Micellar Form of a Ferrocene-Containing Camphor Sulfonamide with Improved Aqueous Solubility and Tumor Curing Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DK164 and Triblock Copolymer
2.3. Analysis of Copolymer and Nanoparticles
2.4. Preparation of Micellar Nanoparticles
2.5. Drug Loading and In Vitro Release
2.6. Cell Culture
2.7. In Vitro Cytotoxicity Assay
2.8. Cytometric Detection of Apoptosis
2.9. Immunofluorescence Microscopy
2.10. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Blank and Drug-Loaded NPs
3.2. Drug Release Study
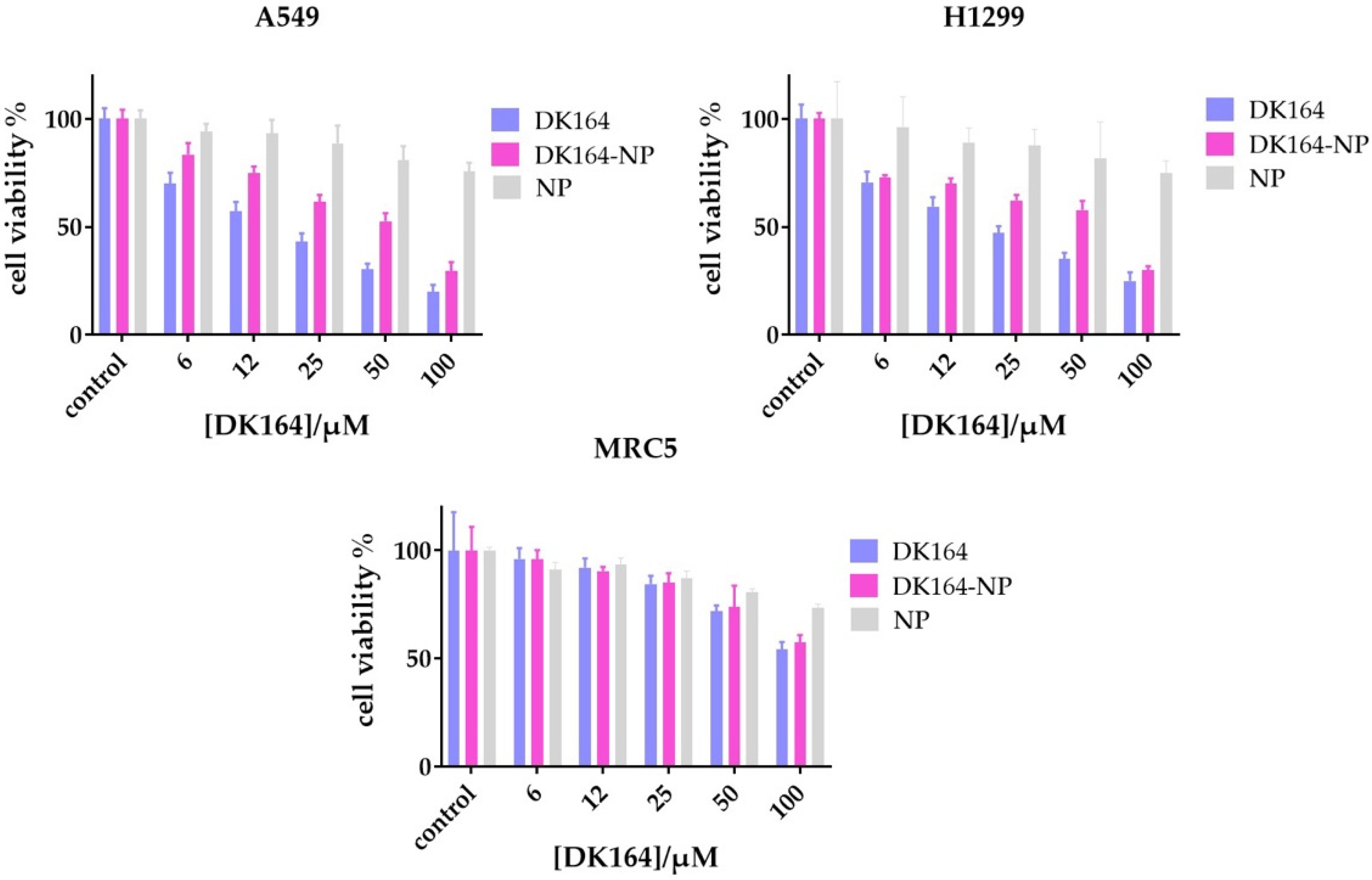
3.3. Evaluation of the In Vitro Cytotoxicity of DK164-NP
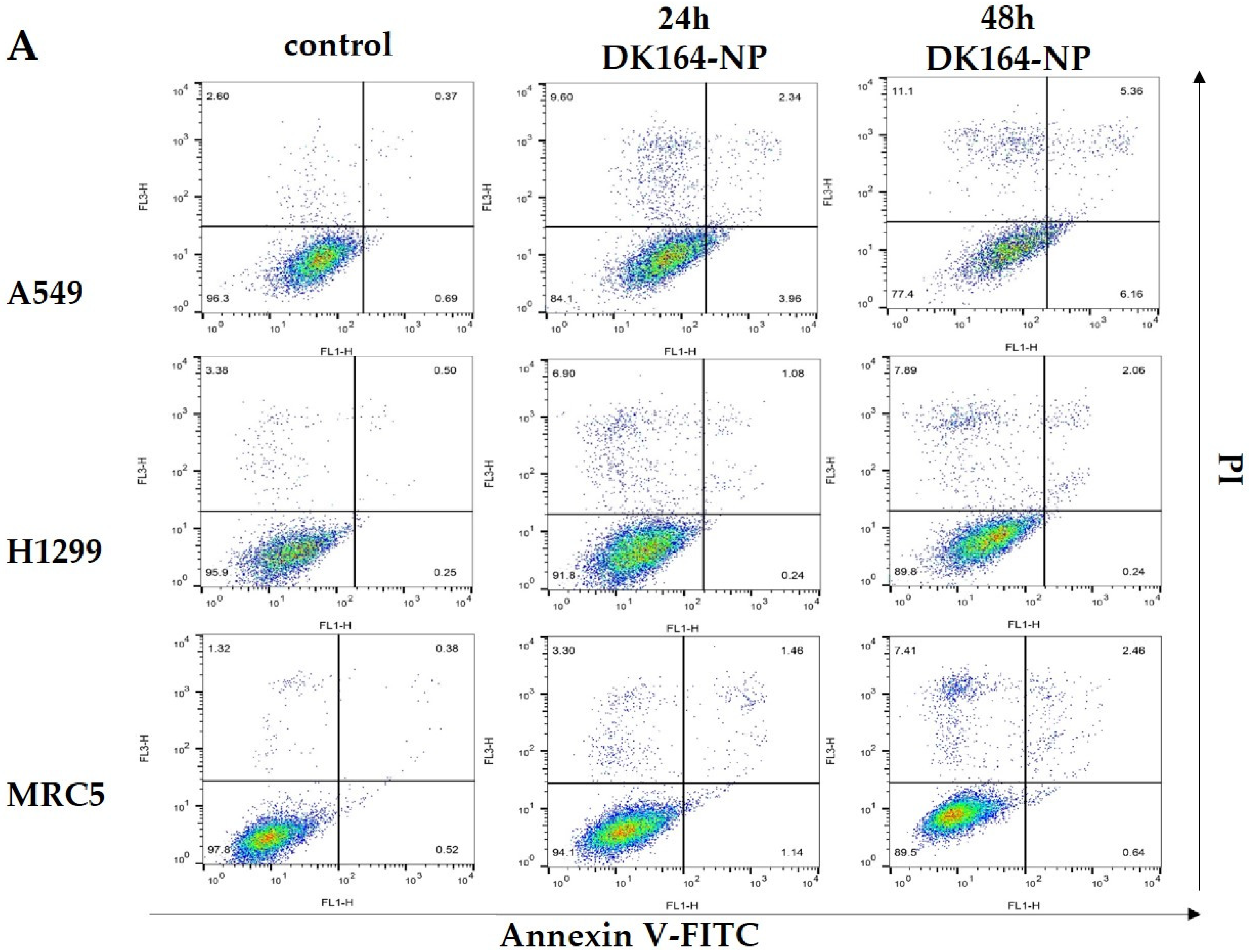
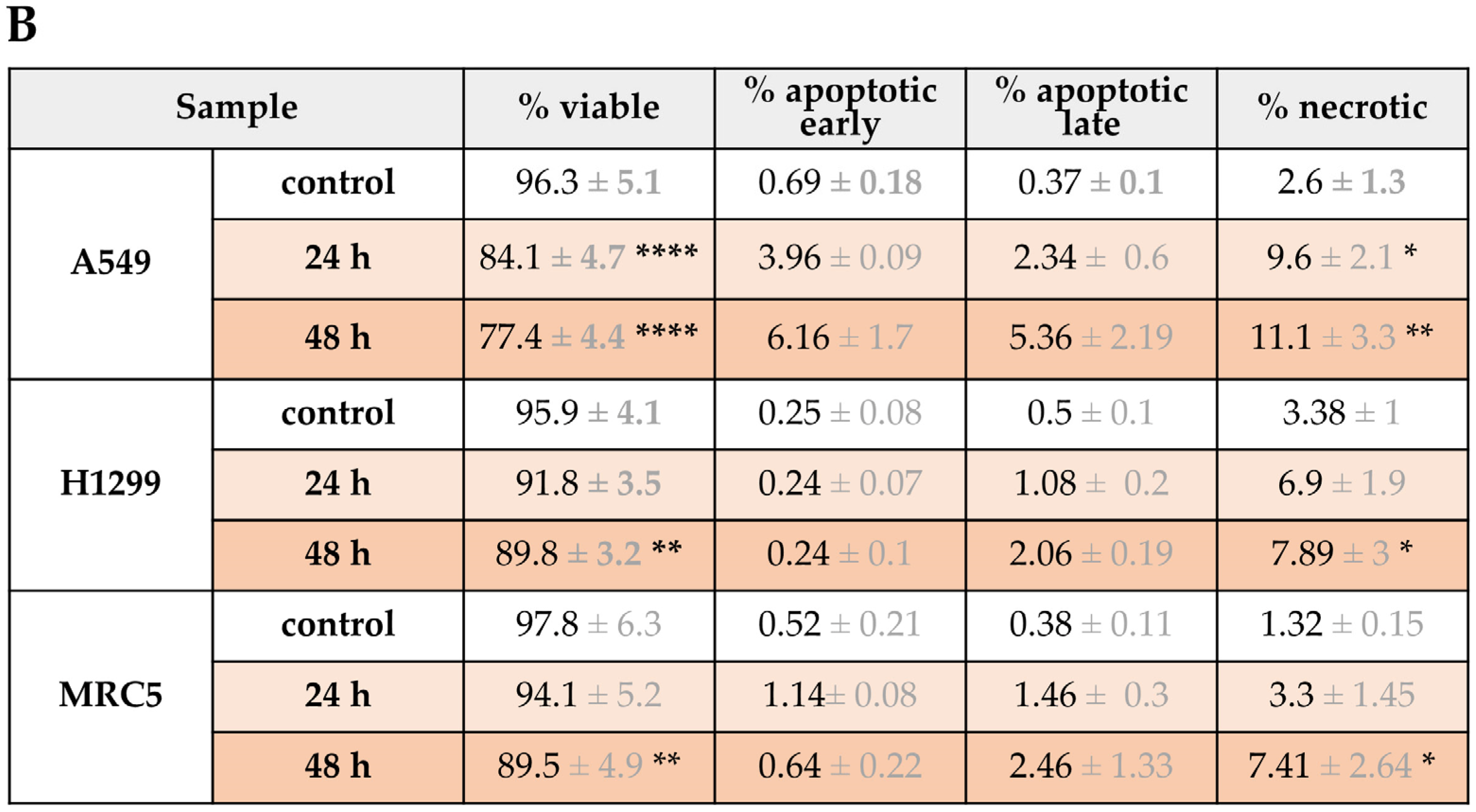
3.4. Flow Cytometry Analysis of Apoptosis
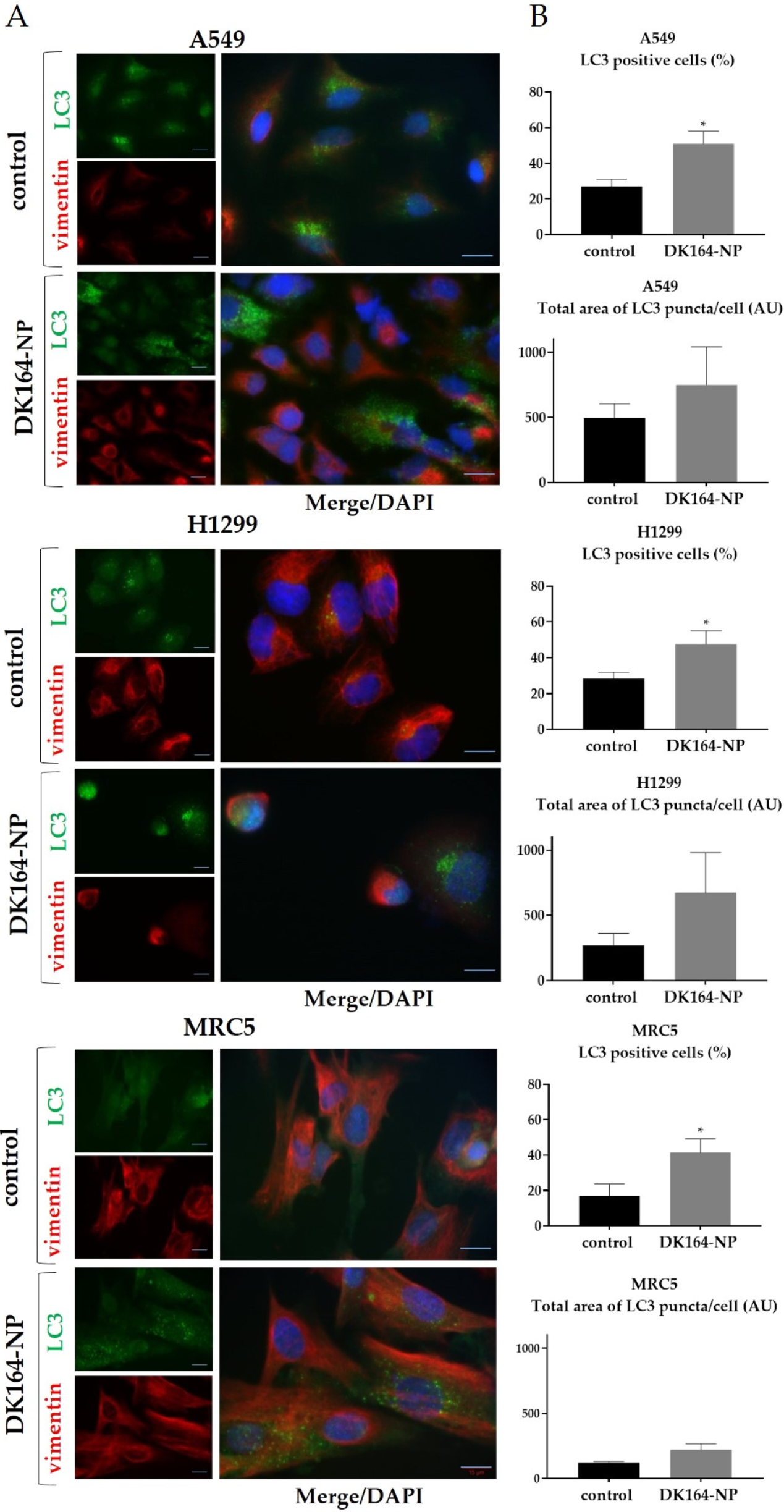
3.5. Influence of DK164-NP on Autophagy
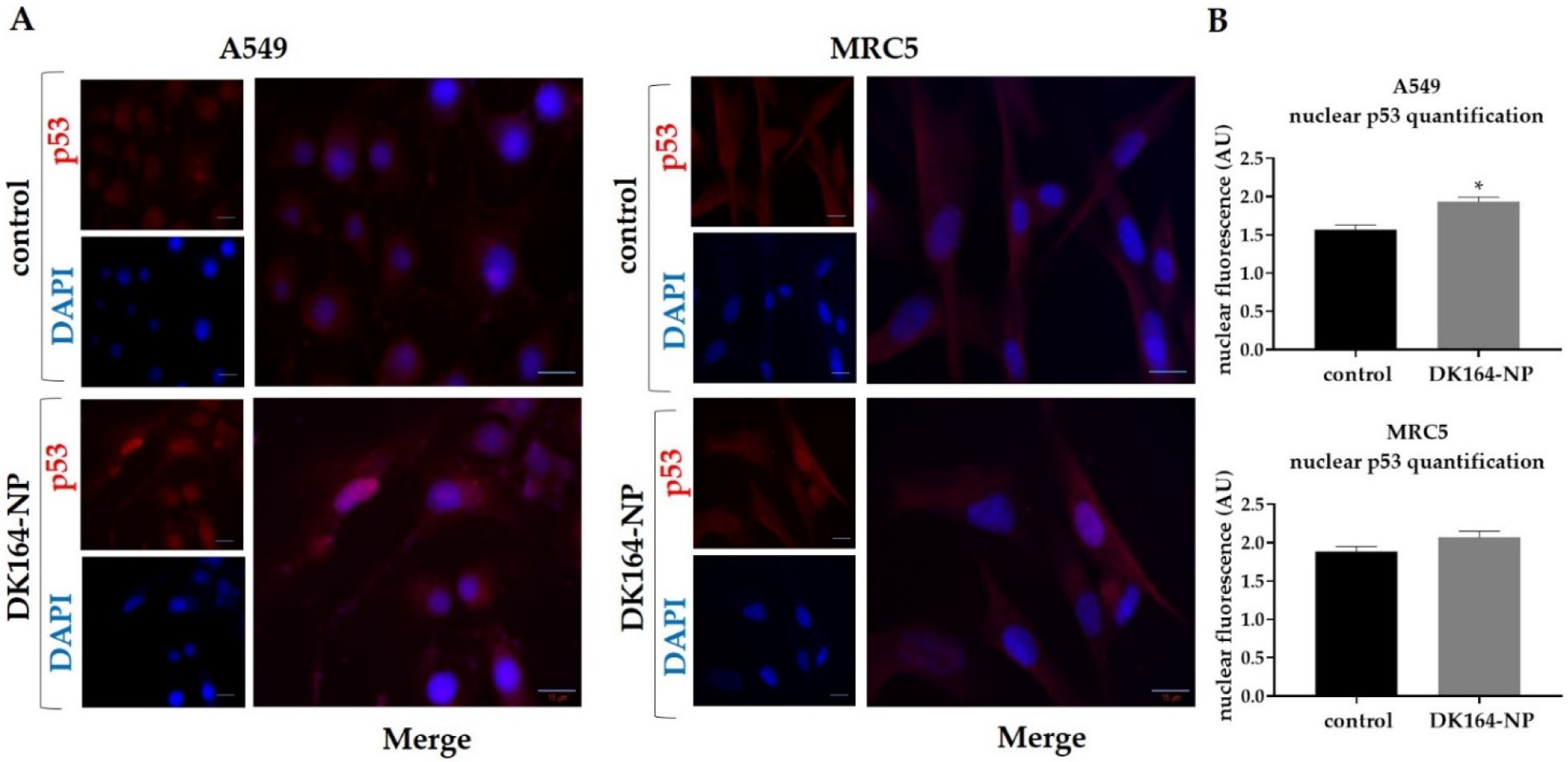
3.6. Effect of DK164-NP on the Dynamics of the Transcription Factors p53 and NFkB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Cancer is One of the Leading Causes of Death. Available online: https://ourworldindata.org/cancer (accessed on 1 February 2023).
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef]
- Chan, H.-K.; Ismail, S. Side effects of chemotherapy among cancer patients in a Malaysian General Hospital: Experiences, perceptions and informational needs from clinical pharmacists. Asian Pac. J. Cancer Prev. 2014, 15, 5305–5309. [Google Scholar] [CrossRef] [PubMed]
- Altun, İ.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar] [PubMed]
