P. Lipid-Based Nanocarrier System for the Effective Delivery of Nutraceuticals. Molecules 2021, 26, 5510. [Google Scholar] [CrossRef]
- Wang, J.; Shi, A.; Agyei, D.; Wang, Q. Formulation of Water-in-Oil-in-Water (W/O/W) Emulsions Containing Trans-Resveratrol. RSC Adv. 2017, 7, 35917–35927. [Google Scholar] [CrossRef] [Green Version]
- Frelichowska, J.; Bolzinger, M.-A.; Pelletier, J.; Valour, J.-P.; Chevalier, Y. Topical Delivery of Lipophilic Drugs from o/w Pickering Emulsions. Int. J. Pharm. 2009, 371, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hiranphinyophat, S.; Otaka, A.; Asaumi, Y.; Fujii, S.; Iwasaki, Y. Particle-Stabilized Oil-in-Water Emulsions as a Platform for Topical Lipophilic Drug Delivery. Colloids Surfaces B Biointerfaces 2021, 197, 111423. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-Delivery of Hydrophobic Curcumin and Hydrophilic Catechin by a Water-in-Oil-in-Water Double Emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, D.; Park, E.; Jang, S.; Cheon, S.Y.; Han, S.; Koo, H. Rhamnolipid-Coated W/O/W Double Emulsion Nanoparticles for Efficient Delivery of Doxorubicin/Erlotinib and Combination Chemotherapy. J. Nanobiotechnology 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Moral, S.; Ochando-Pulido, J.M.; Segura-Carretero, A.; Martinez-Ferez, A. Stabilization of W/O/W Multiple Emulsion Loaded with Hibiscus Sabdariffa Extract through Protein-Polysaccharide Complexes. LWT 2018, 90, 389–395. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, C.; Sun, R.; Ni, S.; Li, Q.; Xia, Q. Development of Novel Composite Antioxidant Multiple Lipid Particles from Combination of W/O/W Multiple Emulsions and Solid Lipid Nanoparticles. Eur. J. lipid Sci. Technol. 2015, 117, 1056–1065. [Google Scholar] [CrossRef]
- Abdelalim, L.R.; Elnaggar, Y.S.R.; Abdallah, O.Y. Oleosomes Encapsulating Sildenafil Citrate as Potential Topical Nanotherapy for Palmar Plantar Erythrodysesthesia with High Ex Vivo Permeation and Deposition. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Cho, H.Y.; Lee, T.; Yoon, J.; Han, Z.; Rabie, H.; Lee, K.-B.; Su, W.W.; Choi, J.-W. Magnetic Oleosome as a Functional Lipophilic Drug Carrier for Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9301–9309. [Google Scholar] [CrossRef]
- Kaur, J.; Aslam, M.; Jha, M.K.; Sarma, A.K. Green Diesel: Integrated Production Processes, Future Perspectives and Techno-Economic Feasibility. In Green Diesel: An Alternative to Biodiesel and Petrodiesel; Springer: Berlin/Heidelberg, Germany, 2022; pp. 205–217. [Google Scholar] [CrossRef]
- Nikiforidis, C. V Structure and Functions of Oleosomes (Oil Bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef]
- Ashique, S.; Singh, A.; Sandhu, N.K. Stability Issues, Probable Approaches for Stabilization and Associated Patents in the Pharmaceutical Field for Oleosome, A Novel Carrier for Drug Delivery. Recent Pat. Nanotechnol. 2022, 16, 207–218. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B.; MacDonald, R.C. Nonlamellar Phases in Cationic Phospholipids, Relevance to Drug and Gene Delivery. ACS Biomater. Sci. Eng. 2015, 1, 130–138. [Google Scholar] [CrossRef]
- Müller, R.H.; Alexiev, U.; Sinambela, P.; Keck, C.M. Nanostructured Lipid Carriers (NLC): The Second Generation of Solid Lipid Nanoparticles. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–185. [Google Scholar] [CrossRef]
- Guo, S.-J.; Ma, C.-G.; Hu, Y.-Y.; Bai, G.; Song, Z.-J.; Cao, X.-Q. Solid Lipid Nanoparticles for Phytosterols Delivery: The Acyl Chain Number of the Glyceride Matrix Affects the Arrangement, Stability, and Release. Food Chem. 2022, 394, 133412. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Jensen, L.B.; Magnussson, E.; Gunnarsson, L.; Vermehren, C.; Nielsen, H.M.; Petersson, K. Corticosteroid Solubility and Lipid Polarity Control Release from Solid Lipid Nanoparticles. Int. J. Pharm. 2010, 390, 53–60. [Google Scholar] [CrossRef]
- Yoon, G.; Park, J.W.; Yoon, I.-S. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs): Recent Advances in Drug Delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S. Nanostructured Lipid Matrices for Improved Microencapsulation of Drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Makwana, V.; Jain, R.; Patel, K.; Nivsarkar, M.; Joshi, A. Solid Lipid Nanoparticles (SLN) of Efavirenz as Lymph Targeting Drug Delivery System: Elucidation of Mechanism of Uptake Using Chylomicron Flow Blocking Approach. Int. J. Pharm. 2015, 495, 439–446. [Google Scholar] [CrossRef]
- Kesavan, K.; Kant, S.; Singh, P.N.; Pandit, J.K. Mucoadhesive Chitosan-Coated Cationic Microemulsion of Dexamethasone for Ocular Delivery: In Vitro and in Vivo Evaluation. Curr. Eye Res. 2013, 38, 342–352. [Google Scholar] [CrossRef]
