Chitosan-Based Nano-Smart Drug Delivery System in Breast Cancer Therapy
Abstract
:1. Introduction
2. General Overview of Breast Cancer
2.1. Features of Breast Cancer
2.2. Breast Cancer Classification
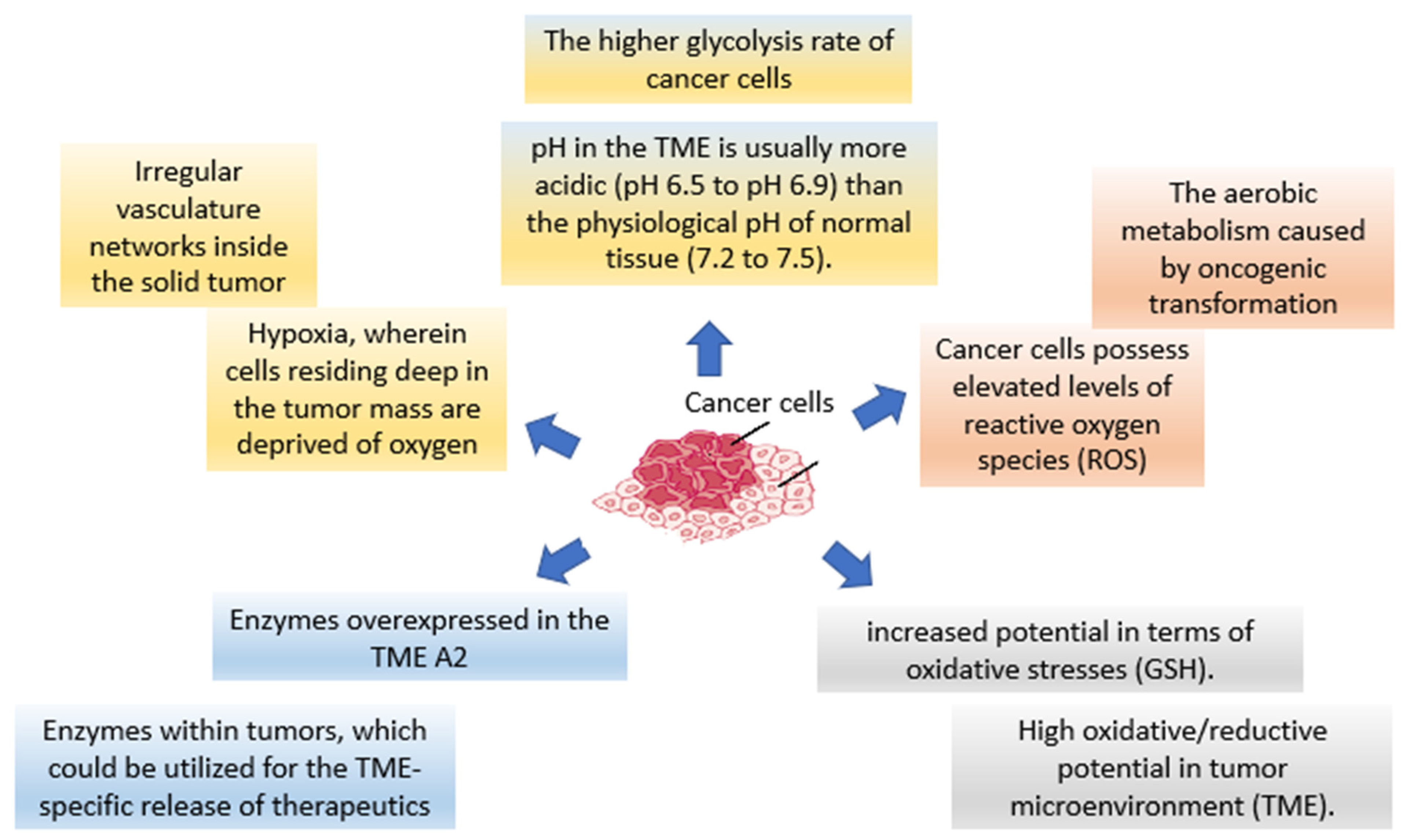
2.3. Tumor Microenvironment
2.4. Folate Receptors
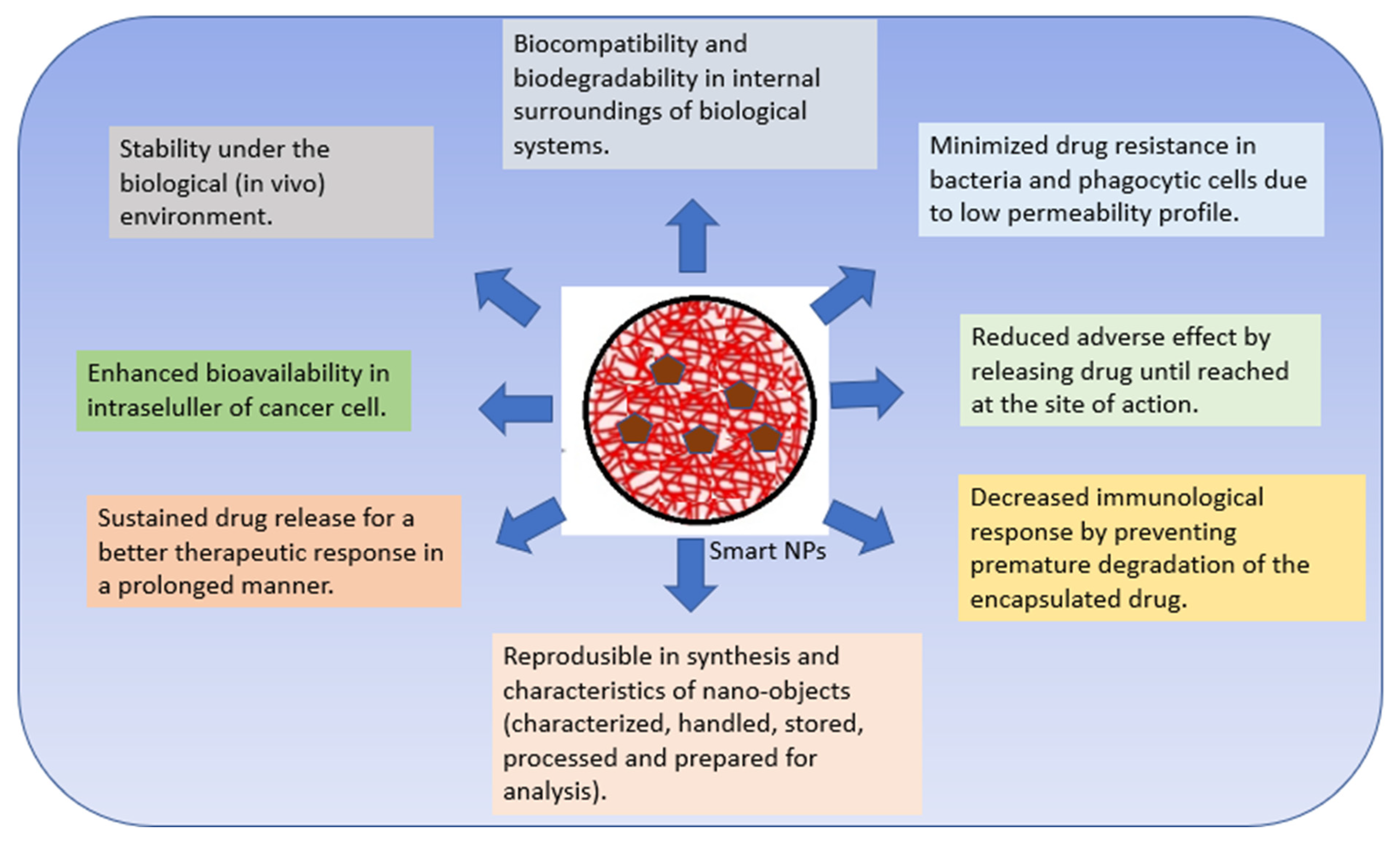
3. Chitosan-Based Nanosystem of Smart Drug Delivery System
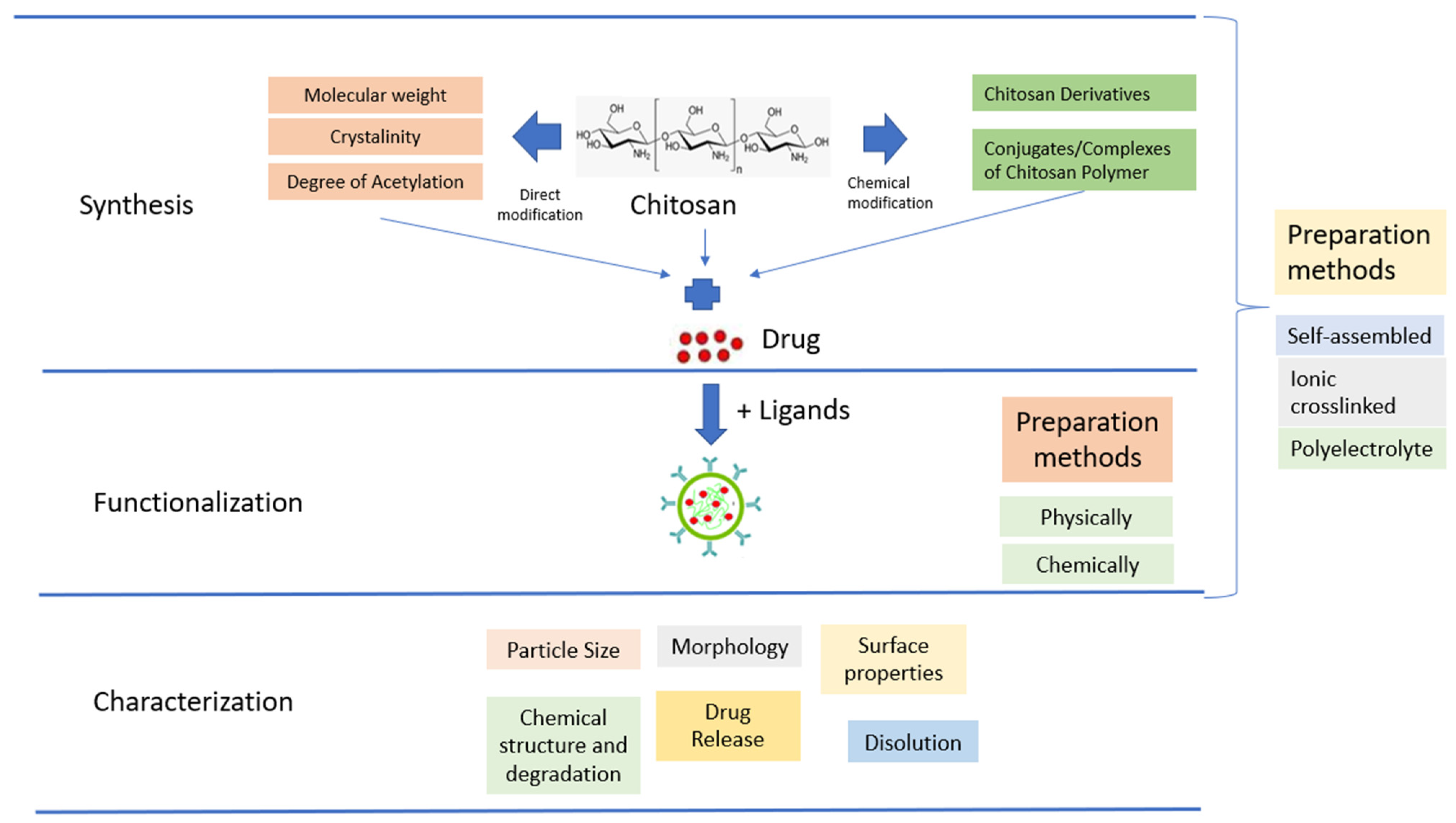
3.1. Synthesis, Functionalization and Characterization of Chitosan Nanoparticles
- Cost-effectiveness [66].
- Drug protection through encapsulation in the core of NPs [69].
- Enhancement of the therapeutic efficacy of therapy, especially in tumor therapy, through passive targeting or enhanced permeation and retention (EPR) effects [70].
- Mechanical properties, targeting ability, and mode of drug release that can also be controlled by modifying the structure of natural materials with polyamines, small molecules, and targeting ligands [56].
- The polymer may be made water-soluble depending on its usage [73].
- A high degradation rate which ensures the material’s safety and its protection of the environment (eco-friendly properties) [76].
3.2. Mechanism of CSNPs for Drug Delivering
3.3. Modification Strategies of CSNPs for BC Therapy
- They have a lower half-life and subtherapeutic tumor concentrations. SiRNA, microRNA, and oligonucleotides for cancer treatment degrade systemically, lowering t ½ [96].
- Oral administration is preferable, easy, and cost-effective. This route must pass across multiple biological barriers, such as the blood-brain barrier and tight junction barrier, and be quickly destroyed by digestive fluids and the liver [97].
- CSNPs may encapsulate or conjugate chemotherapeutic medicines, therapeutic gene nucleic acids, photosensitizers, and cytokines for more reliable cancer target treatment [1].
- Designing CSNPs according to delivery system factors, such as size and size distribution, drug loading capacity, and stability, is possible [102].
- Chitosan has three groups: amino, acetamido, and hydroxy groups; they can provide derivatives of increased solubility and outstanding anticancer activity, offering bioavailability in cancer cells by utilizing sustained release. They also offer increased permeation, transfection, and gelation in situ [103], and easy in vivo biodegradation [104].