- Davies, H. Chemotherapy: An Overview. Care Head Neck Cancer Patients Dent. Hyg. Dent. Ther. 2023, 101–106. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Rosenberg, B.L.; Camp, V.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698. [Google Scholar] [CrossRef] [PubMed]
- Jakupec, M.; Galanski, M.; Keppler, B. Tumour-inhibiting platinum complexes—State of the art and future perspectives. Rev. Physiol. Biochem. Pharmacol. 2003, 146, 1–53. [Google Scholar] [CrossRef]
- Crabtree, R.H. Coordination & Organometallic Chemistry: Principles. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Xu, H. Aminoferrocene-or Ferrocenylaniline-Based ROS Amplifiers with Anticancer Activity. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2022. Available online: https://opus4.kobv.de/opus4-fau/frontdoor/index/index/docId/18972 (accessed on 1 February 2023).
- Ranjan, A.; Sharma, D.; Srivastava, A.K.; Varma, A.; Jayadev, M.S.; Joshi, R.K. Evaluation of anticancer activity of ferrocene based benzothiazole and β-ketooxothioacetal. J. Organomet. Chem. 2022, 979, 122500. [Google Scholar] [CrossRef]
- Jadhav, J.; Das, R.; Kamble, S.; Chowdhury, M.G.; Kapoor, S.; Gupta, A.; Vyas, H.; Shard, A. Ferrocene-based modulators of cancer-associated tumor pyruvate kinase M2. J. Organomet. Chem. 2022, 968, 122338. [Google Scholar] [CrossRef]
- Snegur, L.V.; Rodionov, A.N.; Ostrovskaya, L.A.; Ilyin, M.M.; Simenel, A.A. Ferrocene-modified imidazoles: One-pot oxalyl chloride-assisted synthesis, HPLC enantiomeric resolution, and in vivo antitumor effects. Appl. Organomet. Chem. 2022, 36, e6681. [Google Scholar] [CrossRef]