- Baspinar, Y.; Bertelmann, E.; Pleyer, U.; Buech, G.; Siebenbrodt, I.; Borchert, H.-H. Corneal Permeation Studies of Everolimus Microemulsion. J. Ocul. Pharmacol. Ther. 2008, 24, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, K.; Afzal, S.M.; Surender, G.; Kishan, V. Tween 80 Containing Lipid Nanoemulsions for Delivery of Indinavir to Brain. Acta Pharm. Sin. B 2013, 3, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, H.S.; Mahajan, M.S.; Nerkar, P.P.; Agrawal, A. Nanoemulsion-Based Intranasal Drug Delivery System of Saquinavir Mesylate for Brain Targeting. Drug Deliv. 2014, 21, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Misra, A.; Babbar, A.K.; Mishra, A.K.; Mishra, P.; Pathak, K. Intranasal Nanoemulsion Based Brain Targeting Drug Delivery System of Risperidone. Int. J. Pharm. 2008, 358, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fadeel, D.A.; Kamel, R.; Fadel, M. PEGylated Lipid Nanocarrier for Enhancing Photodynamic Therapy of Skin Carcinoma Using Curcumin: In-Vitro/in-Vivo Studies and Histopathological Examination. Sci. Rep. 2020, 10, 10435. [Google Scholar] [CrossRef]
- Yadav, M.; Schiavone, N.; Guzman-Aranguez, A.; Giansanti, F.; Papucci, L.; Perez de Lara, M.J.; Singh, M.; Kaur, I.P. Atorvastatin-Loaded Solid Lipid Nanoparticles as Eye Drops: Proposed Treatment Option for Age-Related Macular Degeneration (AMD). Drug Deliv. Transl. Res. 2020, 10, 919–944. [Google Scholar] [CrossRef]
- Leonardi, A.; Crasci’, L.; Panico, A.; Pignatello, R. Antioxidant Activity of Idebenone-Loaded Neutral and Cationic Solid–Lipid Nanoparticles. Pharm. Dev. Technol. 2015, 20, 716–723. [Google Scholar] [CrossRef]
- Balguri, S.P.; Adelli, G.R.; Majumdar, S. Topical Ophthalmic Lipid Nanoparticle Formulations (SLN, NLC) of Indomethacin for Delivery to the Posterior Segment Ocular Tissues. Eur. J. Pharm. Biopharm. 2016, 109, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Abrishami, M.; Abrishami, M.; Mahmoudi, A.; Mosallaei, N.; Vakili Ahrari Roodi, M.; Malaekeh-Nikouei, B. Solid Lipid Nanoparticles Improve the Diclofenac Availability in Vitreous after Intraocular Injection. J. Drug Deliv. 2016, 2016, 1368481. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic Solid Lipid Nanoparticles Enhance Ocular Hypotensive Effect of Melatonin in Rabbit. Int. J. Pharm. 2015, 478, 180–186. [Google Scholar] [CrossRef]
- Venishetty, V.K.; Komuravelli, R.; Kuncha, M.; Sistla, R.; Diwan, P.V. Increased Brain Uptake of Docetaxel and Ketoconazole Loaded Folate-Grafted Solid Lipid Nanoparticles. Nanomedicine Nanotechnol. Biol. Med. 2013, 9, 111–121. [Google Scholar] [CrossRef]
- Dal Magro, R.; Ornaghi, F.; Cambianica, I.; Beretta, S.; Re, F.; Musicanti, C.; Rigolio, R.; Donzelli, E.; Canta, A.; Ballarini, E. ApoE-Modified Solid Lipid Nanoparticles: A Feasible Strategy to Cross the Blood-Brain Barrier. J. Control. Release 2017, 249, 103–110. [Google Scholar] [CrossRef]
- He, H.; Yao, J.; Zhang, Y.; Chen, Y.; Wang, K.; Lee, R.J.; Yu, B.; Zhang, X. Solid Lipid Nanoparticles as a Drug Delivery System to across the Blood-Brain Barrier. Biochem. Biophys. Res. Commun. 2019, 519, 385–390. [Google Scholar] [CrossRef]
- Kuo, Y.; Wang, C. Cationic Solid Lipid Nanoparticles with Cholesterol-mediated Surface Layer for Transporting Saquinavir to the Brain. Biotechnol. Prog. 2014, 30, 198–206. [Google Scholar] [CrossRef]
- Sood, S.; Jawahar, N.; Jain, K.; Gowthamarajan, K.; Nainar Meyyanathan, S. Olanzapine Loaded Cationic Solid Lipid Nanoparticles for Improved Oral Bioavailability. Curr. Nanosci. 2013, 9, 26–34. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Rostamkalaei, S.S.; Asadi, M.; Asare-Addo, K.; Nokhodchi, A. The Design of Naproxen Solid Lipid Nanoparticles to Target Skin Layers. Colloids Surfaces B Biointerfaces 2016, 145, 626–633. [Google Scholar] [CrossRef]
- Harde, H.; Agrawal, A.K.; Katariya, M.; Kale, D.; Jain, S. Development of a Topical Adapalene-Solid Lipid Nanoparticle Loaded Gel with Enhanced Efficacy and Improved Skin Tolerability. RSC Adv. 2015, 5, 43917–43929. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Gill, P.; Valizadeh, H.; Nokhodchi, A. Formulation Optimization and in Vitro Skin Penetration of Spironolactone Loaded Solid Lipid Nanoparticles. Colloids Surfaces B Biointerfaces 2015, 128, 473–479. [Google Scholar] [CrossRef]
- Rigon, R.B.; Fachinetti, N.; Severino, P.; Santana, M.H.A.; Chorilli, M. Skin Delivery and in Vitro Biological Evaluation of Trans-Resveratrol-Loaded Solid Lipid Nanoparticles for Skin Disorder Therapies. Molecules 2016, 21, 116. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Jiang, J.; Jiang, L.; Zheng, P.; Wang, F.; Zhou, Y.; Chen, Z.; Li, M.; Lian, M.; Tang, S. Chitosan Mediated Solid Lipid Nanoparticles for Enhanced Liver Delivery of Zedoary Turmeric Oil in Vivo. Int. J. Biol. Macromol. 2020, 149, 108–115. [Google Scholar] [CrossRef]
- Khatri, H.; Chokshi, N.; Rawal, S.; Patel, M.M. Fabrication, Characterization and Optimization of Artemether Loaded PEGylated Solid Lipid Nanoparticles for the Treatment of Lung Cancer. Mater. Res. Express 2019, 6, 45014. [Google Scholar] [CrossRef]