- Multi-functional CSNPs can continue to offer many new opportunities for biomedical applications because they have the ability to interact with complex cellular functions in new ways [50]. There exists a multifunctional DD that combines high specificity against cancer cells with endosomal escape ability.
- Surface modification of CSNPs can be carried out on polymers through physical or chemical methods. Surface modification of CSNPs enhances their tumor-targeting ability through different mechanisms such as a receptor or carrier-mediated transcytosis [105]. CSNPs were more effective than PLGA NPs because they targeted MCF-7 cells [102].
- Modification of chitosan can use variations in molecular weight and the level of acetylation, which will provide different properties according to the needs of the drug delivery system [104].
- Chitosan can be used as a solubility-enhancing polymer backbone. Advances in polymer chemistry have led to the creation of smart polymer systems [105].
- Polymers used for drug administration can respond to stimuli such as temperature, light, or pH. Stimulus-responsive polymers may modify cell adhesion to boost gene expression or enzyme activity [96].
3.4. Stimuli-Responsive NPs
3.5. Multifunctional Delivery Systems
4. CSNPs and Mechanism Anticancer Action
4.1. Targeting
4.2. Cellular Uptake
4.3. Drug Release
4.4. MDR
- NPs can release cargo before reaching target cells. Therefore, they need targeted ligands that can recognize and bind tumor cells without harming healthy ones. Active targeting facilitates drug endocytosis, circumventing cytotoxic drug efflux ABC transporters [3].
- Nanomedicines can encapsulate many molecules, be targeted, and encourage controlled release, thereby boosting combination therapy. The extracellular matrix (ECM), cytokines, and stromal cells impact tumor cell invasion through the stroma. In several cancers, fibrotic stroma inhibit medication distribution and penetration. OA can inhibit matrix metalloproteinases (MMPs), which may reduce tumor stromal cell fibrosis. All these studies suggest OA could boost cancer chemotherapy [60]. The encapsulated flavonoid silybin can operate as an MDR inhibitor by inhibiting the P-gp pump’s “drug-pumping” action [130].