- Raičević, V.; Radulović, N.; Sakač, M. Toward Selective Anticancer Agents: Ferrocene-Steroid Conjugates. Eur. J. Inorg. Chem. 2022, 2022, e202100951. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.-H. Concept of hybrid drugs and recent advancements in anticancer hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef] [PubMed]
- Kamenova-Nacheva, M.; Schröder, M.; Pasheva, E.; Slavchev, I.; Dimitrov, V.; Momekov, G.; Nikolova, R.; Shivachev, B.; Ugrinova, I.; Dobrikov, G.M. Synthesis of ferrocenylmethylidene and arylidene substituted camphane based compounds as potential anticancer agents. New J. Chem. 2017, 41, 9103–9112. [Google Scholar] [CrossRef]
- Schröder, M.; Yusein-Myashkova, S.; Petrova, M.; Dobrikov, G.; Kamenova-Nacheva, M.; Todorova, J.; Pasheva, E.; Ugrinova, I. The Effect of a Ferrocene Containing Camphor Sulfonamide DK-164 on Breast Cancer Cell Lines. Anti-Cancer Agents Med. Chem. 2019, 19, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Petrova, M.; Vlahova, Z.; Dobrikov, G.M.; Slavchev, I.; Pasheva, E.; Ugrinova, I. In Vitro Anticancer Activity of Two Ferrocene-Containing Camphor Sulfonamides as Promising Agents against Lung Cancer Cells. Biomedicines 2022, 10, 1353. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Ki, M.-R.; Abd El-Hafeez, A.A.; Son, R.G.; Pack, S.P. Tailored Functionalized Protein Nanocarriers for Cancer Therapy: Recent Developments and Prospects. Pharmaceutics 2023, 15, 168. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Imbuluzqueta, E.; Sebastián, V.; Blanco-Prieto, M.J. Clinical advances of nanocarrier-based cancer therapy and diagnostics. Expert Opin. Drug Deliv. 2017, 14, 75–92. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef]