- Araújo, J.; Nikolic, S.; Egea, M.A.; Souto, E.B.; Garcia, M.L. Nanostructured Lipid Carriers for Triamcinolone Acetonide Delivery to the Posterior Segment of the Eye. Colloids Surfaces B Biointerfaces 2011, 88, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dandamudi, M.; Rani, S.; Behaeghel, E.; Behl, G.; Kent, D.; O’reilly, N.J.; O’donovan, O.; McLoughlin, P.; Fitzhenry, L. Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease. Pharmaceutics 2021, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.; Kuppusamy, G.; Krishnamurthy, J.; Mahalingam, R.; Singh, S.K.; Gulati, M. Repositioning of Itraconazole for the Management of Ocular Neovascularization through Surface-Modified Nanostructured Lipid Carriers. Assay Drug Dev. Technol. 2019, 17, 178–190. [Google Scholar] [CrossRef]
- Sharif Makhmal Zadeh, B.; Niro, H.; Rahim, F.; Esfahani, G. Ocular Delivery System for Propranolol Hydrochloride Based on Nanostructured Lipid Carrier. Sci. Pharm. 2018, 86, 16. [Google Scholar] [CrossRef] [Green Version]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan Coated Nanostructured Lipid Carriers for Brain Delivery of Proteins by Intranasal Administration. Colloids Surfaces B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Khan, S.A.; Rehman, S.; Nabi, B.; Iqubal, A.; Nehal, N.; Fahmy, U.A.; Kotta, S.; Baboota, S.; Md, S.; Ali, J. Boosting the Brain Delivery of Atazanavir through Nanostructured Lipid Carrier-Based Approach for Mitigating Neuroaids. Pharmaceutics 2020, 12, 1059. [Google Scholar] [CrossRef]
- Zhuang, C.-Y.; Li, N.; Wang, M.; Zhang, X.-N.; Pan, W.-S.; Peng, J.-J.; Pan, Y.-S.; Tang, X. Preparation and Characterization of Vinpocetine Loaded Nanostructured Lipid Carriers (NLC) for Improved Oral Bioavailability. Int. J. Pharm. 2010, 394, 179–185. [Google Scholar] [CrossRef]
- Jawahar, N.; Hingarh, P.K.; Arun, R.; Selvaraj, J.; Anbarasan, A.; Sathianarayanan, S.; Nagaraju, G. Enhanced Oral Bioavailability of an Antipsychotic Drug through Nanostructured Lipid Carriers. Int. J. Biol. Macromol. 2018, 110, 269–275. [Google Scholar] [CrossRef]
- Abd El-Halim, S.M.; Abdelbary, G.A.; Amin, M.M.; Zakaria, M.Y.; Shamsel-Din, H.A.; Ibrahim, A.B. Stabilized Oral Nanostructured Lipid Carriers of Adefovir Dipivoxil as a Potential Liver Targeting: Estimation of Liver Function Panel and Uptake Following Intravenous Injection of Radioiodinated Indicator. DARU J. Pharm. Sci. 2020, 28, 517–532. [Google Scholar] [CrossRef]
- Elmowafy, M.; Ibrahim, H.M.; Ahmed, M.A.; Shalaby, K.; Salama, A.; Hefesha, H. Atorvastatin-Loaded Nanostructured Lipid Carriers (NLCs): Strategy to Overcome Oral Delivery Drawbacks. Drug Deliv. 2017, 24, 932–941. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Liu, S.; Anwaier, G.; Wang, Q.; Shen, W.; Shen, Q.; Qi, R. Formulation and Intestinal Absorption of Naringenin Loaded Nanostructured Lipid Carrier and Its Inhibitory Effects on Nonalcoholic Fatty Liver Disease. Nanomedicine Nanotechnol. Biol. Med. 2021, 32, 102310. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, L.; Zheng, S.; Niu, Y.; Bo, R.; Huang, Y.; Xing, J.; Li, Z.; Wang, D. Cubosome Nanoparticles Potentiate Immune Properties of Immunostimulants. Int. J. Nanomedicine 2016, 11, 3571. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.; Almawash, S.; Al Saqr, A.; Bazeed, A.Y.; Saber, S.; Elagamy, H.I. Bioavailability and Antidiabetic Activity of Gliclazide-Loaded Cubosomal Nanoparticles. Pharmaceuticals 2021, 14, 786. [Google Scholar] [CrossRef]
- Faria, A.R.; Silvestre, O.F.; Maibohm, C.; Adão, R.M.R.; Silva, B.F.B.; Nieder, J.B. Cubosome Nanoparticles for Enhanced Delivery of Mitochondria Anticancer Drug Elesclomol and Therapeutic Monitoring via Sub-Cellular NAD (P) H Multi-Photon Fluorescence Lifetime Imaging. Nano Res. 2019, 12, 991–998. [Google Scholar] [CrossRef]
- Said, M.; Aboelwafa, A.A.; Elshafeey, A.H.; Elsayed, I. Central Composite Optimization of Ocular Mucoadhesive Cubosomes for Enhanced Bioavailability and Controlled Delivery of Voriconazole. J. Drug Deliv. Sci. Technol. 2021, 61, 102075. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin-and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef] [Green Version]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel Piperine-Loaded Tween-Integrated Monoolein Cubosomes as Brain-Targeted Oral Nanomedicine in Alzheimer’s Disease: Pharmaceutical, Biological, and Toxicological Studies. Int. J. Nanomedicine 2015, 10, 5459. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.; Tan, F.H.; Luwor, R.B.; Srinivasa Reddy, T.; Ahmed, N.; Drummond, C.J.; Tran, N. In Vitro and in Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Bio Mater. 2020, 3, 4198–4207. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Gainza, G.; Pastor, M.; Aguirre, J.J.