An Adjuvanted Inactivated SARS-CoV-2 Microparticulate Vaccine Delivered Using Microneedles Induces a Robust Immune Response in Vaccinated Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
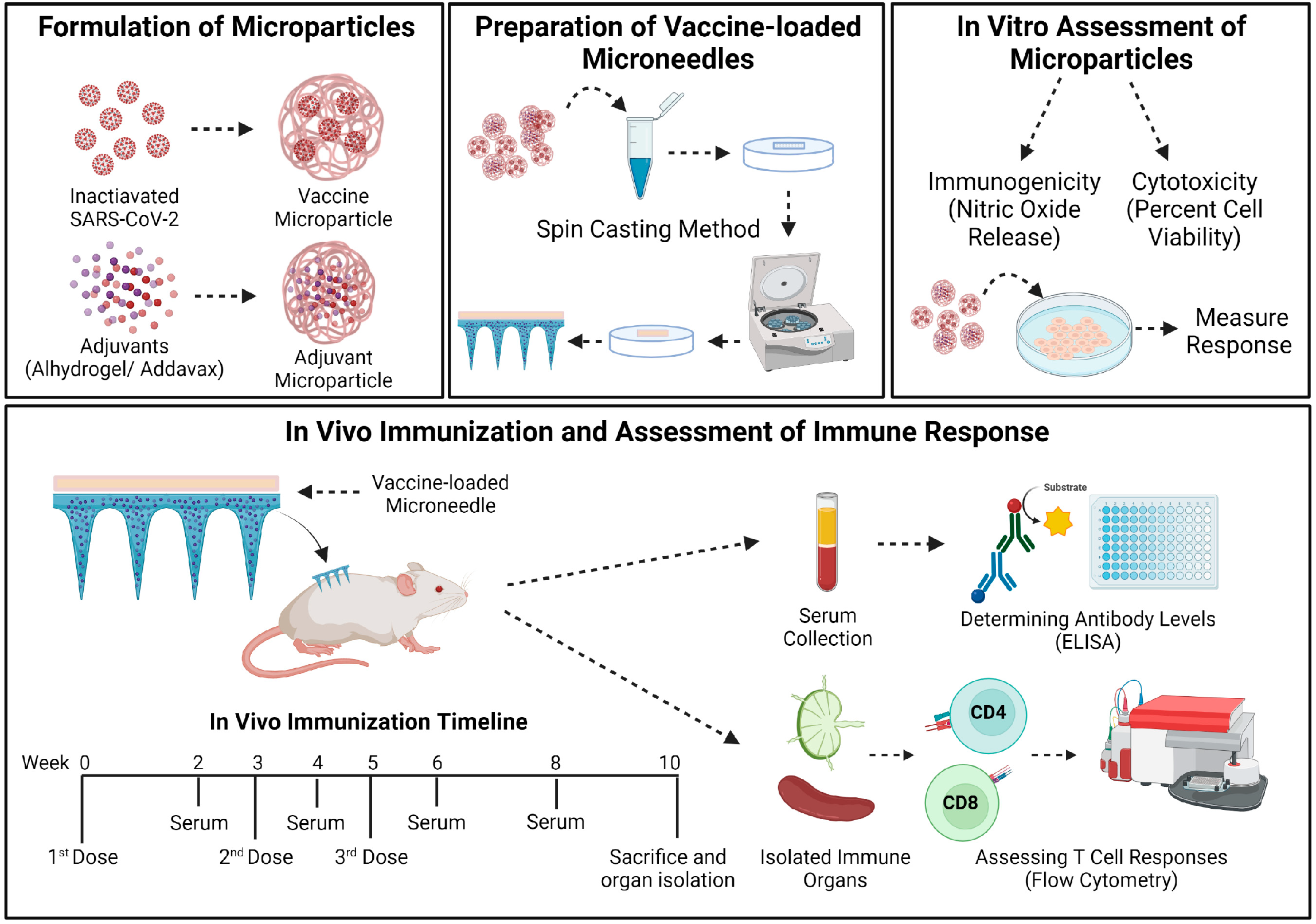
2.2.1. Preparation and Characterization of Microparticles (MP)
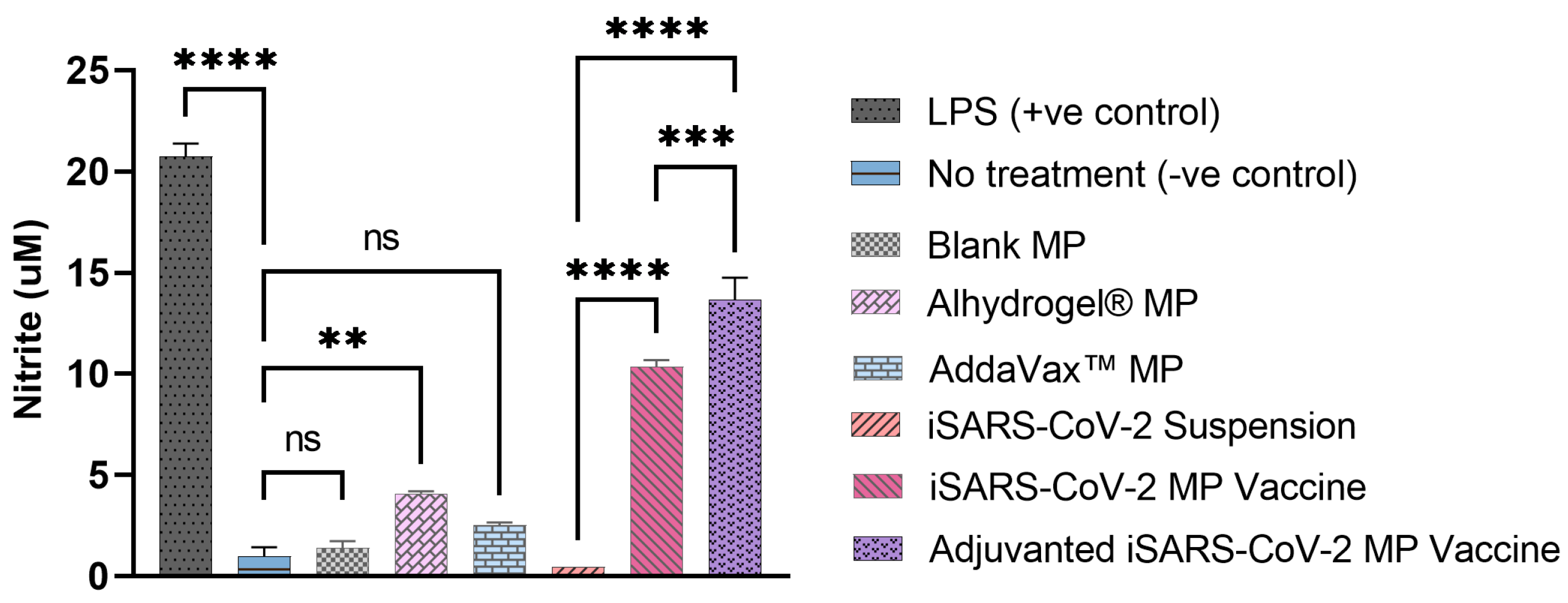
2.2.2. Evaluating the In Vitro Immunostimulatory Activity of the Vaccine MP
2.2.3. Determining the In Vitro Cytotoxicity of the Vaccine MP
2.2.4. Preparation of Vaccine-Loaded MNs
2.2.5. In Vivo Immunization Procedure and Dosing Regimen
2.2.6. Determining the Serum Antibody Levels in Immunized Mice
2.2.7. Evaluating the T-Cell Responses in Immunized Mice
2.2.8. Statistical Analysis
3. Results
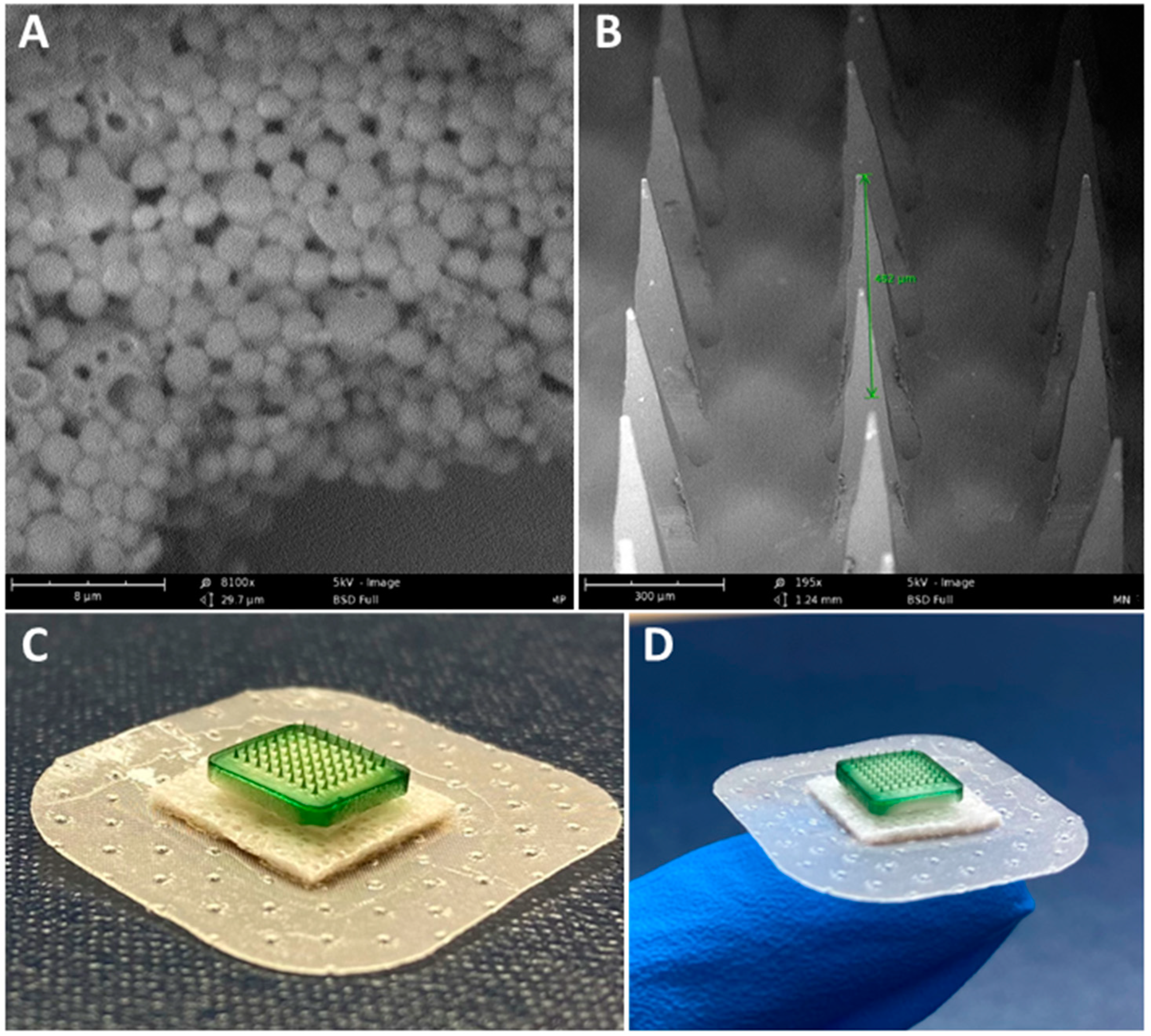
3.1. Characterization of Vaccine MP and MN