- CSNPs bypass MDR because they enter cells by “stealth endocytosis,” preventing drug molecules from being identified by P-glycoprotein (P-gp). Perinuclear NPs can release drugs to avoid efflux pumps [133].
| Drug | Polymer CS or Derivated | Cancer Model | Targeting Ligand | Mechanism | Results | Ref. |
|---|---|---|---|---|---|---|
| Doxorubicin (DOX) | Thiolated glycol-CS (tGCS) | Adriamycin-resistant MCF-7 | siRNA P-gp | Functional siRNA release, in-vivo P-gp downregulation | Subtherapeutic DOX dose inhibited tumor development | [134] |
| DOX and oleanolic acid (OA) co-delivery | Folic acid-CS | MDA-MB-231 | Folate | OA can inhibit MMPs. MRP and P-gp inhibition reverse MRP-mediated efflux | High uptake, and longer circulation than free DOX, reduced DOX-induced tissue damage | [135] |
| DOX | Folic acid-hydroxypropyl-chitosan (HPCS) and oligodeoxynucleotides (ODN) | KB-A-1 DOX-resistant cells | Folate | Inhibition of the MDR 1 gene levels and P-gp levels in vitro and in vivo | Comparatively, ODNs inhibited tumor development by 35% | [136] |
| DOX, Paclitaxel (PTX), and Silybin. | PLGA NPs, followed by a double layer of lipids and chitosan. |
| CD44s | Chitosan and CD44 interactions were the main way that CSNPs were taken up. Silybin can block the P-gp pump to act as an MDR inhibitor | NPs cut the size of the tumor by five times compared to the control group without causing obvious cell death | [130] |
| Doxorubicin hydrochloride and Tariquidar (TQR) | Biotinylated carboxymethyl chitosan hybrid | MCF-7/ADR cells | Biotin as a targeting ligand | TQR prevents P-gp-mediated drug efflux and boosts intracellular drugs. Tumor cells absorbed more biotin ligands | Better cell uptake and nuclear localization than free DOX | [133] |
| Ligustrazine (LZ) | Folate-chitosan NPs (FA-CS-NPs) | MCF-7 (folate receptor-positive) and A549 (folate receptor-negative) cells | Folate | Ligustrazine (LZ) improves the sensitivity of multidrug-resistant cancer cells to chemotherapeutic agents | High cellular uptake specificity by FR-expressing cells. FA-CS-LZ-NPs, are a promising candidate for overcoming MDR | [137] |
| Curcumin (Cur) and DOX | CS-based NPs using genipin (crosslinker) | MCF-7/ADR | Intact positively charged NPs (or an amino group) to the negatively charged DNA in the nucleus | Extended circulation time, enhanced tumor targeting effectiveness, increased tumor inhibition efficacy and decreased expression of MDRP | [138] | |
| siRNA and DOX | CS-coated PF127-TPGS mixed micelle based |
| Folate | Improved anticancer effectiveness and blood circulation | Increased cytotoxicity in native 4T1 and multidrug-resistant 4T1 vs. free DOX | [139] |
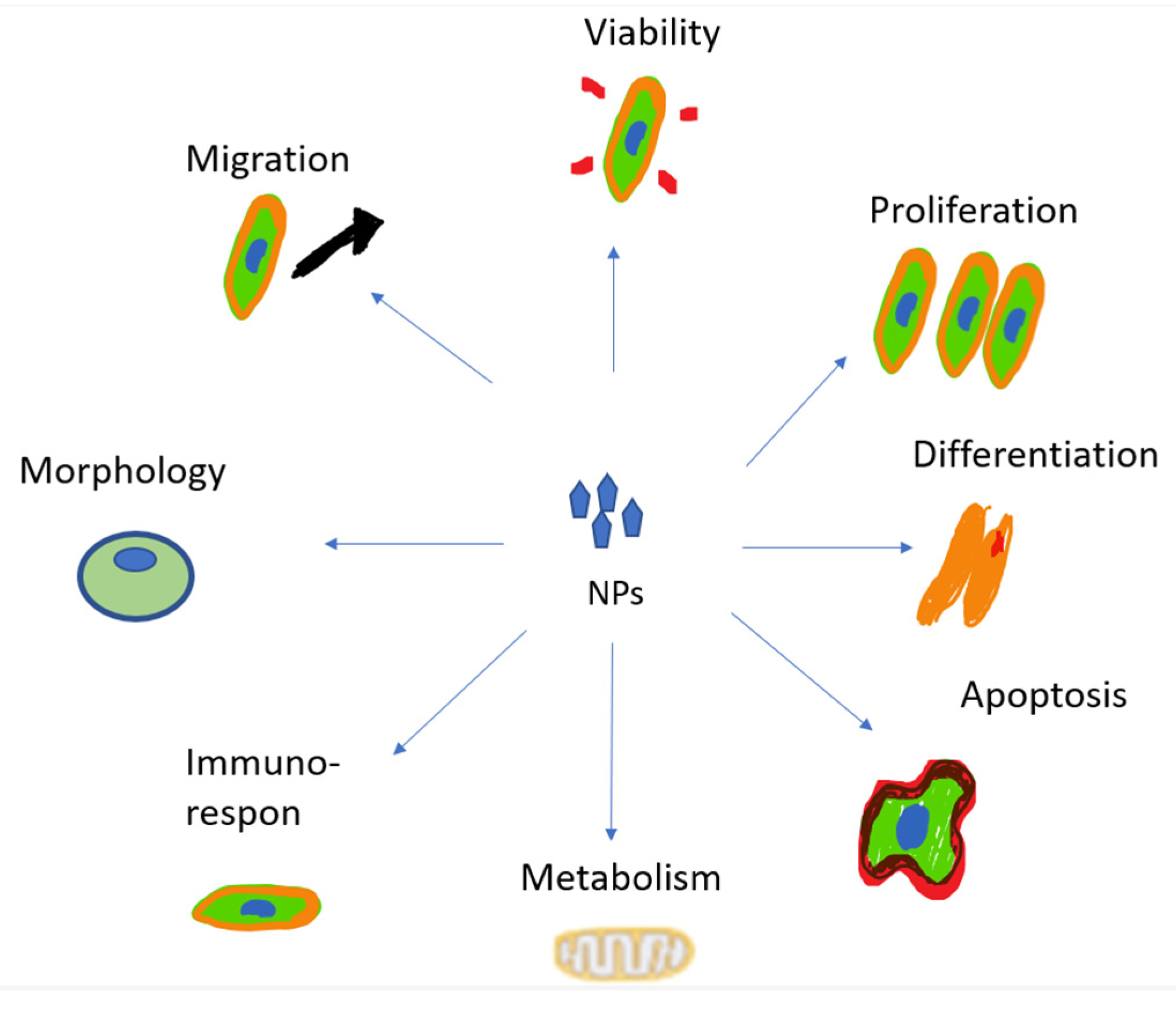
4.5. Cytotoxicity
4.6. Cell Death
| Drug | Polymer CS or Derivated | Cancer Cell Model | Mechanism | Results | Ref. |
|---|---|---|---|---|---|
| DOX | CS- montmorillonite (MMT)-quantum dots | MCF-7 | pH 5.4 controlled DOX release, whereas pH 7.4 had none, suggesting fewer negative effects | NPs had greater cytotoxicity than free DOX in MCF-7 cells | [151] |
| Mebendazole (MBZ) | Folic acid-CSNPs | 4T1 murine TNBC | pH-sensitive CS-FA-MBZ NPs enhance MBZ release in the tumor microenvironment | 15 days after implantation, CS-FA-MBZ implants degraded entirely, reducing tumor volume | [72] |
| Seleno | Seleno-short-chain CS |
| SCC caused apoptosis in MCF-7 and BT-20 cells in vitro by upregulating Bax and downregulating Bcl-2 | MCF-7 and BT-20 cells could undergo in vitro apoptosis when exposed to SSCC via the mitochondrial route | [152] |
| Gold NPs | Chitosan-gold NPs |
| Activation of the p53-p21-mediated cell cycle arrest is concurrent with activation of the Bax-Caspase9-Caspase3-PARP1 axis | Extremely effective against BC cells while having no obvious harmful effects on normal cells | [39] |
| Rutin | Rutin-CS nanoconjugates | TNBC cells | Apoptotic cell death causes DNA synthesis to stop, and DAPI fluorescence micrographic analysis | Triple-negative BC apoptosis | [39] |
| ZnO-NPs | CS-ZnO NPs | MCF 7 | Significant cell cycle arrest at a particular stage of G2/M was achieved with the nanocomplex treatment in a dose-dependent manner. Finally, it was observed that the apoptotic genes and protein expressions of the MCF-7 cell line were up and down-regulated with the treatment of Ch-Ap-ZnONPS when compared to normal cells | The spherical and cubic nanocrystals were found to be lethal against MCF 7 cells whose IC50 value was 42 μg/mL, on MTT assay, in dose-dependent manner (20–80 μg/mL), | [39] |
| Doxorubicin | CS-protamine NPs | MDA-MB-231 | CPNPs-DOX downregulates Bcl-2 relative to free DOX and control. | Treatment with NPs reduces cell viability/count | [153] |
| Ascorbic acid and Oxaliplatin | Non-PEGylated and PEGylated CS NPs (CS NPs) | MCF-7 cells | AA stimulates the internal apoptotic process, whereas OX activates the extrinsic route. Evidence shows that the two pathways are interconnected and that chemicals in one may impact molecules in the other | PEGylation improves AA and OX’s apoptotic effects on MCF-7 cells | [149] |
| Chitooligosaccharides (COS) | Chitooligosaccharides (COS) D3–7 (D-deacetylated unit) and A5 (A-acetylated unit) |
| Apoptosis is promoted by the strong reduction of phosphorylation of EGFR and its downstream signaling pathways FAK, AKT, and MAPK | In a dose-dependent manner, COS significantly reduces the viability of BC cells | [154] |
| Curcumin | Iron (II, III) oxide (Fe3O4) NPs coated with carboxymethyl-CS |
| In MCF-7 cells, combinational therapy-induced cell death (64.51 percent) and sub-G1 cell cycle arrest were observed. In addition, MCF-7 cell proliferation may be inhibited | The IC50 level of MNP-CMC-CUR has been dramatically reduced when compared to free curcumin, as has the metabolic activity of the cells (p 0.05) | [155] |
| Curcumin and Chrysin | Alginate-CS hydrogel | T47D | G2/M causes arrest of the cell cycle | NPs drastically impair viability and trigger apoptosis in cells | [156] |
5. Perspective
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ding, J.; Guo, Y. Recent Advances in Chitosan and its Derivatives in Cancer Treatment. Front. Pharmacol. 2022, 13, 888740. [Google Scholar] [CrossRef] [PubMed]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006. [Google Scholar] [CrossRef]
- Martinelli, C.; Biglietti, M. Nanotechnological approaches for counteracting multidrug resistance in cancer. Cancer Drug Resist. 2020, 3, 1003. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Abdolahinia, E.D.; Barar, J.; Omidi, Y. Smart stimuli-responsive biopolymeric nanomedicines for targeted therapy of solid tumors. Nanomedicine 2020, 15, 2171–2200. [Google Scholar] [CrossRef]