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. A Novel Strategy for the Treatment of Chronic Wounds Based on the Topical Administration of RhEGF-Loaded Lipid Nanoparticles: In Vitro Bioactivity and in Vivo Effectiveness in Healing-Impaired Db/Db Mice. J. Control. Release 2014, 185, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A Magic Lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Han, K.; Peng, X.; Yang, Z.; Qin, L.; Zhu, C.; Huang, X.; Shi, X.; Dian, L.; Lu, M. Nanostructed Cubosomes as Advanced Drug Delivery System. Curr. Pharm. Des. 2013, 19, 6290–6297. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable Drug Delivery Potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Patond, V.B.; Ghonge, A.B.; Narkhede, M.B. Cubosome-Review. Int. J. Trend Sci. Res. Dev 2020, 4, 1116–1120. [Google Scholar]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.C.; Karuppagounder, V. Current Potential and Challenges in the Advances of Liquid Crystalline Nanoparticles as Drug Delivery Systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef]
- Nazaruk, E.; Majkowska-Pilip, A.; Bilewicz, R. Lipidic Cubic-phase Nanoparticles—Cubosomes for Efficient Drug Delivery to Cancer Cells. Chempluschem 2017, 82, 570–575. [Google Scholar] [CrossRef]
- Patel, B.; Thakkar, H.P. Cubosomes: Novel Nanocarriers for Drug Delivery. Nanocarriers Drug Deliv. Syst. Evid. Based Approach 2021, 70, 227–254. [Google Scholar] [CrossRef]
- Rao, S.V.; Sravya, B.N.; Padmalatha, K. A Review on Cubosome: The Novel Drug Delivery System. GSC Biol. Pharm. Sci. 2018, 5, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.-S.; Cho, C.-W. Surface Modification of Solid Lipid Nanoparticles for Oral Delivery of Curcumin: Improvement of Bioavailability through Enhanced Cellular Uptake, and Lymphatic Uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef]
- Choi, S.; Kim, W.; Kim, J. Surface Modification of Functional Nanoparticles for Controlled Drug Delivery. J. Dispers. Sci. Technol. 2003, 24, 475–487. [Google Scholar] [CrossRef]
- Li, Z.; Shan, X.; Chen, Z.; Gao, N.; Zeng, W.; Zeng, X.; Mei, L. Applications of Surface Modification Technologies in Nanomedicine for Deep Tumor Penetration. Adv. Sci. 2021, 8, 2002589. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A Promising Strategy to Overcome Challenges to Cancer-Targeted Nanomedicines: A Review of Challenges to Clinical Transition and Promising Resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- Kebebe, D.; Wu, Y.; Zhang, B.; Yang, J.; Liu, Y.; Li, X.; Ma, Z.; Lu, P.; Liu, Z.; Li, J. Dimeric c (RGD) Peptide Conjugated Nanostructured Lipid Carriers for Efficient Delivery of Gambogic Acid to Breast Cancer. Int. J. Nanomedicine 2019, 14, 6179–6195. [Google Scholar] [CrossRef] [Green Version]
- Mussi, S.V.; Torchilin, V.P. Recent Trends in the Use of Lipidic Nanoparticles as Pharmaceutical Carriers for Cancer Therapy and Diagnostics. J. Mater. Chem. B 2013, 1, 5201–5209. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Torres, D.; Martín-Pastor, M.; Alonso, M.J. Application of NMR Spectroscopy to the Characterization of PEG-Stabilized Lipid Nanoparticles. Langmuir 2004, 20, 8839–8845. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, C.-Y.; Chai, G.; Du, Y.-Z.; Hu, F.-Q. Improved Transport and Absorption through Gastrointestinal Tract by PEGylated Solid Lipid Nanoparticles. Mol. Pharm. 2013, 10, 1865–1873. [Google Scholar] [CrossRef]
- Göppert, T.M.; Müller, R.H. Adsorption Kinetics of Plasma Proteins on Solid Lipid Nanoparticles for Drug Targeting. Int. J. Pharm. 2005, 302, 172–186. [Google Scholar] [CrossRef]
- Kumar, S.; Randhawa, J.K. High Melting Lipid Based Approach for Drug Delivery: Solid Lipid Nanoparticles. Mater. Sci. Eng. C 2013, 33, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.W.; Batrakova, E.V.; Waltner, T.O.; Alakhov, V.Y.; Kabanov, A.V. Interactions of Pluronic Block Copolymers with Brain Microvessel Endothelial Cells: Evidence of Two Potential Pathways for Drug Absorption. Bioconjug. Chem. 1997, 8, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Teng, Z.; Li, Y.; Wang, Q. Solid Lipid Nanoparticles for Oral Drug Delivery: Chitosan Coating Improves Stability, Controlled Delivery, Mucoadhesion and Cellular Uptake. Carbohydr. Polym. 2015, 122, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Yostawonkul, J.; Surassmo, S.; Iempridee, T.; Pimtong, W.; Suktham, K.; Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R. Surface Modification of Nanostructure Lipid Carrier (NLC) by Oleoyl-Quaternized-Chitosan as a Mucoadhesive Nanocarrier. Colloids Surfaces B Biointerfaces 2017, 149, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, D.; Jiang, S.; Shi, K.; Huang, Y.; Li, R.; Xu, Q. Methazolamide-Loaded Solid Lipid Nanoparticles Modified with Low-Molecular Weight Chitosan for the Treatment of Glaucoma: Vitro and Vivo Study. J. Drug Target. 2014, 22, 849–858. [Google Scholar] [CrossRef]
- Sohaib, M.; Shah, S.U.; Shah, K.U.; Khan, N.R.; Irfan, M.M.; Niazi, Z.R.; Alqahtani, A.A.; Alasiri, A.; Walbi, I.A.; Mahmood, S. Physicochemical Characterization of Chitosan-Decorated Finasteride Solid Lipid Nanoparticles for Skin Drug Delivery. Biomed Res. Int. 2022. [Google Scholar] [CrossRef]