3.2. Vaccine MP Show Enhanced Immunostimulatory Activity In Vitro
3.3. Vaccine MP Are Non-Cytotoxic to DCs
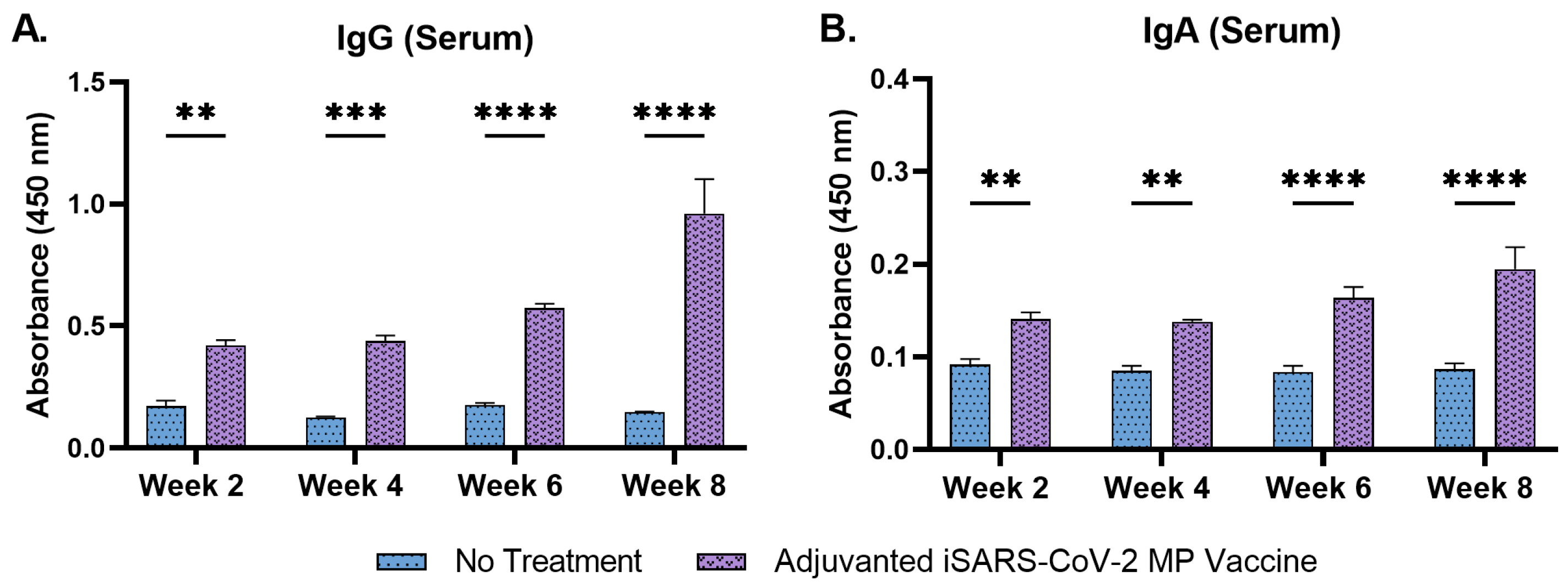
3.4. Adjuvanted Vaccine Increased Antibody Levels in Immunized Mice
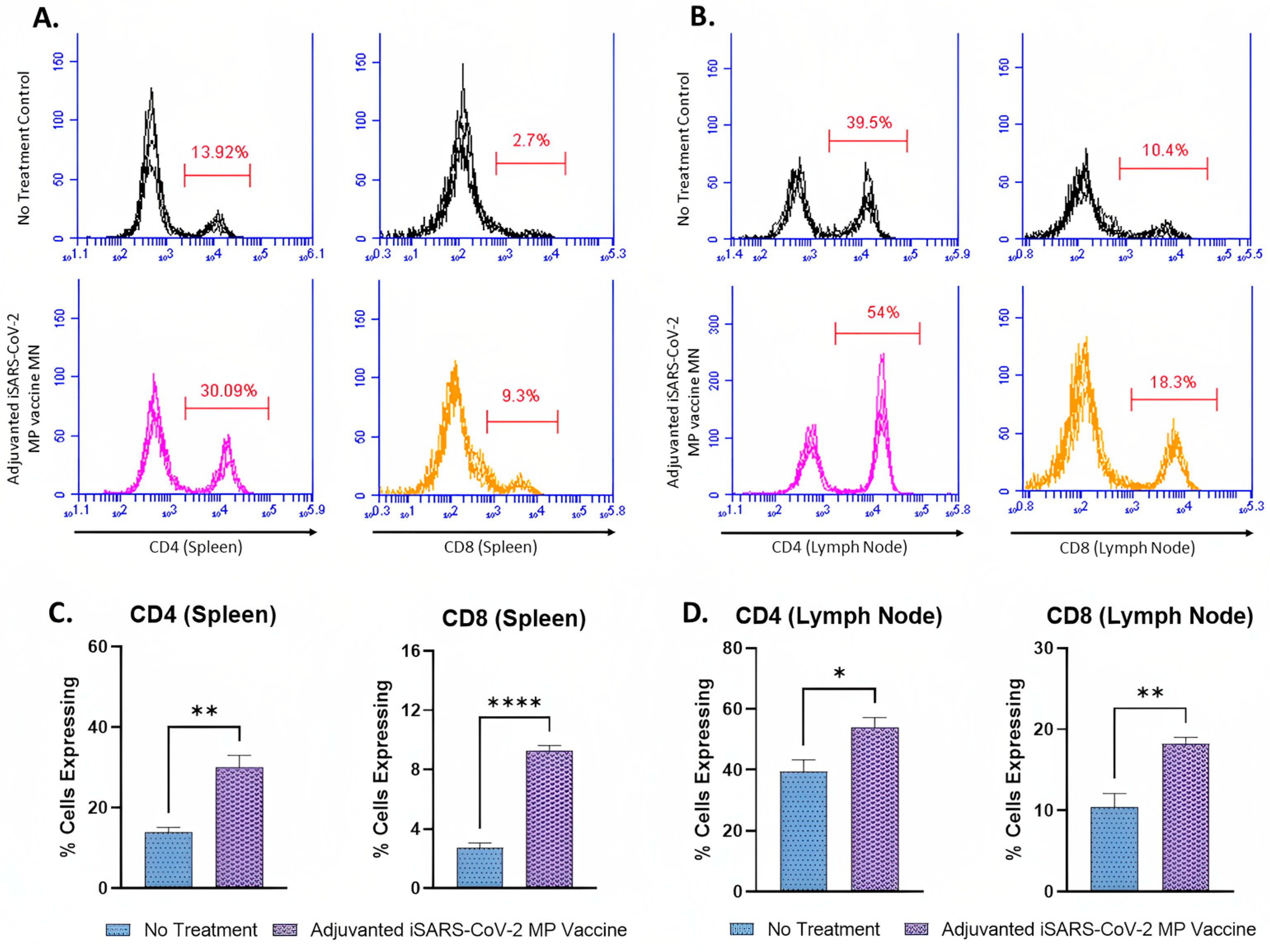
3.5. Adjuvanted Vaccine Produced T-Cell Responses in Immunized Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. COVID Data Tracker. Centers for Disease Control and Prevention 2020. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 22 February 2021).
- CDC. COVID-19 Vaccination. Centers for Disease Control and Prevention 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html (accessed on 6 January 2022).
- COVID-19 Vaccines|FDA, n.d. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 19 December 2022).
