Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review
Abstract
:1. Introduction
2. Instability of Peptide and the Possible Causes of Degradation
2.1. Hydrolytic Pathways
2.1.1. Chain Cleavage of the Peptide Backbone
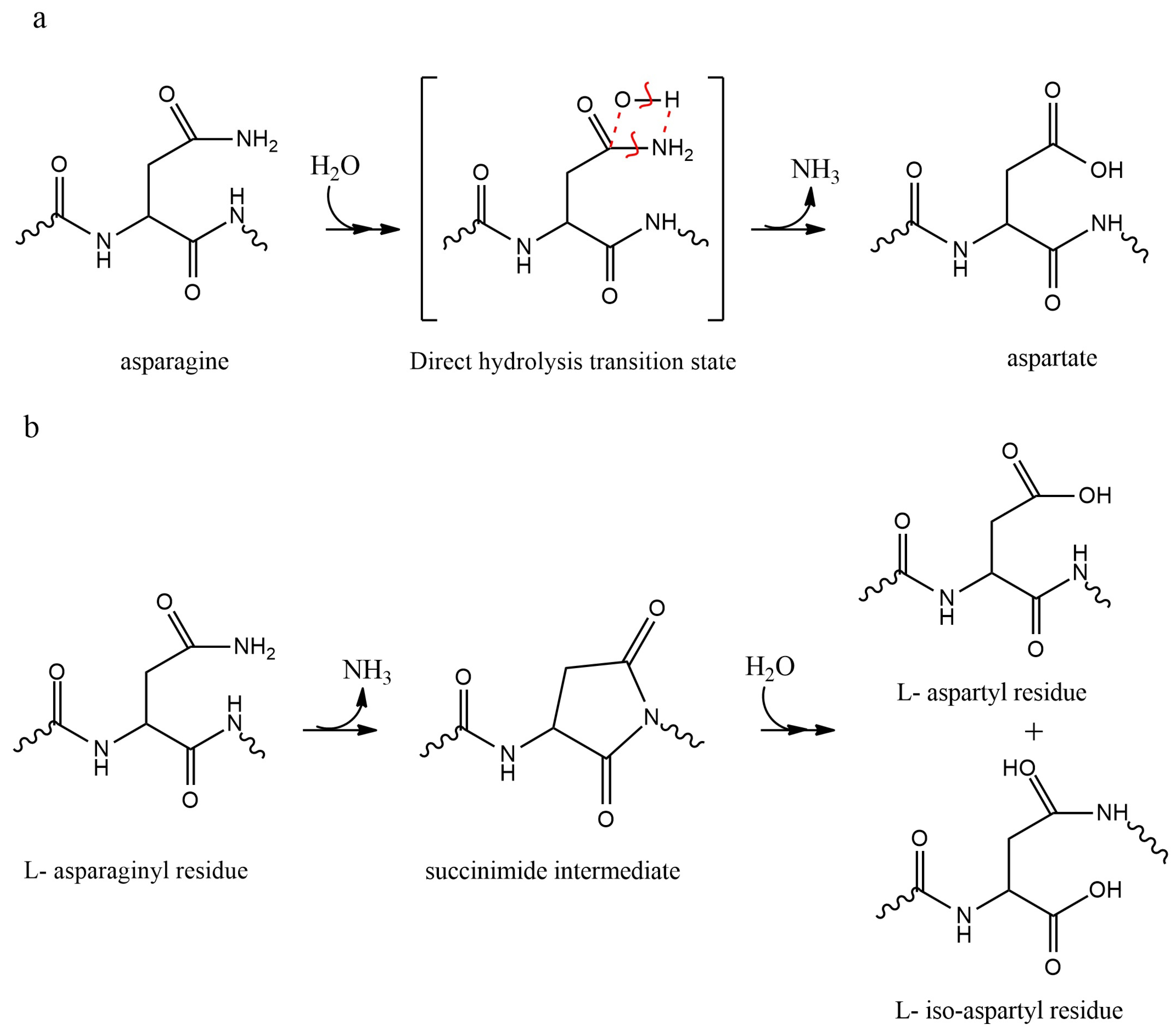
2.1.2. Deamidation of Asn and Gln Residues
2.1.3. Isomerization of Asp Residues
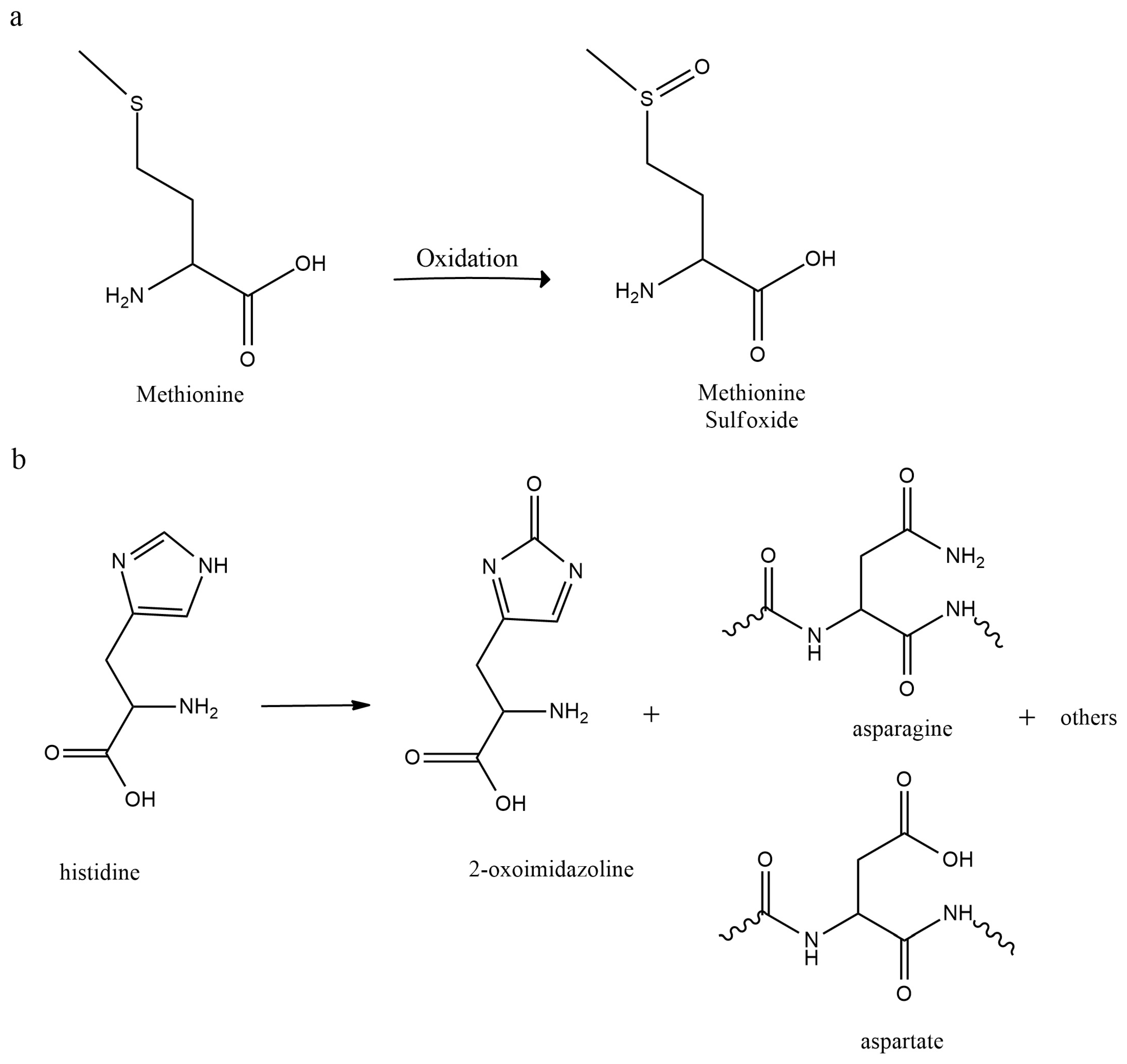
2.2. Oxidative Pathways