- Rosiere, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New Folate-Grafted Chitosan Derivative to Improve Delivery of Paclitaxel-Loaded Solid Lipid Nanoparticles for Lung Tumor Therapy by Inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef]
- Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; Cavaliere, C.; Laganà, A. Effect of DOPE and Cholesterol on the Protein Adsorption onto Lipid Nanoparticles. J. Nanoparticle Res. 2013, 15, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; He, N.; Yang, T.; Cai, S.; Zhang, Y.; Lin, J.; Huang, M.; Chen, W.; Zhang, Y.; Hong, Z. Fucoxanthin Loaded in Palm Stearin-and Cholesterol-Based Solid Lipid Nanoparticle-Microcapsules, with Improved Stability and Bioavailability in Vivo. Mar. Drugs 2022, 20, 237. [Google Scholar] [CrossRef]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating Endosomal Escape of Polymorphic Lipid Nanoparticles That Boost mRNA Delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.-Q.; Ho, W.; Li, F.; Gao, M.; Bai, X.; Xu, X. Enzyme-Catalyzed One-Step Synthesis of Ionizable Cationic Lipids for Lipid Nanoparticle-Based mRNA COVID-19 Vaccines. ACS Nano 2022, 16, 18936–18950. [Google Scholar] [CrossRef]
- Li, R.; Pu, C.; Sun, Y.; Sun, Q.; Tang, W. Interaction between Soybean Oleosome-Associated Proteins and Phospholipid Bilayer and Its Influence on Environmental Stability of Luteolin-Loaded Liposomes. Food Hydrocoll. 2022, 130, 107721. [Google Scholar] [CrossRef]
- Kang, X.; Chen, H.; Li, S.; Jie, L.; Hu, J.; Wang, X.; Qi, J.; Ying, X.; Du, Y. Magnesium Lithospermate B Loaded PEGylated Solid Lipid Nanoparticles for Improved Oral Bioavailability. Colloids Surfaces B Biointerfaces 2018, 161, 597–605. [Google Scholar] [CrossRef]
- Yadav, D.; Dewangan, H.K. PEGYLATION: An Important Approach for Novel Drug Delivery System. J. Biomater. Sci. Polym. Ed. 2021, 32, 266–280. [Google Scholar] [CrossRef]
- Kamel, R.; Abbas, H.; Shaffie, N.M. Development and Evaluation of PLA-Coated Co-Micellar Nanosystem of Resveratrol for the Intra-Articular Treatment of Arthritis. Int. J. Pharm. 2019, 569, 118560. [Google Scholar] [CrossRef]
- Huang, J.; Lu, Y.; Wang, H.; Liu, J.; Liao, M.; Hong, L.; Tao, R.; Ahmed, M.M.; Liu, P.; Liu, S. The Effect of Lipid Nanoparticle PEGylation on Neuroinflammatory Response in Mouse Brain. Biomaterials 2013, 34, 7960–7970. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Zhang, T.; Ma, Z.; Wu, B. Effects of PEGylated Lipid Nanoparticles on the Oral Absorption of One BCS II Drug: A Mechanistic Investigation. Int. J. Nanomedicine 2014, 9, 5503. [Google Scholar] [CrossRef] [Green Version]
- Ban, C.; Jo, M.; Lim, S.; Choi, Y.J. Control of the Gastrointestinal Digestion of Solid Lipid Nanoparticles Using PEGylated Emulsifiers. Food Chem. 2018, 239, 442–452. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Pan, J. Baicalin-Loaded PEGylated Lipid Nanoparticles: Characterization, Pharmacokinetics, and Protective Effects on Acute Myocardial Ischemia in Rats. Drug Deliv. 2016, 23, 3696–3703. [Google Scholar] [CrossRef] [Green Version]
- Ho, E.A.; Osooly, M.; Strutt, D.; Masin, D.; Yang, Y.; Yan, H.; Bally, M. Characterization of Long-Circulating Cationic Nanoparticle Formulations Consisting of a Two-Stage PEGylation Step for the Delivery of SiRNA in a Breast Cancer Tumor Model. J. Pharm. Sci. 2013, 102, 227–236. [Google Scholar] [CrossRef]
- Dang, H.; Dong, C.; Zhang, L. Sustained Latanoprost Release from PEGylated Solid Lipid Nanoparticle-Laden Soft Contact Lens to Treat Glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, H.; Shu, L.; Zhang, Y.; Okeke, C.; Zhang, L.; Li, J.; Li, N. Preparation and Evaluation of Baicalin-Loaded Cationic Solid Lipid Nanoparticles Conjugated with OX26 for Improved Delivery across the BBB. Drug Dev. Ind. Pharm. 2015, 41, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wu, X.; Gao, X.; Yu, Y.; Lei, H.; Zhu, Z.; Shi, Y.; Chen, Y.; Qin, M.; Wang, W. Maleimide–Thiol Adducts Stabilized through Stretching. Nat. Chem. 2019, 11, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Scoble, J.A.; Li, N.; Lovrecz, G.; Waddington, L.J.; Tran, N.; Muir, B.W.; Coia, G.; Kirby, N.; Drummond, C.J. Epidermal Growth Factor Receptor-Targeted Lipid Nanoparticles Retain Self-Assembled Nanostructures and Provide High Specificity. Nanoscale 2015, 7, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ghatak, S.; El Masry, M.S.; Das, A.; Liu, Y.; Roy, S.; Lee, R.J.; Sen, C.K. Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function Following Burn Wound. Mol. Ther. 2018, 26, 2178–2188. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K. Poloxamer: A Versatile Tri-Block Copolymer for Biomedical Applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, A.; Mohamed, M.; Daftardar, S.B.; Patel, M.; Boddu, S.H.S.; Nesamony, J. Solid Lipid Nanoparticles in Drug Delivery: Opportunities and Challenges. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–330. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic Block Copolymers in Drug Delivery: Advances in Formulation Structure and Performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef]