- Science Brief: Emerging SARS-CoV-2 Variants|CDC n.d. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-emerging-variants.html (accessed on 2 November 2021).
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prausnitz, M. A Phase I Study of the Safety, Reactogenicity, Acceptability and Immunogenicity of Inactivated Influenza Vaccine Delivered by Microneedle Patch or by Hypodermic Needle. 2019. Available online: clinicaltrials.gov (accessed on 7 March 2023).
- Kim, Y.-C.; Prausnitz, M.R. Enabling skin vaccination using new delivery technologies. Drug Deliv. Transl. Res. 2011, 1, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braz Gomes, K.; D’Souza, B.; Vijayanand, S.; Menon, I.; D’Souza, M.J. A dual-delivery platform for vaccination using antigen-loaded nanoparticles in dissolving microneedles. Int. J. Pharm. 2022, 613, 121393. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, T.; Liang, B.; Deng, H.; Wang, H.; Feng, X.; Quan, X.; Wang, X.; Li, S.; Lu, S.; et al. Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting. Front. Immunol. 2021, 12, 802858. [Google Scholar] [CrossRef]
- Satarker, S.; Nampoothiri, M. Structural Proteins in Severe Acute Respiratory Syndrome Coronavirus-2. Arch. Med. Res. 2020, 51, 482–491. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef]
- Batéjat, C.; Grassin, Q.; Manuguerra, J.-C.; Leclercq, I. Heat inactivation of the severe acute respiratory syndrome coronavirus 2. J. Biosaf. Biosecur. 2021, 3, 1–3. [Google Scholar] [CrossRef]
- Li, X.N.; Huang, Y.; Wang, W.; Jing, Q.L.; Zhang, C.H.; Qin, P.Z.; Guan, W.J.; Gan, L.; Li, Y.L.; Liu, W.H.; et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: A test-negative case-control real-world study. Emerg. Microbes Infect. 2021, 10, 1751–1759. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Ding, C.; He, J.; Zhang, X.; Jiang, C.; Sun, Y.; Zhang, Y.; Chen, Q.; He, H.; Li, W.; Xie, J.; et al. Crucial Mutations of Spike Protein on SARS-CoV-2 Evolved to Variant Strains Escaping Neutralization of Convalescent Plasmas and RBD-Specific Monoclonal Antibodies. Front. Immunol. 2021, 12, 693775. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Magazine, N.; Zhang, T.; Wu, Y.; McGee, M.C.; Veggiani, G.; Huang, W. Mutations and Evolution of the SARS-CoV-2 Spike Protein. Viruses 2022, 14, 640. [Google Scholar] [CrossRef] [PubMed]
- Viral Targets for Vaccines against COVID-19|Nature Reviews Immunology n.d. Available online: https://www.nature.com/articles/s41577-020-00480-0 (accessed on 19 December 2022).