- Hoang, B.; Ernsting, M.J.; Tang, W.-H.S.; Bteich, J.; Undzys, E.; Kiyota, T.; Li, S.-D. Cabazitaxel-conjugated nanoparticles for docetaxel-resistant and bone metastatic prostate cancer. Cancer Lett. 2017, 410, 169–179. [Google Scholar] [CrossRef]
- Sumer Bolu, B.; Golba, B.; Sanyal, A.; Sanyal, R. Trastuzumab targeted micellar delivery of docetaxel using dendron-polymer conjugates. Biomater. Sci. 2020, 8, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002, 54, 759–779. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, Y.D.; Zarebkohan, A.; Salehi, R.; Rahimi, F.; Torchilin, V.P.; Hamblin, M.R.; Seifalian, A. An update on dual targeting strategy for cancer treatment. J. Control. Release 2022, 349, 67–96. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Huda, S.; Alam, M.A.; Sharma, P.K. Smart nanocarriers-based drug delivery for cancer therapy: An innovative and developing strategy. J. Drug Deliv. Sci. Technol. 2020, 60, 102018. [Google Scholar] [CrossRef]
- D’Angelo, N.A.; Noronha, M.A.; Câmara, M.C.; Kurnik, I.S.; Feng, C.; Araujo, V.H.; Santos, J.H.; Feitosa, V.; Molino, J.V.; Rangel-Yagui, C.O. Doxorubicin nanoformulations on therapy against cancer: An overview from the last 10 years. Biomater. Adv. 2022, 133, 112623. [Google Scholar] [CrossRef]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A. Smart nanocarriers as an emerging platform for cancer therapy: A review. Molecules 2021, 27, 146. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Wei, X.; Zhou, S. Polymer-based drug delivery systems for cancer treatment. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3525–3550. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kokhaei, P.; Ghorbani, M.; Eslami, M. Application of different nanoparticles in the diagnosis of colorectal cancer. Gene Rep. 2020, 21, 100896. [Google Scholar] [CrossRef]
- Grancharov, G.; Atanasova, M.-D.; Aluani, D.; Yoncheva, K.; Tzankova, V.; Trusheva, B.; Forys, A.; Trzebicka, B.; Petrov, P.D. Functional block copolymers bearing pendant cinnamyl groups for enhanced solubilization of caffeic acid phenethyl ester. Polym. J. 2020, 52, 435–447. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nikravan, G.; Haddadi-Asl, V.; Salami-Kalajahi, M. Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles. e-Polymers 2019, 19, 203–214. [Google Scholar] [CrossRef]
- Lorin, S.; Hamaï, A.; Mehrpour, M.; Codogno, P. Autophagy regulation and its role in cancer. Semin. Cancer Biol. 2013, 23, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy Levy, J.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.G.; Sargent, J.M.; Elgie, A.W.; Williamson, C.J.; Lewandowicz, G.M.; Chappatte, O.; Hill, J.G. Chemosensitivity testing predicts survival in ovarian cancer. Eur. J. Gynaecol. Oncol. 2001, 22, 278–282. [Google Scholar] [PubMed]
- Ugurel, S.; Tilgen, W.; Reinhold, U. Chemosensitivity testing in malignant melanoma. Recent Results Cancer Res.. Fortschr. Der Krebsforschung. Prog. Dans Les Rech. Sur Le Cancer 2003, 161, 81–92. [Google Scholar] [CrossRef]
