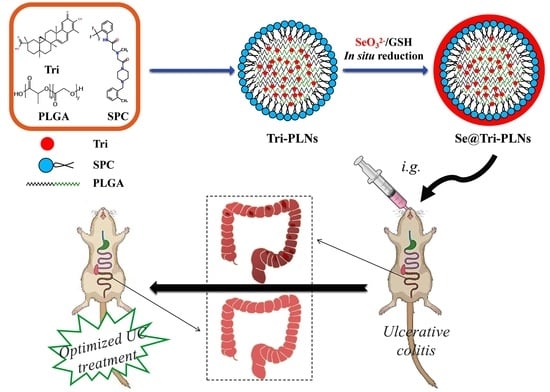
Selenized Polymer-Lipid Hybrid Nanoparticles for Oral Delivery of Tripterine with Ameliorative Oral Anti-Enteritis Activity and Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Line and Animals
2.3. Preparation of Se@Tri-PLNs
2.4. Characterization of Nanocarriers
2.5. Biorelevant Stability Study
2.6. In Vitro Release Study
2.7. Cytotoxicity Assay
2.8. Cellular Uptake and Internalization
2.9. Cellular Trafficking Pathway
2.10. Oral Pharmacokinetics
2.11. In Vivo Anti-Enteritis Activity Evaluation
3. Results and Discussion
3.1. Preparation and Characterization of Se@Tri-PLNs
3.2. Gastrointestinal Stability
3.3. In Vitro Drug Release
3.4. Cytotoxicity
3.5. Cellular Uptake, Internalization, and Transport Mechanisms
3.6. Enhanced Bioavailability
3.7. Ameliorative In Vivo Anti-Enteritis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Mattos, B.R.; Garcia, M.P.; Nogueira, J.B.; Paiatto, L.N.; Albuquerque, C.G.; Souza, C.L.; Fernandes, L.G.; Tamashiro, W.M.; Simioni, P.U. Inflammatory bowel disease: An overview of immune mechanisms and biological treatments. Mediators Inflamm. 2015, 2015, 493012. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.; Carvello, M.; Kotze, P.G.; Spinelli, A. Robotic surgery in inflammatory bowel disease. Curr. Drug Targets 2021, 22, 112–116. [Google Scholar] [CrossRef]
- Santos, J.D.M.; Peña-Sánchez, J.N.; Fowler, S.A. Patients’ perspectives on medication for inflammatory bowel disease: A mixed-method systematic review. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.C.; Sauk, J.S.; Limketkai, B.N.; Kwaan, M.R. Declining rates of surgery for inflammatory bowel disease in the era of biologic therapy. J. Gastrointest. Surg. 2021, 25, 211–219. [Google Scholar] [CrossRef]
- Cascão, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A spectrum of treatment opportunities in chronic diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Sun, Y.; Shi, P.; Dong, J.N.; Zuo, L.G.; Wang, H.G.; Gong, J.F.; Li, Y.; Gu, L.L.; Li, N.; et al. Celastrol ameliorates experimental colitis in IL-10 deficient mice via the up-regulation of autophagy. Int. Immunopharmacol. 2015, 26, 221–228. [Google Scholar] [CrossRef]
- Li, M.; Guo, W.; Dong, Y.; Wang, W.; Tian, C.; Zhang, Z.; Yu, T.; Zhou, H.; Gui, Y.; Xue, K.; et al. Beneficial effects of celastrol on immune balance by modulating gut microbiota in experimental ulcerative colitis mice. Genom. Proteom. Bioinform. 2022, 20, 288–303. [Google Scholar] [CrossRef]
- Qi, X.; Qin, J.; Ma, N.; Chou, X.; Wu, Z. Solid self-microemulsifying dispersible tablets of celastrol: Formulation development, charaterization and bioavailability evaluation. Int. J. Pharm. 2014, 472, 40–47. [Google Scholar] [CrossRef]
- Song, J.; Shi, F.; Zhang, Z.; Zhu, F.; Xue, J.; Tan, X.; Zhang, L.; Jia, X. Formulation and evaluation of celastrol-loaded liposomes. Molecules 2011, 16, 7880–7892. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Jia, Y.; Zhang, P.; Yang, H.; Cong, X.; An, L.; Xiao, C. Celastrol self-stabilized nanoparticles for effective treatment of melanoma. Int. J. Nanomed. 2020, 15, 1205–1214. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Zhang, Q.; He, P.; Zhou, B.; He, K.; Sun, X.; Lei, G.; Gong, T.; Zhang, Z. Targeted apoptosis of macrophages and osteoclasts in arthritic joints is effective against advanced inflammatory arthritis. Nat. Commun. 2021, 12, 2174. [Google Scholar] [CrossRef]
- Cao, X.; Hu, Y.; Luo, S.; Wang, Y.; Gong, T.; Sun, X.; Fu, Y.; Zhang, Z. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm. Sin. B 2019, 9, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Prestidge, C.A. Polymer-lipid hybrid systems: Merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opin. Drug Deliv. 2016, 13, 691–707. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, W.; Xie, Q.; Wang, H.; Ma, Z.; Wu, B.; Zhang, X. Selenium nanoparticles as versatile carriers for oral delivery of insulin: Insight into the synergic antidiabetic effect and mechanism. Nanomedicine 2017, 13, 1965–1974. [Google Scholar] [CrossRef]