- Fan, S.; Sun, W.; Fan, L.; Wu, N.; Sun, W.; Ma, H.; Chen, S.; Li, Z.; Li, Y.; Zhang, J.; et al. The highly conserved RNA-binding specificity of nucleocapsid protein facilitates the identification of drugs with broad anti-coronavirus activity. Comput. Struct. Biotechnol. J. 2022, 20, 5040–5044. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Lu, S.; Corbett, K.D. Structural Basis for SARS-CoV-2 Nucleocapsid Protein Recognition by Single-Domain Antibodies. Front. Immunol. 2021, 12, 719037. [Google Scholar] [CrossRef]
- Lopandić, Z.; Protić-Rosić, I.; Todorović, A.; Glamočlija, S.; Gnjatović, M.; Ćujic, D.; Gavrović-Jankulović, M. IgM and IgG Immunoreactivity of SARS-CoV-2 Recombinant M Protein. Int. J. Mol. Sci. 2021, 22, 4951. [Google Scholar] [CrossRef]
- Waeckerle-Men, Y.; Uetz-von Allmen, E.; Gander, B.; Scandella, E.; Schlosser, E.; Schmidtke, G.; Merkle, H.P.; Groettrup, M. Encapsulation of proteins and peptides into biodegradable poly(D,L-lactide-co-glycolide) microspheres prolongs and enhances antigen presentation by human dendritic cells. Vaccine 2006, 24, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Shastri, P.N.; Kim, M.-C.; Quan, F.-S.; D’Souza, M.J.; Kang, S.-M. Immunogenicity and protection of oral influenza vaccines formulated into microparticles. J. Pharm. Sci. 2012, 101, 3623–3635. [Google Scholar] [CrossRef] [Green Version]
- Gomes, K.B.; Menon, I.; Bagwe, P.; Bajaj, L.; Kang, S.-M.; D’Souza, M.J. Enhanced Immunogenicity of an Influenza Ectodomain Matrix-2 Protein Virus-like Particle (M2e VLP) Using Polymeric Microparticles for Vaccine Delivery. Viruses 2022, 14, 1920. [Google Scholar] [CrossRef]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable Particles as Vaccine Delivery Systems: Size Matters. AAPS J. 2012, 15, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.; Williamson, D.; Titball, R. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Park, K. Effects of the Microparticle Shape on Cellular Uptake. Mol. Pharm. 2016, 13, 2164–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.-F.; Pollock, K.; Brewer, J. Effects of PLGA microparticles on antigen presentation of bone marrow-derived dendritic cells in vitro. Chin. J. Biomed. Eng. 2005, 24, 35–37. [Google Scholar]
- Snapper, C.M. Distinct Immunologic Properties of Soluble Versus Particulate Antigens. Front. Immunol. 2018, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Menon, I.; Moo Kang, S.; D’Souza, M. Nanoparticle formulation of the fusion protein virus like particles of respiratory syncytial virus stimulates enhanced in vitro antigen presentation and autophagy. Int. J. Pharm. 2022, 623, 121919. [Google Scholar] [CrossRef]
- Kale, A.; Joshi, D.; Menon, I.; Bagwe, P.; Patil, S.; Vijayanand, S.; Gomes, K.B.; D’Souza, M. Novel microparticulate Zika vaccine induces a significant immune response in a preclinical murine model after intramuscular administration. Int. J. Pharm. 2022, 624, 121975. [Google Scholar] [CrossRef]
- Vijayanand, S.; Patil, S.; Joshi, D.; Menon, I.; Braz Gomes, K.; Kale, A.; Bagwe, P.; Yacoub, S.; Uddin, M.N.; D’Souza, M.J. Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus. Vaccines 2022, 10, 1491. [Google Scholar] [CrossRef]
- Escobar-García, J.D.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M. Room Temperature Nanoencapsulation of Bioactive Eicosapentaenoic Acid Rich Oil within Whey Protein Microparticles. Nanomaterials 2021, 11, 575. [Google Scholar] [CrossRef]