- Xu, J.M.; Song, S.T.; Tang, Z.M.; Jiang, Z.F.; Liu, X.Q.; Zhou, L.; Zhang, J.; Liu, X.W. Predictive chemotherapy of advanced breast cancer directed by MTT assay in vitro. Breast Cancer Res. Treat. 1999, 53, 77–85. [Google Scholar] [CrossRef]
- Nakamura, R.; Saikawa, Y.; Kubota, T.; Kumagai, A.; Kiyota, T.; Ohashi, M.; Yoshida, M.; Otani, Y.; Kumai, K.; Kitajima, M. Role of the MTT chemosensitivity test in the prognosis of gastric cancer patients after postoperative adjuvant chemotherapy. Anticancer Res. 2006, 26, 1433–1437. [Google Scholar]
- Noguchi, K.; Iwahashi, M.; Tani, M.; Nakamura, M.; Nakamori, M.; Nakatani, Y.; Ueda, K.; Ishida, K.; Naka, T.; Ojima, T. Evaluation of chemosensitivity testing with highly purified tumor cells in 435 patients with gastric carcinoma using an MTT assay. Anticancer Res. 2005, 25, 931–937. [Google Scholar]
- Makin, G.; Hickman, J.A. Apoptosis and cancer chemotherapy. Cell Tissue Res. 2000, 301, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.S.; Zong, W.X. Chemotherapeutic approaches for targeting cell death pathways. Oncol. 2006, 11, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Lichan, C.; Yanyun, Z.; Shu-Feng, Z. Role of Apoptosis in Cancer Resistance to Chemotherapy. In Current Understanding of Apoptosis; Yusuf, T., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Jahangiri, A.; DeLay, M.; Aghi, M.K. Tumor cell autophagy as an adaptive response mediating resistance to treatments such as antiangiogenic therapy. Cancer Res. 2012, 72, 4294–4299. [Google Scholar] [CrossRef] [PubMed]