- Wong, K.H.; Lu, A.; Chen, X.; Yang, Z. Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment. Molecules 2020, 25, 3620. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Neerooa, B.N.H.M.; Ooi, L.-T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.M.; Pushpamalar, J.; Teow, S.-Y. Development of Polymer-Assisted Nanoparticles and Nanogels for Cancer Therapy: An Update. Gels 2021, 7, 60. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2020, 5, e1291. [Google Scholar] [CrossRef]
- Ahmed Eltayeb, M.; Faggad, A.; Sharafeldin Abbadi, O.; Mohammed Ali Elhassan, M. Characteristics of Breast Cancer at First Presentation in Sudanese Patients Attending the National Cancer Institute–University of Gezira (NCI–UG). Arch. Breast Cancer 2020, 7, 104–110. [Google Scholar] [CrossRef]
- Shadabfar, M.; Abdouss, M.; Khonakdar, H.A. Synthesis, characterization, and evaluation of a magnetic molecular imprinted polymer for 5-fluorouracil as an intelligent drug delivery system for breast cancer treatment. J. Mater. Sci. 2020, 55, 12287–12304. [Google Scholar] [CrossRef]
- Leea, B.K.; Yuna, Y.H.; Parka, K. Smart Nanoparticles for Drug Delivery: Boundaries and Opportunities. Chem. Eng. Sci. 2011, 23, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangopadhyay, S.; Nikam, R.R.; Gore, K.R. Folate Receptor-Mediated siRNA Delivery: Recent Developments and Future Directions for RNAi Therapeutics. Nucleic Acid Ther. 2021, 31, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Abuwatfa, W.H.; Al-Sayah, M.H.; Husseini, G.A. Gold-Nanoparticle Hybrid Nanostructures for Multimodal Cancer Therapy. Nanomaterials 2022, 12, 3706. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 1–29. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 7. [Google Scholar] [CrossRef]
- Charelli, L.E.; de Mattos, G.C.; de Jesus Sousa-Batista, A.; Pinto, J.C.; Balbino, T.A. Polymeric nanoparticles as therapeutic agents against coronavirus disease. J. Nanopart. Res. 2022, 24, 1–15. [Google Scholar] [CrossRef]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef]
- Xiao, X.; Teng, F.; Shi, C.; Chen, J.; Wu, S.; Wang, B.; Meng, X.; Essiet Imeh, A.; Li, W. Polymeric nanoparticles—Promising carriers for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 59. [Google Scholar] [CrossRef]
- Karam, M.; Fahs, D.; Maatouk, B.; Safi, B.; Jaffa, A.A.; Mhanna, R. Polymeric nanoparticles in the diagnosis and treatment of myocardial infarction: Challenges and future prospects. Mater. Today Bio 2022, 14, 100249. [Google Scholar] [CrossRef] [PubMed]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Surface Engineering of Nanomaterials with Polymers, Biomolecules, and Small Ligands for Nanomedicine. Materials 2022, 15, 3251. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Desai, V.M.; Singhvi, G. Surface Modification of Lipid-Based Nanocarriers: A Potential Approach to Enhance Targeted Drug Delivery. ACS Omega 2022, 8, 74–86. [Google Scholar] [CrossRef]
- Komsthöft, T.; Bovone, G.; Bernhard, S.; Tibbitt, M.W. Polymer functionalization of inorganic nanoparticles for biomedical applications. Curr. Opin. Chem. Eng. 2022, 37, 100849. [Google Scholar] [CrossRef]
- Yang, T.; Wang, A.; Nie, D.; Fan, W.; Jiang, X.; Yu, M.; Guo, S.; Zhu, C.; Wei, G.; Gan, Y. Ligand-switchable nanoparticles resembling viral surface for sequential drug delivery and improved oral insulin therapy. Nat. Commun. 2022, 13, 6649. [Google Scholar] [CrossRef]
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef]
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef]
- Rofeal, M.; Abdelmalek, F.; Steinbüchel, A. Naturally-Sourced Antibacterial Polymeric Nanomaterials with Special Reference to Modified Polymer Variants. Int. J. Mol. Sci. 2022, 23, 4101. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up polymeric-based nanoparticles drug delivery systems: Development and challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Nagati, V.; Tenugu, S.; Pasupulati, A.K. Chapter 4—Stability of therapeutic nano-drugs during storage and transportation as well as after ingestion in the human body. In Nanotechnology in Biomedicine; Das Talukdar, A., Dey Sarker, S., Patra, J.K.B.T.-A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–102. ISBN 978-0-323-88450-1. [Google Scholar]
- Hu, C.M.J.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Alexander-bryant, A.A.; Vanden Berg-foels, W.S. Bioengineering Strategies for Designing Targeted Cancer Therapies, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 118, ISBN 9780124071735. [Google Scholar]
- Sun, X.; Liu, J.; Ji, H.; Yang, M.; Lu, Y. Clinicopathological characteristics and prognosis of breast cancer in young women—A single center study in a developing country. Cancer Manag. Res. 2021, 13, 1601–1607. [Google Scholar] [CrossRef]
- Fatemian, T.; Chowdhury, E.H. Cytotoxicity enhancement in breast cancer cells with carbonate apatite-facilitated intracellular delivery of anti-cancer drugs. Toxics 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsharzadeh, M.; Hashemi, M.; Mokhtarzadeh, A.; Abnous, K.; Ramezani, M. Recent advances in co-delivery systems based on polymeric nanoparticle for cancer treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1095–1110. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, A.; Roy, B.; Shaw, P.; Mondal, P.; Mondal, M.K.; Chowdhury, P.; Bhattacharya, S.; Chattopadhyay, A. Chitosan-gold nanoparticles trigger apoptosis in human breast cancer cells in vitro. Nucleus 2021, 64, 79–92. [Google Scholar] [CrossRef]
- Barcelo-Bovea, V.; Dominguez-Martinez, I.; Joaquin-Ovalle, F.; Amador, L.A.; Castro-Rivera, E.; Medina-álvarez, K.; McGoron, A.; Griebenow, K.; Ferrer-Acosta, Y. Optimization and characterization of protein nanoparticles for the targeted and smart delivery of cytochrome c to non-small cell lung carcinoma. Cancers 2020, 12, 1215. [Google Scholar] [CrossRef]
- Lodhi, M.S.; Khalid, F.; Khan, M.T.; Samra, Z.Q.; Muhammad, S.; Zhang, Y.J.; Mou, K. A Novel Method of Magnetic Nanoparticles Functionalized with Anti-Folate Receptor Antibody and Methotrexate for Antibody Mediated Targeted Drug Delivery. Molecules 2022, 27, 261. [Google Scholar] [CrossRef]
- Tashima, T. Delivery of Drugs into Cancer Cells Using Antibody—Drug Conjugates Based on Receptor-Mediated Endocytosis and the Enhanced Permeability and Retention Effect Fab Fc Linker Drug. Antibodies 2022, 11, 78. [Google Scholar] [CrossRef]
- Granja, A.; Nunes, C.; Sousa, C.T.; Reis, S. Folate receptor-mediated delivery of mitoxantrone-loaded solid lipid nanoparticles to breast cancer cells. Biomed. Pharmacother. 2022, 154, 113525. [Google Scholar] [CrossRef]