- Göppert, T.M.; Müller, R.H. Protein Adsorption Patterns on Poloxamer-and Poloxamine-Stabilized Solid Lipid Nanoparticles (SLN). Eur. J. Pharm. Biopharm. 2005, 60, 361–372. [Google Scholar] [CrossRef]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Multifunctional Albumin–MnO2 Nanoparticles Modulate Solid Tumor Microenvironment by Attenuating Hypoxia, Acidosis, Vascular Endothelial Growth Factor and Enhance Radiation Response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef]
- Gordijo, C.R.; Abbasi, A.Z.; Amini, M.A.; Lip, H.Y.; Maeda, A.; Cai, P.; O’Brien, P.J.; DaCosta, R.S.; Rauth, A.M.; Wu, X.Y. Design of Hybrid MnO2-Polymer-Lipid Nanoparticles with Tunable Oxygen Generation Rates and Tumor Accumulation for Cancer Treatment. Adv. Funct. Mater. 2015, 25, 1858–1872. [Google Scholar] [CrossRef]
- Wang, T.; Ma, X.; Lei, Y.; Luo, Y. Solid Lipid Nanoparticles Coated with Cross-Linked Polymeric Double Layer for Oral Delivery of Curcumin. Colloids Surfaces B Biointerfaces 2016, 148, 1–11. [Google Scholar] [CrossRef]
- Sánchez, C.C.; Patino, J.M.R. Interfacial, Foaming and Emulsifying Characteristics of Sodium Caseinate as Influenced by Protein Concentration in Solution. Food Hydrocoll. 2005, 19, 407–416. [Google Scholar] [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Influence of PH and Pectin Type on Properties and Stability of Sodium-Caseinate Stabilized Oil-in-Water Emulsions. Food Hydrocoll. 2006, 20, 607–618. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Ladavière, C. A Close Collaboration of Chitosan with Lipid Colloidal Carriers for Drug Delivery Applications. J. Control. Release 2017, 256, 121–140. [Google Scholar] [CrossRef]
- Fonte, P.; Andrade, F.; Araújo, F.; Andrade, C.; das Neves, J.; Sarmento, B. Chitosan-Coated Solid Lipid Nanoparticles for Insulin Delivery. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 508, pp. 295–314. ISBN 0076-6879. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin Research Revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.; Kumar, A.C.K.; Priya, V.K.; Yadav, C.M.; Raju, P.Y. Formulation and Evaluation of Chitosan Solid Lipid Nanoparticles of Carbamazepine. Lipids Health Dis. 2012, 11, 72. [Google Scholar] [CrossRef] [Green Version]
- Dharmala, K.; Yoo, J.W.; Lee, C.H. Development of Chitosan–SLN Microparticles for Chemotherapy: In Vitro Approach through Efflux-Transporter Modulation. J. Control. Release 2008, 131, 190–197. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Oliveira, I.F.; da Silva, V.M.; Prata, A.S.; Hubinger, M.D. Chitosan Coated Nanostructured Lipid Carriers (NLCs) for Loading Vitamin D: A Physical Stability Study. Int. J. Biol. Macromol. 2018, 119, 902–912. [Google Scholar] [CrossRef]
- Rassu, G.; Soddu, E.; Cossu, M.; Gavini, E.; Giunchedi, P.; Dalpiaz, A. Particulate Formulations Based on Chitosan for Nose-to-Brain Delivery of Drugs. A Review. J. Drug Deliv. Sci. Technol. 2016, 32, 77–87. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan Microspheres as a Potential Carrier for Drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; ISBN 0123822408. [Google Scholar]
- Cheng, X.; Lee, R.J. The Role of Helper Lipids in Lipid Nanoparticles (LNPs) Designed for Oligonucleotide Delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. mRNA Vaccine for Cancer Immunotherapy. Mol. Cancer 2021, 20, 1–23. [Google Scholar] [CrossRef]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic Delivery of Nucleic Acids: The Case of Ionizable Lipid Nanoparticles. Bioeng. Transl. Med. 2021, 6, e10213. [Google Scholar] [CrossRef]
- Kuo, Y.-C. Loading Efficiency of Stavudine on Polybutylcyanoacrylate and Methylmethacrylate-Sulfopropylmethacrylate Copolymer Nanoparticles. Int. J. Pharm. 2005, 290, 161–172. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lin, T.-W. Electrophoretic Mobility, Zeta Potential, and Fixed Charge Density of Bovine Knee Chondrocytes, Methyl Methacrylate–Sulfopropyl Methacrylate, Polybutylcyanoacrylate, and Solid Lipid Nanoparticles. J. Phys. Chem. B 2006, 110, 2202–2208. [Google Scholar] [CrossRef]
- Seyfoddin, A.; Shaw, J.; Al-Kassas, R. Solid Lipid Nanoparticles for Ocular Drug Delivery. Drug Deliv. 2010, 17, 467–489. [Google Scholar] [CrossRef]
- Liu, D.; Liu, C.; Zou, W.; Zhang, N. Enhanced Gastrointestinal Absorption of N 3-O-Toluyl-Fluorouracil by Cationic Solid Lipid Nanoparticles. J. Nanoparticle Res. 2010, 12, 975–984. [Google Scholar] [CrossRef]
- Cui, S.; Wang, Y.; Gong, Y.; Lin, X.; Zhao, Y.; Zhi, D.; Zhou, Q.; Zhang, S. Correlation of the Cytotoxic Effects of Cationic Lipids with Their Headgroups. Toxicol. Res. 2018, 7, 473–479. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.N.; Omidfar, K. Drug Targeting Using Solid Lipid Nanoparticles. Chem. Phys. Lipids 2014, 181, 56–61. [Google Scholar] [CrossRef]