- Carew, J.S.; Nawrocki, S.T.; Kahue, C.N.; Zhang, H.; Yang, C.; Chung, L.; Houghton, J.A.; Huang, P.; Giles, F.J.; Cleveland, J.L. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl–mediated drug resistance. Blood J. Am. Soc. Hematol. 2007, 110, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Firat, E.; Weyerbrock, A.; Gaedicke, S.; Grosu, A.-L.; Niedermann, G. Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote γ-irradiation-induced cell death in primary stem-like glioma cells. PLoS ONE 2012, 7, e47357. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Yuan, Z.; Zhang, Q.; Long, Z.; Chen, J.; Tang, Z.; Zhu, Y.; Chen, S.; Xu, J.; Yan, M. Aurora kinase A inhibition-induced autophagy triggers drug resistance in breast cancer cells. Autophagy 2012, 8, 1798–1810. [Google Scholar] [CrossRef]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of p53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Hu, J.; Cao, J.; Topatana, W.; Juengpanich, S.; Li, S.; Zhang, B.; Shen, J.; Cai, L.; Cai, X.; Chen, M. Targeting mutant p53 for cancer therapy: Direct and indirect strategies. J. Hematol. Oncol. 2021, 14, 157. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Li, F.; Sethi, G. Targeting transcription factor NF-κB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2010, 1805, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Wang, M.; Wang, L.; Cheng, B.-F.; Lin, X.-Y.; Feng, Z.-W. NF-κB regulates caspase-4 expression and sensitizes neuroblastoma cells to Fas-induced apoptosis. PLoS ONE 2015, 10, e0117953. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Marsaud, V.; Bouclier, C.; Top, S.; Vessieres, A.; Pigeon, P.; Gref, R.; Legrand, P.; Jaouen, G.; Renoir, J.M. Nanoparticles loaded with ferrocenyl tamoxifen derivatives for breast cancer treatment. Int. J. Pharm. 2008, 347, 128–135. [Google Scholar] [CrossRef]