- Mahalunkar, S.; Yadav, A.S.; Gorain, M.; Pawar, V.; Braathen, R.; Weiss, S.; Bogen, B.; Gosavi, S.W.; Kundu, G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019, 14, 8285–8302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosleh-Shirazi, S.; Abbasi, M.; Moaddeli, M.R.; Vaez, A.; Shafiee, M.; Kasaee, S.R.; Amani, A.M.; Hatam, S. Nanotechnology Advances in the Detection and Treatment of Cancer: An Overview. Nanotheranostics 2022, 6, 400–423. [Google Scholar] [CrossRef] [PubMed]
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical nanotechnology: From the bench to the market. Futur. J. Pharm. Sci. 2022, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef]
- Brewer, E.; Coleman, J.; Lowman, A. Emerging technologies of polymeric nanoparticles in cancer drug delivery. J. Nanomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Applications of “Smart Polymers” as Biomaterials, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123746269. [Google Scholar]
- Harwansh, R.K.; Deshmukh, R.; Barkat, M.A.; Rahman, M.A. Bioinspired Polymeric-based Core-shell Smart Nano-systems. Pharm. Nanotechnol. 2019, 7, 181–205. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Ellah, N.H.A.; El Hamid, B.N.A. Temperature and pH Dual-Stimuli Responsive Polymeric Carriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081019955. [Google Scholar]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Chabalenge, B.; Mwila, C.; Kalungia, A.C.; Nkanga, C.I.; Bapolisi, A.M.; Walker, R.B. Biocompatibility of biomaterials for nanoencapsulation: Current approaches. Nanomaterials 2020, 10, 1649. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [Green Version]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Parodi, A.; Buzaeva, P.; Nigovora, D.; Baldin, A.; Kostyushev, D.; Chulanov, V.; Savvateeva, L.V.; Zamyatnin, A.A. Nanomedicine for increasing the oral bioavailability of cancer treatments. J. Nanobiotechnol. 2021, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef] [PubMed]
- Al-Rooqi, M.M.; Hassan, M.M.; Moussa, Z.; Obaid, R.J.; Suman, N.H.; Wagner, M.H.; Natto, S.S.A.; Ahmed, S.A. Advancement of chitin and chitosan as promising biomaterials. J. Saudi Chem. Soc. 2022, 26, 101561. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Mennini, N. Multiple Roles of Chitosan in Mucosal Drug Delivery: An Updated Review. Mar. Drugs 2022, 20, 335. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, D.; Liu, D.; Su, J.; Jin, Y.; Wang, D.; Han, B.; Jiang, Z.; Liu, B. Applications of Chitosan and its Derivatives in Skin and Soft Tissue Diseases. Front. Bioeng. Biotechnol. 2022, 10, 894667. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Li, N.; Lin, J.; Liu, C.; Zhang, Q.; Li, R.; Wang, C.; Zhao, C.; Lu, L.; Zhou, C.; Tian, J.; et al. Temperature- and pH-responsive injectable chitosan hydrogels loaded with doxorubicin and curcumin as long-lasting release platforms for the treatment of solid tumors. Front. Bioeng. Biotechnol. 2022, 10, 1043939. [Google Scholar] [CrossRef]
- Yang, J.I.; Lee, H.L.; Yun, J.J.; Kim, J.; So, K.H.; Jeong, Y.I.; Kang, D.H. pH and Redox-Dual Sensitive Chitosan Nanoparticles Having Methyl Ester and Disulfide Linkages for Drug Targeting against Cholangiocarcinoma Cells. Materials 2022, 15, 3795. [Google Scholar] [CrossRef]
- Guo, K.; Liu, Y.; Ding, M.; Sun, Q.; Shubhra, Q.T.H. Enhanced drug release from a pH-responsive nanocarrier can augment colon cancer treatment by blocking PD-L1 checkpoint and consuming tumor glucose. Mater. Des. 2022, 219, 110824. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A.; Hasnain, M.S.; Nayak, A.K. Chapter 7—Chitosan-Based Drug Delivery Systems in Cancer Therapeutics; Hasnain, M.S., Beg, S., Nayak, A.K.B.T.-C., Eds.; Academic Press: Beijing, China, 2022; pp. 159–193. ISBN 978-0-12-819336-5. [Google Scholar]
- Nawaz, R.; Naqvi, S.T.R.; Fatima, B.; Zulfiqar, N.; Farooq, M.U.; ul Haq, M.N.; Hussain, D.; Javeed, A.; Rasul, A.; Jafri, L.; et al. Cost-effective fabrication, antibacterial application and cell viability studies of modified nonwoven cotton fabric. Sci. Rep. 2022, 12, 2493. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.N.; Calabuig-Fariñas, S.; de Mena, M.L.; de Bremond, M.J.G.S.; González, C.G.; Martínez, S.T.; García-García, J.Á.; González-Cruz, V.I.; Herrero, C.C. Update on systemic treatment in early triple negative breast cancer. Ther. Adv. Vaccines 2018, 9, 259–261. [Google Scholar]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K. Chitosan Nanoparticle Encapsulation of Antibacterial Essential Oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Kuen, C.Y.; Masarudin, M.J. Chitosan Nanoparticle-Based System: A New Insight into the Promising Controlled Release System for Lung Cancer Treatment. Molecules 2022, 27, 473. [Google Scholar] [CrossRef] [PubMed]
- Cuggino, J.C.; Gatti, G.; Picchio, M.L.; Maccioni, M.; Gugliotta, L.M.; Alvarez Igarzabal, C.I. Dually responsive nanogels as smart carriers for improving the therapeutic index of doxorubicin for breast cancer. Eur. Polym. J. 2019, 116, 445–452. [Google Scholar] [CrossRef]
- Kefayat, A.; Hosseini, M.; Ghahremani, F.; Jolfaie, N.A.; Rafienia, M. Biodegradable and biocompatible subcutaneous implants consisted of pH-sensitive mebendazole-loaded/folic acid-targeted chitosan nanoparticles for murine triple-negative breast cancer treatment. J. Nanobiotechnol. 2022, 20, 169. [Google Scholar] [CrossRef]
- Kashif, S.A.; Park, J.K. Enzymatically Hydrolyzed Water-Soluble Chitosan as a Potent Anti-Microbial Agent. Macromol. Res. 2019, 27, 551–557. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, H. Improved antitumor efficacy of paclitaxel with nano-formulation in breast cancer. Nanotechnol. Rev. 2017, 6, 291–299. [Google Scholar] [CrossRef]
- Choukaife, H.; Seyam, S.; Alallam, B.; Doolaanea, A.A.; Alfatama, M. Current Advances in Chitosan Nanoparticles Based Oral Drug Delivery for Colorectal Cancer Treatment. Int. J. Nanomed. 2022, 17, 3933–3966. [Google Scholar] [CrossRef]
- Shukla, R.; Vasdev, N.; Ruwali, M.; Hasnain, M.S.; Beg, S. Chapter 17—Chitosan for Delivery of Biomolecules; Hasnain, M.S., Beg, S., Nayak, A.K.B.T.-C., Eds.; Academic Press: Beijing, China, 2022; pp. 433–460. ISBN 978-0-12-819336-5. [Google Scholar]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; ul Shah, M.Z.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. Int. J. Nanomed. 2020, 15, 2699–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghomrasni, N.B.; Chivas-Joly, C.; Devoille, L.; Hochepied, J.F.; Feltin, N. Challenges in sample preparation for measuring nanoparticles size by scanning electron microscopy from suspensions, powder form and complex media. Powder Technol. 2020, 359, 226–237. [Google Scholar] [CrossRef]