- Deng, W.; Wang, H.; Wu, B.; Zhang, X. Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycemic activity to synergistically potentiate the antidiabetic effect. Acta Pharm. Sin. B 2019, 9, 74–86. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, C.; Feng, W.; Li, Y.; Zhu, M.; Sun, S.; Zhang, X. Selenium-deposited tripterine phytosomes ameliorate the antiarthritic efficacy of the phytomedicine via a synergistic sensitization. Int. J. Pharm. 2020, 578, 119104. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Q.; Yan, L.; Ren, Y.; Fan, J.; Zhang, X.; Zhu, S. Phytosomal tripterine with selenium modification attenuates the cytotoxicity and restrains the inflammatory evolution via inhibiting nlrp3 inflammasome activation and pyroptosis. Int. Immunopharmacol. 2022, 108, 108871. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Yin, Y.; Song, X. Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycemic effect. Int. J. Nanomed. 2017, 12, 8671–8680. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xing, C.; Huang, D.; Zhou, C.; Ding, B.; Guo, Z.; Peng, Z.; Wang, D.; Zhu, X.; Liu, S.; et al. Eradication of tumor growth by delivering novel photothermal selenium-coated tellurium nanoheterojunctions. Sci. Adv. 2020, 6, eaay6825. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Qi, J.; Lu, Y.; He, W.; Li, X.; Wu, W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine 2014, 10, 167–176. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Ye, Y.; Zhang, T.; Wang, H.; Ma, Z.; Wu, B. Nanostructured lipid carriers used for oral delivery of oridonin: An effect of ligand modification on absorption. Int. J. Pharm. 2015, 479, 391–398. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Protective effects of berberine hydrochloride on dss-induced ulcerative colitis in rats. Int. Immunopharmacol. 2019, 68, 242–251. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Du, M.; Ouyang, Y.; Meng, F.; Zhang, X.; Ma, Q.; Zhuang, Y.; Liu, H.; Pang, M.; Cai, T.; Cai, Y. Polymer–lipid hybrid nanoparticles: A novel drug delivery system for enhancing the activity of psoralen against breast cancer. Int. J. Pharm. 2019, 561, 274–282. [Google Scholar] [CrossRef]
- Gan, Z.; Huang, C.; Shen, Y.; Zhou, Q.; Han, D.; Ma, J.; Liu, S.; Zhang, Y. Preparation of carbon nitride nanoparticles by nanoprecipitation method with high yield and enhanced photocatalytic activity. Chin. Chem. Lett. 2020, 31, 513–516. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Zhou, X.; Liu, H.; Sun, H.; Ma, Z.; Wu, B. Enhancement of oral bioavailability of tripterine through lipid nanospheres: Preparation, characterization, and absorption evaluation. J. Pharm. Sci. 2014, 103, 1711–1719. [Google Scholar] [CrossRef]
- Wu, S.; Sun, K.; Wang, X.; Wang, D.; Wan, X.; Zhang, J. Protonation of epigallocatechin-3-gallate (EGCG) results in massive aggregation and reduced oral bioavailability of EGCG-dispersed selenium nanoparticles. J. Agric. Food Chem. 2013, 61, 7268–7275. [Google Scholar] [CrossRef]
- Shan, W.G.; Wang, H.G.; Wu, R.; Zhan, Z.J.; Ma, L.F. Synthesis and anti-tumor activity study of water-soluble PEG-celastrol coupling derivatives as self-assembled nanoparticles. Bioorg. Med. Chem. Lett. 2019, 29, 685–687. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Liu, L.; Zhang, Y.; Luo, Y.; Zhang, X.; Yang, P.; Zhang, M.; Yu, W.; Qu, S. Elucidation of the intestinal absorption mechanism of celastrol using the Caco-2 cell transwell model. Planta Med. 2016, 82, 1202–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, B.; Yan, R.; Li, R.; Zhang, X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int. J. Nanomed. 2018, 13, 7473–7490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, J.; Zhong, X.; Gu, H.; Wang, X.; Li, J.; Li, J.; Wu, Y.; Zhang, C.; Zhang, J. Colonic delivery of celastrol-loaded layer-by-layer liposomes with pectin/trimethylated chitosan coating to enhance its anti-ulcerative colitis effects. Pharmaceutics 2021, 13, 2005. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liang, X.; Ma, P.; Tao, Y.; Wu, X.; Wu, X.; Chu, X.; Gui, S. Phytantriol-based in situ liquid crystals with long-term release for intra-articular administration. AAPS PharmSciTech 2015, 16, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Freag, M.S.; Saleh, W.M.; Abdallah, O.Y. Self-assembled phospholipid-based phytosomal nanocarriers as promising platforms for improving oral bioavailability of the anticancer celastrol. Int. J. Pharm. 2018, 535, 18–26. [Google Scholar] [CrossRef]