- Thomas, H.; Fries, F.; Gmelch, M.; Bärschneider, T.; Kroll, M.; Vavaleskou, T.; Reineke, S. Purely Organic Microparticles Showing Ultralong Room Temperature Phosphorescence. ACS Omega 2021, 6, 13087–13093. [Google Scholar] [CrossRef]
- Kaplan, I.; Yüksel, H.; Evliyaoğlu, O.; Basarali, M.K.; Toprak, G.; Çolpan, L.; Şen, V. Effects of Storage Temperature and Time on Stability of Serum Tacrolimus and Cyclosporine A Levels in Whole Blood by LC-MS/MS. Int. J. Anal. Chem. 2015, 2015, 956389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, D.J.; Kaplan, D.L. Poly (lactic-co-glycolic acid) controlled release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833. [Google Scholar] [CrossRef]
- Adjuvants and Vaccines|Vaccine Safety|CDC 2020. Available online: https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html (accessed on 1 July 2022).
- Alhydrogel® Adjuvant 2%. InvivoGen 2016. Available online: https://www.invivogen.com/alhydrogel (accessed on 1 July 2022).
- Brewer, J.M. (How) do Aluminium Adjuvants Work? Immunol. Lett. 2006, 102, 10–15. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccin. Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Nian, X.; Zhang, J.; Deng, T.; Liu, J.; Gong, Z.; Lv, C.; Yao, L.; Li, J.; Huang, S.; Yang, X. AddaVax Formulated with PolyI:C as a Potential Adjuvant of MDCK-based Influenza Vaccine Enhances Local, Cellular, and Antibody Protective Immune Response in Mice. AAPS PharmSciTech 2021, 22, 270. [Google Scholar] [CrossRef]
- AddaVaxTM. InvivoGen 2016. Available online: https://www.invivogen.com/addavax (accessed on 1 July 2022).
- Wilkins, A.L.; Kazmin, D.; Napolitani, G.; Clutterbuck, E.A.; Pulendran, B.; Siegrist, C.A.; Pollard, A.J. AS03- and MF59-Adjuvanted Influenza Vaccines in Children. Front. Immunol. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, D.; Gala, R.P.; Uddin, M.N.; D’Souza, M.J. Novel ablative laser mediated transdermal immunization for microparticulate measles vaccine. Int. J. Pharm. 2021, 606, 120882. [Google Scholar] [CrossRef]
- Gala, R.P.; Zaman, R.U.; D’Souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, D.; Chbib, C.; Uddin, M.N.; D’Souza, M.J. Evaluation of Microparticulate (S)-4,5-Dihydroxy-2,3-pentanedione (DPD) as a Potential Vaccine Adjuvant. AAPS J. 2021, 23, 84. [Google Scholar] [CrossRef]
- Braz Gomes, K.; D’Sa, S.; Allotey-Babington, G.L.; Kang, S.-M.; D’Souza, M.J. Transdermal Vaccination with the Matrix-2 Protein Virus-like Particle (M2e VLP) Induces Immunity in Mice against Influenza A Virus. Vaccines 2021, 9, 1324. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Chapter 3—Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar] [CrossRef]
- Uehara, E.U.; Shida, B.D.; de Brito, C.A. Role of nitric oxide in immune responses against viruses: Beyond microbicidal activity. Inflamm. Res. 2015, 64, 845–852. [Google Scholar] [CrossRef]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdan, C.; Röllinghoff, M.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P. Nitric oxide and immune response. Indian J. Biochem. Biophys. 2007, 44, 310–319. [Google Scholar]
- Frontiers|Redefining the Role of Langerhans Cells as Immune Regulators within the Skin n.d. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2017.01941/full (accessed on 4 August 2022).