- Hakke, V.; Sonawane, S.; Anandan, S.; Sonawane, S.; Ashokkumar, M. Process intensification approach using microreactors for synthesizing nanomaterials—A critical review. Nanomaterials 2021, 11, 98. [Google Scholar] [CrossRef]
- Langevin, D.; Raspaud, E.; Mariot, S.; Knyazev, A.; Stocco, A.; Salonen, A.; Luch, A.; Haase, A.; Trouiller, B.; Relier, C.; et al. Towards reproducible measurement of nanoparticle size using dynamic light scattering: Important controls and considerations. NanoImpact 2018, 10, 161–167. [Google Scholar] [CrossRef]
- Abere, D.V.; Ojo, S.A.; Paredes-Epinosa, M.B.; Hakami, A. Derivation of composites of chitosan-nanoparticles from crustaceans source for nanomedicine: A mini review. Biomed. Eng. Adv. 2022, 4, 100058. [Google Scholar] [CrossRef]
- Mondal, A.; Dhar, A.K.; Banerjee, S.; Hasnain, M.S.; Nayak, A.K. Chapter 2—Antimicrobial Uses of Chitosan; Hasnain, M.S., Beg, S., Nayak, A.K.B.T.-C., Eds.; Academic Press: Beijing, China, 2022; pp. 13–36. ISBN 978-0-12-821058-1. [Google Scholar]
- Genedy, H.H.; Delair, T.; Montembault, A. Chitosan Based MicroRNA Nanocarriers. Pharmaceuticals 2022, 15, 1036. [Google Scholar] [CrossRef]
- Manivong, S.; Garcia Ac, A.; Patten, S.A.; Fernandes, J.C.; Benderdour, M.; Banquy, X.; Moldovan, F.; Roullin, V.G. Chitosan-Based Nanogels: Synthesis and Toxicity Profile for Drug Delivery to Articular Joints. Nanomaterials 2022, 12, 1337. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Kedir, W.M.; Abdi, G.F.; Goro, M.M.; Tolesa, L.D. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: A review. Heliyon 2022, 8, e10196. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Cytotoxicity Enhancement in MCF-7 Breast Cancer Cells with Depolymerized Chitosan Delivery of α-Mangostin. Polymers 2022, 14, 3139. [Google Scholar] [CrossRef]
- Lu, D.; Lu, T. Anticancer drug development, challenge and dilemma. Nurse Care Open Acces J. 2020, 7, 72–75. [Google Scholar] [CrossRef]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14, 1860. [Google Scholar] [CrossRef]
- Karaosmanoglu, S.; Zhang, Y.; Zhou, W.; Ouyang, D. Synthesis of Carrier-Free Paclitaxel—Curcumin Nanoparticles: The Role of Curcuminoids. Bioengineering 2022, 9, 815. [Google Scholar] [CrossRef]
- Gote, V.; Nookala, A.R.; Bolla, P.K.; Pal, D. Drug resistance in metastatic breast cancer: Tumor targeted nanomedicine to the rescue. Int. J. Mol. Sci. 2021, 22, 4673. [Google Scholar] [CrossRef]
- Bennet, D.; Kim, S. Polymer Nanoparticles for Smart Drug Delivery. In Application of Nanotechnology in Drug Delivery; Intech: Rijeka, Croatia, 2014. [Google Scholar] [CrossRef] [Green Version]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Mathew, A.; Benny, S.J.; Boby, J.M.; Sirohi, B. Value-Based Care in Systemic Therapy: The Way Forward. Curr. Oncol. 2022, 29, 5792–5799. [Google Scholar] [CrossRef]
- Ho, K.S. Targeted Drug Delivery to Breast Cancer Using Polymeric Nanoparticle Micelles. Ph. D. Thesis, University of Toronto, Toronto, ON, Canada, 2012. [Google Scholar]
- Aguilar, M.R.; San Román, J. Smart Polymers and their Applications. In Smart Polymers and Their Applications; Woodhead Publishing: Sawston, UK, 2014; pp. 1–568. [Google Scholar] [CrossRef]
- Nokhodi, F.; Nekoei, M.; Goodarzi, M.T. Hyaluronic acid-coated chitosan nanoparticles as targeted-carrier of tamoxifen against MCF7 and TMX-resistant MCF7 cells. J. Mater. Sci. Mater. Med. 2022, 33, 24. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Alsuraifi, A.; Curtis, A.; Lamprou, D.A.; Hoskins, C. Stimuli responsive polymeric systems for cancer therapy. Pharmaceutics 2018, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Lara-Velazquez, M.; Alkharboosh, R.; Norton, E.S.; Ramirez-Loera, C.; Freeman, W.D.; Guerrero-Cazares, H.; Forte, A.J.; Quiñones-Hinojosa, A.; Sarabia-Estrada, R. Chitosan-Based Non-viral Gene and Drug Delivery Systems for Brain Cancer. Front. Neurol. 2020, 11, 740. [Google Scholar] [CrossRef]
- Klausner, E.A.; Zhang, Z.; Chapman, R.L.; Multack, R.F.; Volin, M. V Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials 2010, 31, 1814–1820. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Advances in Chitosan-Based CRISPR/Cas9 Delivery Systems. Pharmaceutics 2022, 14, 1840. [Google Scholar] [CrossRef]
- Santos-Carballal, B.; Fernández, E.F.; Goycoolea, F.M. Chitosan in non-viral gene delivery: Role of structure, characterization methods, and insights in cancer and rare diseases therapies. Polymers 2018, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent advances in chitosan-based carriers for gene delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, H.N. Chitosan-Based Nanocarriers for Gene Delivery. In Nanoengineering of Biomaterials; Wiley Online Library: Hoboken, NJ, USA, 2022; pp. 91–105. ISBN 9783527832095. [Google Scholar]
- Aranda-Barradas, M.E.; Trejo-López, S.E.; Del Real, A.; Álvarez-Almazán, S.; Méndez-Albores, A.; García-Tovar, C.G.; González-Díaz, F.R.; Miranda-Castro, S.P. Effect of molecular weight of chitosan on the physicochemical, morphological, and biological properties of polyplex nanoparticles intended for gene delivery. Carbohydr. Polym. Technol. Appl. 2022, 4, 100228. [Google Scholar] [CrossRef]
- Brighenti, R.; Li, Y.; Vernerey, F.J. Smart Polymers for Advanced Applications: A Mechanical Perspective Review. Front. Mater. 2020, 7, 1–18. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Pethe, A.M.; Yadav, K.S. Polymers, responsiveness and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M.; Kagan, C.R.; Millstone, J.E. Reproducibility in Nanocrystal Synthesis? Watch out for Impurities! ACS Nano 2020, 14, 6359–6361. [Google Scholar] [CrossRef]
- Amirani, E.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B. Effects of chitosan and oligochitosans on the phosphatidylinositol 3-kinase-AKT pathway in cancer therapy. Int. J. Biol. Macromol. 2020, 164, 456–467. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Z.; Zheng, C.; Liu, Y.; Hao, R.; Ji, X.; Xi, Q.; Shen, J.; Li, Z. Chitosan oligosaccharide regulates AMPK and STAT1 pathways synergistically to mediate PD-L1 expression for cancer chemoimmunotherapy. Carbohydr. Polym. 2022, 277, 118869. [Google Scholar] [CrossRef]
- Narmani, A.; Jafari, S.M. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr. Polym. 2021, 272, 118464. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Ricci, M.; Fujita, Y.; Zundert, I. Van Polymeric Engineering of Nanoparticles for Highly Efficient Multifunctional Drug Delivery Systems. Sci. Rep. 2019, 9, 2666. [Google Scholar] [CrossRef]