- Zhan, S.; Paik, A.; Onyeabor, F.; Ding, B.; Prabhu, S.; Wang, J. Oral bioavailability evaluation of celastrol-encapsulated silk fibroin nanoparticles using an optimized LC-MS/MS method. Molecules 2020, 25, 3422. [Google Scholar] [CrossRef]
- Fan, N.; Zhao, J.; Zhao, W.; Zhang, X.; Song, Q.; Shen, Y.; Shum, H.C.; Wang, Y.; Rong, J. Celastrol-loaded lactosylated albumin nanoparticles attenuate hepatic steatosis in non-alcoholic fatty liver disease. J. Control. Release 2022, 347, 44–54. [Google Scholar] [CrossRef]
- Krug, P.; Mielczarek, L.; Wiktorska, K.; Kaczyńska, K.; Wojciechowski, P.; Andrzejewski, K.; Ofiara, K.; Szterk, A.; Mazur, M. Sulforaphane-conjugated selenium nanoparticles: Towards a synergistic anticancer effect. Nanotechnology 2019, 30, 065101. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Balbaa, M. Synergistic effect of nano-selenium and metformin on type 2 diabetic rat model: Diabetic complications alleviation through insulin sensitivity, oxidative mediators and inflammatory markers. PLoS ONE 2019, 14, e0220779. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Zhang, W.; Sun, J.; Gao, R. Therapeutic and immune function improvement of vitamin D combined with IFN-α on mouse with hepatitis B infection. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418775250. [Google Scholar] [CrossRef] [Green Version]
- Shaker, M.E.; Ashamallah, S.A.; Houssen, M.E. Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokines and intestinal homeostasis. Chem. Biol. Interact. 2014, 210, 26–33. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, C.; Shen, J.; Xia, T.; Yang, J.; He, Y. The natural compound celastrol inhibits necroptosis and alleviates ulcerative colitis in mice. Int. Immunopharmacol. 2015, 29, 552–559. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and anti-inflammatory properties of selenium-containing agents: Their role in the regulation of defense mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef]











| Inhibitor | Concentration | Function |
|---|---|---|
| Hypertonic sucrose | 0.5 M | Nonspecific inhibition to clathrin-mediated endocytosis |
| Chlorpromazine | 25 μM | Specific inhibition to clathrin-mediated endocytosis |
| Simvastatin | 25 μM | Nonspecific inhibition to caveolin-mediated endocytosis |
| Genistein | 25 μM | Specific inhibition to caveolin-mediated endocytosis |
| PK Parameters | Tri Suspensions | Tri-PLNs | Se@Tri-PLNs |
|---|---|---|---|
| Cmax (ng/mL) | 293.12 ± 37.42 | 298.81 ± 22.50 | 237.50 ± 26.82 ** |
| Tmax (h) | 4.00 ± 0.00 | 6.00 ± 2.00 | 7.33 ± 1.15 * |
| AUC0–∞ (ng/mL·h) | 2186.46 ± 202.22 | 6130.28 ± 483.05 ** | 8675.95 ± 322.14 ** |
| t1/2 (h) | 5.05 ± 1.46 | 13.76 ± 1.58 ** | 13.77 ± 2.35 ** |
| Group | Thymus Index | Spleen Index |
|---|---|---|
| Control | 0.1769 ± 0.0155 | 0.4731 ± 0.0618 |
| DSS | 0.1187 ± 0.0126 | 0.2508 ± 0.0350 |
| DSS + Tri | 0.1177 ± 0.0087 | 0.2822 ± 0.0260 |
| DSS + Tri-PLNs | 0.1497 ± 0.0384 | 0.3681 ± 0.0350 |
| DSS + Se@Tri-PLNs | 0.1631 ± 0.0060 | 0.4131 ± 0.0536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Qi, C.; Ruan, S.; Cao, G.; Ma, Z.; Zhang, X. Selenized Polymer-Lipid Hybrid Nanoparticles for Oral Delivery of Tripterine with Ameliorative Oral Anti-Enteritis Activity and Bioavailability. Pharmaceutics 2023, 15, 821. https://doi.org/10.3390/pharmaceutics15030821
Ren Y, Qi C, Ruan S, Cao G, Ma Z, Zhang X. Selenized Polymer-Lipid Hybrid Nanoparticles for Oral Delivery of Tripterine with Ameliorative Oral Anti-Enteritis Activity and Bioavailability. Pharmaceutics. 2023; 15(3):821. https://doi.org/10.3390/pharmaceutics15030821
Chicago/Turabian StyleRen, Yuehong, Chunli Qi, Shuxian Ruan, Guangshang Cao, Zhiguo Ma, and Xingwang Zhang. 2023. "Selenized Polymer-Lipid Hybrid Nanoparticles for Oral Delivery of Tripterine with Ameliorative Oral Anti-Enteritis Activity and Bioavailability" Pharmaceutics 15, no. 3: 821. https://doi.org/10.3390/pharmaceutics15030821