- Mazzini, L.; Martinuzzi, D.; Hyseni, I.; Benincasa, L.; Molesti, E.; Casa, E.; Lapini, G.; Piu, P.; Trombetta, C.M.; Marchi, S.; et al. Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J. Immunol. Methods 2021, 489, 112937. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Aleebrahim-Dehkordi, E.; Molavi, B.; Mokhtari, M.; Deravi, N.; Fathi, M.; Fazel, T.; Mohebalizadeh, M.; Koochaki, P.; Shobeiri, P.; Hasanpour-Dehkordi, A. T helper type (Th1/Th2) responses to SARS-CoV-2 and influenza A (H1N1) virus: From cytokines produced to immune responses. Transpl. Immunol. 2022, 70, 101495. [Google Scholar] [CrossRef] [PubMed]
- Carty, S.A.; Riese, M.J.; Koretzky, G.A. Chapter 21—T-Cell Immunity. In Hematology, 7th ed.; Hoffman, R., Benz, E.J., Silberstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E., Abutalib, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–239. [Google Scholar] [CrossRef]
- Ganneru, B.; Jogdand, H.; Daram, V.K.; Das, D.; Molugu, N.R.; Prasad, S.D.; Kannappa, S.V.; Ella, K.M.; Ravikrishnan, R.; Awasthi, A.; et al. Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation. IScience 2021, 24, 102298. [Google Scholar] [CrossRef] [PubMed]
- Getahun, A.; Dahlström, J.; Wernersson, S.; Heyman, B. IgG2a-Mediated Enhancement of Antibody and T Cell Responses and Its Relation to Inhibitory and Activating Fcγ Receptors1. J. Immunol. 2004, 172, 5269–5276. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayanand, S.; Patil, S.; Menon, I.; Braz Gomes, K.; Kale, A.; Bagwe, P.; Uddin, M.N.; Zughaier, S.M.; D’Souza, M.J. An Adjuvanted Inactivated SARS-CoV-2 Microparticulate Vaccine Delivered Using Microneedles Induces a Robust Immune Response in Vaccinated Mice. Pharmaceutics 2023, 15, 895. https://doi.org/10.3390/pharmaceutics15030895
Vijayanand S, Patil S, Menon I, Braz Gomes K, Kale A, Bagwe P, Uddin MN, Zughaier SM, D’Souza MJ. An Adjuvanted Inactivated SARS-CoV-2 Microparticulate Vaccine Delivered Using Microneedles Induces a Robust Immune Response in Vaccinated Mice. Pharmaceutics. 2023; 15(3):895. https://doi.org/10.3390/pharmaceutics15030895
Chicago/Turabian StyleVijayanand, Sharon, Smital Patil, Ipshita Menon, Keegan Braz Gomes, Akanksha Kale, Priyal Bagwe, Mohammad N. Uddin, Susu M. Zughaier, and Martin J. D’Souza. 2023. "An Adjuvanted Inactivated SARS-CoV-2 Microparticulate Vaccine Delivered Using Microneedles Induces a Robust Immune Response in Vaccinated Mice" Pharmaceutics 15, no. 3: 895. https://doi.org/10.3390/pharmaceutics15030895
APA StyleVijayanand, S., Patil, S., Menon, I., Braz Gomes, K., Kale, A., Bagwe, P., Uddin, M. N., Zughaier, S. M., & D’Souza, M. J. (2023). An Adjuvanted Inactivated SARS-CoV-2 Microparticulate Vaccine Delivered Using Microneedles Induces a Robust Immune Response in Vaccinated Mice. Pharmaceutics, 15(3), 895. https://doi.org/10.3390/pharmaceutics15030895