- Tabatt, K.; Sameti, M.; Olbrich, C.; Müller, R.H.; Lehr, C.-M. Effect of Cationic Lipid and Matrix Lipid Composition on Solid Lipid Nanoparticle-Mediated Gene Transfer. Eur. J. Pharm. Biopharm. 2004, 57, 155–162. [Google Scholar] [CrossRef]

| Type of LNPs | Target | Drug | Lipid and Surfactant | Therapeutic Effect | Reference |
|---|---|---|---|---|---|
| Nano emulsion (liquid-core LNPs) | Eye | Dexamethasone | Isopropyl myristate, Tween 80, propylene glycol | Treatment of acute and chronic eye disease such as uveitis | [63] |
| Eye | Everolimus | Triacetin, poloxamer 184, propylene glycol | Immunosuppressive drug to prevent corneal graft rejection | [64] | |
| Brain | Indinavir | Soybean oil, Tween 80, EPC-80 (egg yolk lecithin), oleic acid, α-tocopherol | Treatment of human immunodeficiency virus (HIV) infection | [65] | |
| Brain | Saquinavir mesylate | Capmul MCM, Tween 80, PEG 400, isopropyl myristate | Treatment of human immunodeficiency virus (HIV) infection | [66] | |
| Brain | Risperidone | Capmul MCM, Tween 80, transcutol, propylene glycol | Antipsychotic drug | [67] | |
| Skin | Curcumin | Tefose 1500 mixed PEG-6 stearate and PEG-32 stearate), Span 85, Span 20, Tween 80, Tween 20 | Targeted therapies for skin cancer | [68] | |
| Breast | Carmustine | Olive oil, 1,2-dioleoyl-sn-glycero-3-phosphocholine (phospholipid) | Targeted therapies for breast cancer | [50] | |
| SLN | Eye | Atorvastatin | Compritol® 888 ATO, Phospholipon 90 H, poloxamer 188, PEG 400 | Treatment of age-related macular degeneration | [69] |
| Eye | Melatonin | Stearic or palmitic acid, cationic lipid, Didecyldimethylammonium bromide, Softisan 100, Tween 80 | Increase the ocular hypotensive effect of drugs and treat anti-glaucoma | [70] | |
| Eye | Indomethacin | Compritol ATO 888, Tween 80, poloxamer 188, glycerin | Treatment of posterior segment of eye disease | [71] | |
| Eye | Diclofenac | Compritol 888 ATO, Precirol 5 ATO, hydrogenated soy PC, poloxamer 188 | Improve analgesic and anti-inflammatory drug toxicity | [72] | |
| Eye | Idebenone | Stearic acid or palmitic acid, Softisan 100, Tween 80, didecyldimethylammonium bromide | Leber’s hereditary optic neuropathy | [73] | |
| Brain | Docetaxel, ketoconazole | Glyceryl monostearate, soy lecithin, vitamin E, Tween 80 | Brain-targeted anticancer drug that penetrates the blood–brain barrier | [74] | |
| Brain | Apolipoprotein E-derived peptide | Dynasan 116, Epikuron 200 | Penetrate the blood–brain barrier (BBB) | [75] | |
| Brain | β-elemene | Glyceryl monostearate, glycerol tristearate, sodium cholate | Blood–brain barrier penetration and neurotherapy | [76] | |
| Brain | Saquinavir | Cacao butter, cholesterol, stearylamine, esterquat 1, Tween 80 | treatment of human immunodeficiency virus (HIV) infection | [77] | |
| Brain | Olanzapine | Stearic acid or glyceryl monostearate, soy lecithin, poloxamer 188, stearyl amine | A psychotropic agent that belongs to the thienobenzodiazepine class and is indicated for acute and maintenance treatment of schizophrenia | [78] | |
| Skin | Naproxen | Glyceryl monostearate, Span 80, Tween 80 | Reduce side effects of systemic absorption of drugs and increase drug concentration at the site of action/treatment of rheumatic diseases and related pain conditions | [79] | |
| Skin | Adapalene | Steric acid, trimyristin, glyceryl monostearate, glyceryl monooleate, Compritol 888 ATO, Precirol ATO 5, Brij 78, Pluronic F68, Tween 80, Span 20 | Treatment of acne | [80] | |
| Skin | Spironolactone | Stearic acid, Tween 80, Span 80, Span 60 | Treatment of skin disorders | [81] | |
| Skin | Resveratrol | Stearic acid, soy phosphatidylcholine, poloxamer 407 | Treatment of skin disorder | [82] | |
| Liver | Zedoary turmeric oil | Glycerin monostearate, glycerol, Tween 80 | Strong antitumor activity | [83] | |
| Lung | Artemether | Glyceryl monostearate, Compritol 888 ATO, stearyl amine, MPEG2000-DSPE, Cremophor EL, poloxamer 188, poloxamer 407, Solutol HS | Improve oral bioavailability and treat lung cancer | [84] | |
| NLC | Eye | Triamcinolone acetonide | Precirol® ATO 5, Squalene®, Lutrol® F68, Monoolein | Treatment of posterior segment diseases | [85] |
| Eye | Dexamethasone | Labrafac™ lipophile WL1349, Tween 80, Cholesterol | Treatment of dry eye disease (DED) or keratoconjunctivitis sicca | [86] | |
| Eye | Itraconazole | Tripalmitin, transcutol HP Chitosan, Tween 80 | Anti-neovascularization effect and treatment of diabetic retinopathy (DR) | [87] | |
| Eye | Propranolol hydrochloride | Compritol ATO 888, oleic acid, Transcutol P, Tween 80, Span 20 | Treatment of posterior segment of the eye disease | [88] | |
| Brain | Insulin | Precirol ATO5, Miglyol, Tween 80, poloxamer 188 | Penetrate the blood–brain barrier to treat the central nervous system | [89] | |
| Brain | Atazanavir | Precirol ATO5, Lauroglycol 90, Cremophor RH 40 | Treatment of neuro-AIDS | [90] | |