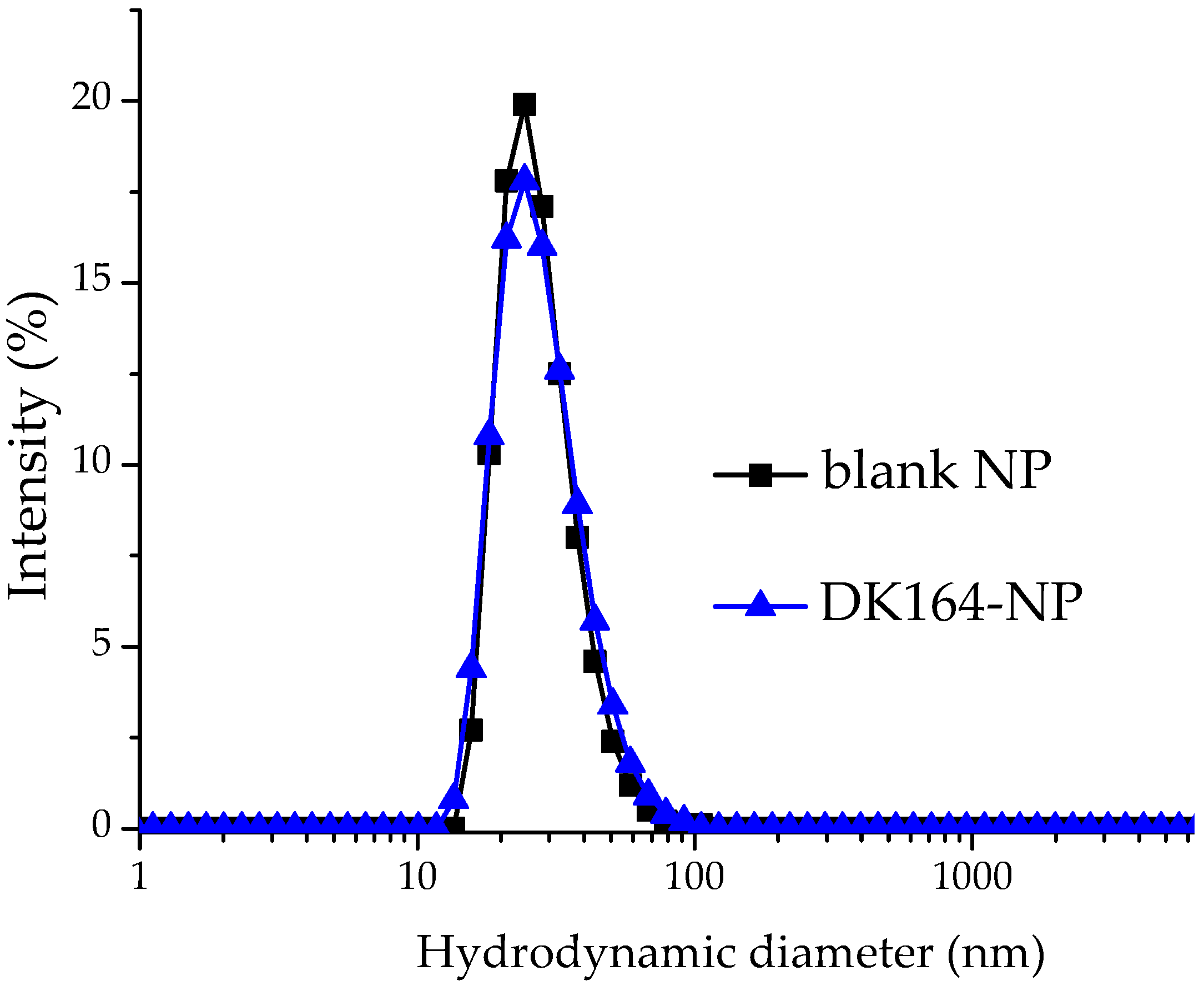


| Sample | Dh (nm) | DI | ξ-Potential (mV) | EE (%) |
|---|---|---|---|---|
| Blank micelles | 42.3 ± 4.2 | 0.21 ± 0.05 | −0.82 ± 0.04 | - |
| DK164-NP | 44.7 ± 2.4 | 0.24 ± 0.06 | −1.83 ± 0.40 | 98 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, M.; Petrova, M.; Dobrikov, G.M.; Grancharov, G.; Momekova, D.; Petrov, P.D.; Ugrinova, I. Micellar Form of a Ferrocene-Containing Camphor Sulfonamide with Improved Aqueous Solubility and Tumor Curing Potential. Pharmaceutics 2023, 15, 791. https://doi.org/10.3390/pharmaceutics15030791
Schröder M, Petrova M, Dobrikov GM, Grancharov G, Momekova D, Petrov PD, Ugrinova I. Micellar Form of a Ferrocene-Containing Camphor Sulfonamide with Improved Aqueous Solubility and Tumor Curing Potential. Pharmaceutics. 2023; 15(3):791. https://doi.org/10.3390/pharmaceutics15030791
Chicago/Turabian StyleSchröder, Maria, Maria Petrova, Georgi M. Dobrikov, Georgy Grancharov, Denitsa Momekova, Petar D. Petrov, and Iva Ugrinova. 2023. "Micellar Form of a Ferrocene-Containing Camphor Sulfonamide with Improved Aqueous Solubility and Tumor Curing Potential" Pharmaceutics 15, no. 3: 791. https://doi.org/10.3390/pharmaceutics15030791
APA StyleSchröder, M., Petrova, M., Dobrikov, G. M., Grancharov, G., Momekova, D., Petrov, P. D., & Ugrinova, I. (2023). Micellar Form of a Ferrocene-Containing Camphor Sulfonamide with Improved Aqueous Solubility and Tumor Curing Potential. Pharmaceutics, 15(3), 791. https://doi.org/10.3390/pharmaceutics15030791