- Patra, S.; Madhuri, R.; Sharma, P.K. Stimuli-Responsive Polymers for Treatment of Diabetes Mellitus; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081019955. [Google Scholar]
- Huang, S.; Ding, X. Precise Design Strategies of Nanotechnologies for Controlled Drug Delivery. J. Funct. Biomater. 2022, 13, 188. [Google Scholar] [CrossRef]
- Sandhiya, V.; Ubaidulla, U. Enhancing cellular uptake and membrane permeability of gallic acid for breast cancer therapy via folate-tagged PEGylated iron oxide nanoparticles has theronastic agent. Bull. Natl. Res. Cent. 2022, 46, 234. [Google Scholar] [CrossRef]
- Frigaard, J.; Jensen, J.L.; Galtung, H.K.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An Overview of the Cytotoxicity of Chitosan Based Nanoparticles. Front. Pharmacol. 2022, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.G.; Chan, C.Y. Multiple Roles of Actin in Exo- and Endocytosis. Front. Synaptic Neurosci. 2022, 14, 841704. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Somasundaram, A.; Shin, W.; Ge, L.; Villareal, S.; Chan, C.Y.; Ashery, U.; Shupliakov, O.; Taraska, J.W.; Wu, L.G. Clathrin-mediated endocytosis cooperates with bulk endocytosis to generate vesicles. iScience 2022, 25, 103809. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W.; Biology, C.; Biology, C. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2017, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Macedo, L.B.; Nogueira-Librelotto, D.R.; Mathes, D.; de Vargas, J.M.; da Rosa, R.M.; Rodrigues, O.E.D.; Vinardell, M.P.; Mitjans, M.; Rolim, C.M.B. Overcoming MDR by Associating Doxorubicin and pH-Sensitive PLGA Nanoparticles Containing a Novel Organoselenium Compound—An In Vitro Study. Pharmaceutics 2022, 14, 80. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, F.; Li, K.; Xu, J.; Li, P.; Fan, Y. pH-responsive mesoporous Fe2O3–Au nanomedicine delivery system with magnetic targeting for cancer therapy. Med. Nov. Technol. Devices 2022, 15, 100127. [Google Scholar] [CrossRef]
- Lou, S.; Zhao, Z.; Dezort, M.; Lohneis, T.; Zhang, C. Multifunctional Nanosystem for Targeted and Controlled Delivery of Multiple Chemotherapeutic Agents for the Treatment of Drug-Resistant Breast Cancer. ACS Omega 2018, 3, 9210–9219. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Feng, J.; Qin, B.; Zhang, C.; Zhu, C.; Liu, W.; Wang, Y.; Liu, W.; Huang, L.; et al. Multifunctional nanoparticles co-loaded with Adriamycin and MDR-targeting siRNAs for treatment of chemotherapy-resistant esophageal cancer. J. Nanobiotechnol. 2022, 20, 166. [Google Scholar] [CrossRef]
- Luque-Bolivar, A.; Pérez-Mora, E.; Villegas, V.E.; Rondón-Lagos, M. Resistance and overcoming resistance in breast cancer. Breast Cancer Targets Ther. 2020, 12, 211–229. [Google Scholar] [CrossRef]
- Wu, J.L.; He, X.Y.; Jiang, P.Y.; Gong, M.Q.; Zhuo, R.X.; Cheng, S.X. Biotinylated carboxymethyl chitosan/CaCO3hybrid nanoparticles for targeted drug delivery to overcome tumor drug resistance. RSC Adv. 2016, 6, 69083–69093. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Song, S.; Lee, S.J.; Park, S.G.; Kim, K.S.; Kim, M.G.; Son, S.; Koo, H.; Kwon, I.C.; Jeong, J.H.; et al. Cancer-targeted MDR-1 siRNA delivery using self-cross-linked glycol chitosan nanoparticles to overcome drug resistance. J. Control. Release 2015, 198, 1–9. [Google Scholar] [CrossRef]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Zheng, H.; Li, S.; Zhu, L.M. A Novel Chitosan-Based Nanomedicine for Multi-Drug Resistant Breast Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 369, ISBN 1352922738. [Google Scholar]
- Wang, J.; Tao, X.; Zhang, Y.; Wei, D.; Ren, Y. Reversion of multidrug resistance by tumor targeted delivery of antisense oligodeoxynucleotides in hydroxypropyl-chitosan nanoparticles. Biomaterials 2010, 31, 4426–4433. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ma, H.; Shao, M.; Fan, Q.; Lv, H.; Peng, J.; Hao, T.; Li, D.; Zhao, C.; Zong, X. Synthesis of folate-chitosan nanoparticles loaded with ligustrazine to target folate receptor positive cancer cells. Mol. Med. Rep. 2017, 16, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Xie, P.; Luo, M.; Li, Q.; Li, L.; Zhang, J.; Zheng, Q.; Chen, H.; Nan, K. Efficiency against multidrug resistance by co-delivery of doxorubicin and curcumin with a legumain-sensitive nanocarrier. Nano Res. 2018, 11, 3619–3635. [Google Scholar] [CrossRef]
- Butt, A.M.; Amin, M.C.; Katas, H.; Abdul Murad, N.A.; Jamal, R.; Kesharwani, P. Doxorubicin and siRNA Codelivery via Chitosan-Coated pH-Responsive Mixed Micellar Polyplexes for Enhanced Cancer Therapy in Multidrug-Resistant Tumors. Mol. Pharm. 2016, 13, 4179–4190. [Google Scholar] [CrossRef]
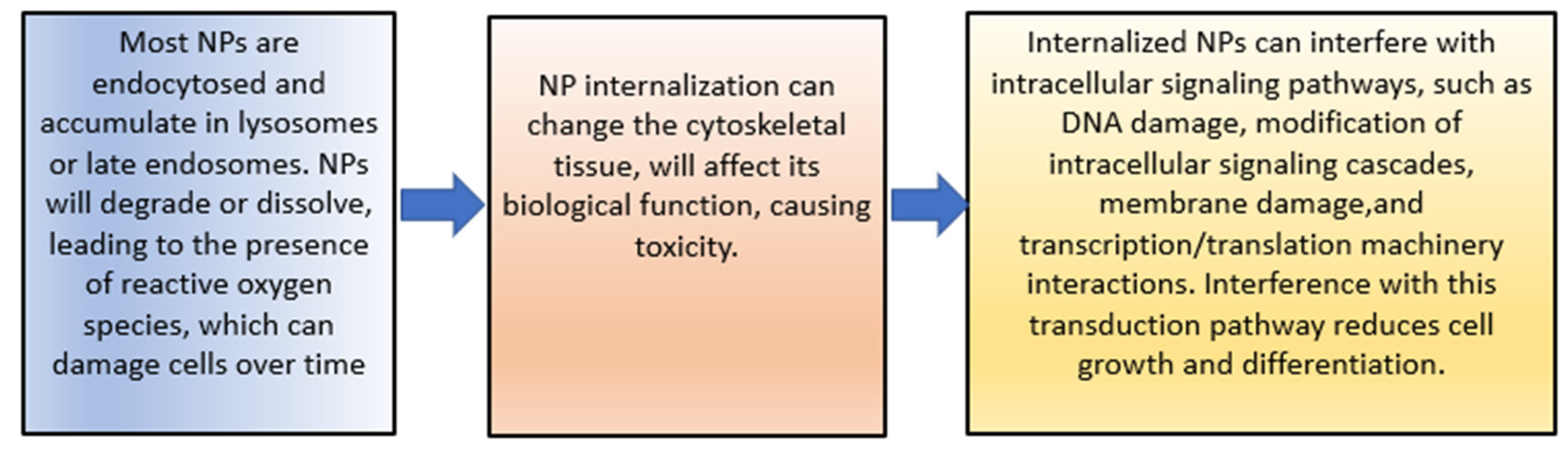
- Liu, X.; Yang, Z.; Sun, J.; Ma, T.; Hua, F.; Shen, Z. A brief review of cytotoxicity of nanoparticles on mesenchymal stem cells in regenerative medicine. Int. J. Nanomed. 2019, 14, 3875–3892. [Google Scholar] [CrossRef] [Green Version]
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef]
- Habibullah, M.M.; Mohan, S.; Syed, N.K.; Makeen, H.A.; Jamal, Q.M.S.; Alothaid, H.; Bantun, F.; Alhazmi, A.; Hakamy, A.; Kaabi, Y.A.; et al. Human Growth Hormone Fragment 176–191 Peptide Enhances the Toxicity of Doxorubicin-Loaded Chitosan Nanoparticles Against MCF-7 Breast Cancer Cells. Drug Des. Devel. Ther. 2022, 16, 1963–1974. [Google Scholar] [CrossRef]
- Lin, G.; Huang, J.; Zhang, M.; Chen, S.; Zhang, M. Chitosan-Crosslinked Low Molecular Weight PEI-Conjugated Iron Oxide Nanoparticle for Safe and Effective DNA Delivery to Breast Cancer Cells. Nanomaterials 2022, 12, 584. [Google Scholar] [CrossRef]
- San, H.H.M.; Alcantara, K.P.; Bulatao, B.P.I.; Chaichompoo, W.; Nalinratana, N.; Suksamrarn, A.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Development of Turmeric Oil—Loaded Chitosan/Alginate Nanocapsules for Cytotoxicity Enhancement against Breast Cancer. Polymers 2022, 14, 1835. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.H.; Moni, S.S.; Madkhali, O.A.; Bakkari, M.A.; Alshahrani, S.; Alqahtani, S.S.; Alhakamy, N.A.; Mohan, S.; Ghazwani, M.; Bukhary, H.A.; et al. Characterization of cisplatin-loaded chitosan nanoparticles and rituximab-linked surfaces as target-specific injectable nano-formulations for combating cancer. Sci. Rep. 2022, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Homayouni Tabrizi, M. Fabrication of folic acid-conjugated chitosan-coated PLGA nanoparticles for targeted delivery of Peganum harmala smoke extract to breast cancer cells. Nanotechnology 2022, 33, 495101. [Google Scholar] [CrossRef]