| Brain | Vinpocetine | Compritol 888 ATO, Monostearin, Miglyol 812N, Solutol HS-15 or poloxamer 188, lecithin | Treatment of chronic cerebral vascular ischemia, acute stroke, senile cerebral dysfunction, and Alzheimer’s disease | [91] | |
| Brain | Olanzapine | Glyceryl tripalmitate, castor oil, Pluronic F-68, soy lecithin | Treatment of schizophrenia | [92] | |
| Liver | Adefovir dipivoxil | Precirol ATO5, Capmul MCM, Cremophor RH 40, poloxamer 188, egg yolk lecithin | Treatment of hepatitis B virus infection | [93] | |
| Liver | Atorvastatin | Gelucire® 43/01, Compritol® 888 ATO, Capryol® PGMC, Pluronic® F68, Tween® 80 | Decrement of cholesterol and triglyceride (fats) levels in the blood | [94] | |
| Liver | Naringenin | Stearic acid, monostearin, oleic acid, poloxamer 188, soybean lecithin | Inhibition of nonalcoholic fatty liver disease | [95] | |
| Cubosome | Peritoneal macrophage | Antigen, Polysaccharide | Phytantriol, propylene glycol, Pluronic F127 | Increase the ability of immunostimulants | [96] |
| antidiabetic activity | Gliclazide | Glyceryl monooleate, poloxamer 407 | Improve antidiabetic activity | [97] | |
| Cell | Elesclomol copper complex | Monoolein, poloxamer 407 (PF127) | Anticancer drug for skin cancer, intractable solid cancer, and blood cancer | [98] | |
| Eye | Voriconazole | Monoolein, Pluronic F127 | Treatment of fungal keratitis | [99] | |
| Brain | Curcumin | Monoolein, fish oil, PEG1000 | Treatment of neurodegenerative disease | [100] | |
| Brain | Piperine | Glyceryl monooleate, Tween 80, poloxamer 407, Cremophor | Treatment of Alzheimer’s disease | [101] | |
| Skin | Paclitaxel | Monoolein, DSPE-PEG-ma, Pluronic F127 | Treatment of skin cancer | [102] |
| Surface Modifier | Function | Reference |
|---|---|---|
| PEGylation | - Increase the stability | [117] [118] [119] [120] [121] [122] |
| - Increase the residence time in the body | ||
| - Increase drug stability | ||
| - Increase the absorption rate for oral administration | ||
| - Increase drug penetration and accumulation rate in cells | ||
| - Increase resistance to digestive enzymes | ||
| - Increase drug-loading capacity | ||
| - Drug release control | ||
| - Decrease particles aggregation | ||
| - Decrease immunogenicity by stealthing LNPs from reticuloendothelial system (RES) | ||
| - Based on the EPR effect, it imparts (passive targeting ability) to LNPs for tumor cells | ||
| - Targeting ability can be imparted to LNPs through antibody conjugation (based on chemical treatment) | ||
| Block co-polymer | - Increase the stability | [123] [124] [125] |
| - Increase the residence time in the body | ||
| - Increase the cellular uptake and targeting ability | ||
| - Increases the adsorption rate for apoE, which increases the uptake rate of LNPs in the brain | ||
| Chitosan coating | - Increase the stability of LNPs (especially in acidic environment) | [83] [89] [126] [127] [128] [129] [130] |
| - Increase the residence time in the body | ||
| - Increase the absorption rate for oral administration | ||
| - Increase mucosal adhesion | ||
| - Increase delivery to the lungs via inhalation | ||
| - Increase drug delivery to brain | ||
| - Increase permeability to corneal cells | ||
| - Increase skin penetration | ||
| - Increase intracellular penetration | ||
| - Increase sustained release time | ||
| - Increase drug-loading capacity | ||
| - By positively charging the membranes of LNPs, allowing higher contact with cells that have negatively charged membranes | ||
| Functional lipid | - Increase the stability | [33] [77] [78] [131] [132] [133] [134] |
| - Increase the residence time in the body | ||
| - Increase drug delivery to brain (cholesterol) | ||
| - Increase uptake by hepatocytes (cholesterol) | ||
| - Increase drug-loading capacity (cationic lipid) | ||
| - Increase endosome escape ability (β-sitosterol) | ||
| - Decrease clearance interference by immune cells (through neutralizing the negative charge of the LNP membrane) (cationic lipid) | ||
| Surfactant protein | - Increase the structural stability of the membrane | [50] [135] |
| - Increase resistance to various environmental stress (i.e., ion, pH, and temperature) | ||
| - Antibody conjugation for increasing targeting ability of LNPs based on non-chemical treatment through genetic modification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. https://doi.org/10.3390/pharmaceutics15030772
Seo Y, Lim H, Park H, Yu J, An J, Yoo HY, Lee T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics. 2023; 15(3):772. https://doi.org/10.3390/pharmaceutics15030772
Chicago/Turabian StyleSeo, Yoseph, Hayeon Lim, Hyunjun Park, Jiyun Yu, Jeongyun An, Hah Young Yoo, and Taek Lee. 2023. "Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications" Pharmaceutics 15, no. 3: 772. https://doi.org/10.3390/pharmaceutics15030772
APA StyleSeo, Y., Lim, H., Park, H., Yu, J., An, J., Yoo, H. Y., & Lee, T. (2023). Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics, 15(3), 772. https://doi.org/10.3390/pharmaceutics15030772