- Fong, S.S.; Foo, Y.Y.; Saw, W.S.; Leo, B.F.; Teo, Y.Y.; Chung, I.; Goh, B.T.; Misran, M.; Imae, T.; Chang, C.C.; et al. Chitosan-Coated-PLGA Nanoparticles Enhance the Antitumor and Antimigration Activity of Stattic—A STAT3 Dimerization Blocker. Int. J. Nanomed. 2022, 17, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Shakeran, Z.; Varshosaz, J.; Keyhanfar, M.; Mohammad-Beigi, H.; Rahimi, K.; Sutherland, D.S. Co-delivery of STAT3 siRNA and methotrexate in breast cancer cells. Artif. Cells Nanomed. Biotechnol. 2022, 50, 29–39. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ramzy, A.; Mandour, A.A.; Nasr, S.; Abdelnaser, A.; Bakowsky, U.; Azzazy, H.M.E.S. PEGylated Chitosan Nanoparticles Encapsulating Ascorbic Acid and Oxaliplatin Exhibit Dramatic Apoptotic Effects against Breast Cancer Cells. Pharmaceutics 2022, 14, 407. [Google Scholar] [CrossRef]
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-tumor Activity. Front. Oncol. 2020, 10, 578418. [Google Scholar] [CrossRef]
- Rahmani, E.; Pourmadadi, M.; Ghorbanian, S.A.; Yazdian, F.; Rashedi, H.; Navaee, M. Preparation of a pH-responsive chitosan-montmorillonite-nitrogen-doped carbon quantum dots nanocarrier for attenuating doxorubicin limitations in cancer therapy. Eng. Life Sci. 2022, 22, 634–649. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Y.; Fu, S.; Zhang, J.; Wang, W.; Yan, Z.; Guo, H.; Liu, A. Seleno-short-chain chitosan induces apoptosis in human breast cancer cells through mitochondrial apoptosis pathway in vitro. Cell Cycle 2018, 17, 1579–1590. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hakeem, M.A.; Abdel-Haseb, O.M.; Abdel-Ghany, S.E.; Cevik, E.; Sabit, H. Doxorubicin loaded on chitosan-protamine nanoparticles triggers apoptosis via downregulating Bcl-2 in breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101423. [Google Scholar] [CrossRef]
- Mallakuntla, M.K.; Penugurti, V.; Manavathi, B.; Podile, A.R. Chitooligosaccharides induce apoptosis in human breast cancer cells. Carbohydr. Polym. Technol. Appl. 2021, 2, 100077. [Google Scholar] [CrossRef]
- Pazouki, N.; Irani, S.; Olov, N.; Atyabi, S.M.; Bagheri-Khoulenjani, S. Fe3O4 nanoparticles coated with carboxymethyl chitosan containing curcumin in combination with hyperthermia induced apoptosis in breast cancer cells. Prog. Biomater. 2022, 11, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Abbasalizadeh, F.; Alizadeh, E.; Bagher Fazljou, M.S.; Torbati, M.; Akbarzadeh, A. Anticancer Effect of Alginate-chitosan Hydrogel Loaded with Curcumin and Chrysin on Lung and Breast Cancer Cell Lines. Curr. Drug Deliv. 2022, 19, 600–613. [Google Scholar] [PubMed]

| Drug | Polymer CS or Derivated | Cancer Model | Mechanism | Results | Ref. |
|---|---|---|---|---|---|
| α-mangostin (AMG) | Depolymerized chitosan | MCF-7 | Enhance the physicochemical characteristics | Enhancement of the cytotoxicity of AMG | [92] |
| DNA delivery | An iron oxide core coated with low-molecular-weight (800 Da) polyethyleneimine crosslinked with chitosan |
| Nano-size, positive surface charges for DNA condensation, protection, and serum stability | The ability to deliver DNA for DNA transfection in vitro | [143] |
| Z. multiflora EO | Low -molecular-weight CS |
| Increases cellular uptake, solubility, and biological and pharmacological activities | CSNPs containing Z. multiflora EO were more powerful than non-formulated Z. multiflora EO in prior investigations | [143] |
| Tamoxifen (TMX) | Hyaluronic acid-coated chitosan NPs |
| HA-conjugating to CD44 receptors increased nanoparticle drug uptake | NPs with acidic pH (5–6) released more TMX at pH 7.4. HA-CS NPs were more cytotoxic than CS NPs and free drugs | [102] |
| Turmeric oil (TO) | CS-alginate NPs (CS/Alg-NPs) |
| NPs-induced apoptosis in normal and malignant cells can proceed via ROS production, activating caspase 9, and causing the mitochondrial intrinsic apoptosis pathway | Increasing BC cell cytotoxicity | [144] |
| Human growth hormone (hGH) | Gum Arabica chitosan NPs |
| Increase doxorubicin-loaded CSNP toxicity by binding to BC target proteins | Dual-loaded CSNPs had a stronger anti-proliferative effect against MCF-7 than doxorubicin-loaded CSNPs | [142] |
| Cisplatin | Cisplatin-loaded CSNPs and cisplatin-loaded CSNPs surface linked to rituximab | MCF-7 | The inhibition was ascribed to simple passive penetration via cell membrane pores and delayed breakdown inside cells, resulting in sustained action at the lowest drug dose | A novel cisplatin–DNA tetrahedron-body-expressed nano drug exhibited more cytotoxicity than cisplatin against HER2-overexpressing BC cells | [145] |
| Peganum harmala smoke extract (PSE) | PLGA-NPs coated with folic acid-CS (PCF-NPs) | MCF-7 | MCF-7 cells undergo apoptosis when P53, Cas-3, and Cas-9 genes are upregulated | Selective toxicity on MCF-7 cells | [146] |
| Stattic (S) | CS-coated-poly(lactic-co-glycolic acid) |
| Increased accumulation in the mouse primary tumor and newly formed metastatic foci with high angiogenesis activity | Post-entrapment, the drug’s antimetastatic characteristics improved physicochemically, in vitro and in vivo | [147] |
| Co-delivery of MTX and STAT3 siRNA | Mesoporous silica NPs were functionalized with CS | MCF7 cells | Additional free MTX boosted cellular absorption of modified NPs, involving the DHFR receptor. NPs with and without drugs have variable protein corona compositions, influencing cellular absorption | BC cell viability was dramatically reduced as compared to single treatments alone | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herdiana, Y.; Wathoni, N.; Gozali, D.; Shamsuddin, S.; Muchtaridi, M. Chitosan-Based Nano-Smart Drug Delivery System in Breast Cancer Therapy. Pharmaceutics 2023, 15, 879. https://doi.org/10.3390/pharmaceutics15030879
Herdiana Y, Wathoni N, Gozali D, Shamsuddin S, Muchtaridi M. Chitosan-Based Nano-Smart Drug Delivery System in Breast Cancer Therapy. Pharmaceutics. 2023; 15(3):879. https://doi.org/10.3390/pharmaceutics15030879
Chicago/Turabian StyleHerdiana, Yedi, Nasrul Wathoni, Dolih Gozali, Shaharum Shamsuddin, and Muchtaridi Muchtaridi. 2023. "Chitosan-Based Nano-Smart Drug Delivery System in Breast Cancer Therapy" Pharmaceutics 15, no. 3: 879. https://doi.org/10.3390/pharmaceutics15030879
APA StyleHerdiana, Y., Wathoni, N., Gozali, D., Shamsuddin, S., & Muchtaridi, M. (2023). Chitosan-Based Nano-Smart Drug Delivery System in Breast Cancer Therapy. Pharmaceutics, 15(3), 879. https://doi.org/10.3390/pharmaceutics15030879