2.2.1. Autoxidation
2.2.2. Metal Induced Oxidation
2.2.3. Light-Induced Oxidation
2.2.4. Peroxide Oxidation
2.3. β-Elimination
2.4. Disulfide Exchange
2.5. Dimerization, Aggregation, and Precipitation
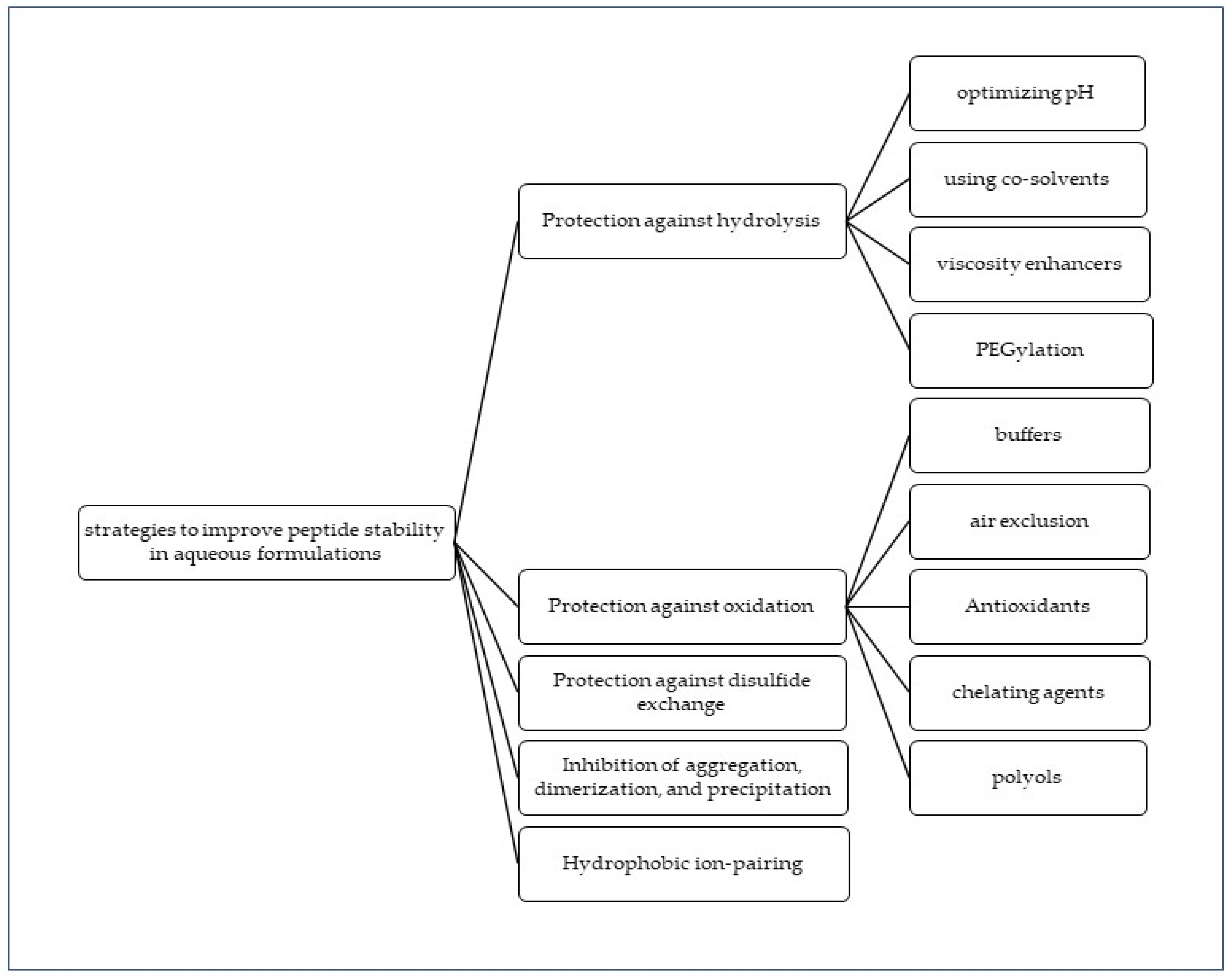
3. Strategies to Optimize Peptide Stability in Aqueous Formulations
3.1. Protection against Hydrolysis
3.1.1. pH Optimization
3.1.2. The Use of Co-Solvents
3.1.3. Viscosity Enhancement
3.1.4. PEGylation
3.2. Protection against Oxidation
3.2.1. Buffers
3.2.2. Air Exclusion
3.2.3. Antioxidants
3.2.4. Chelating Agents
3.2.5. Polyols
3.3. Protection against Disulfide Exchange Reaction
3.4. Inhibition of Aggregation, Dimerization, and Precipitation
3.5. Hydrophobic Ion-Pairing (HIP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- du Vigneaud, V.; Ressler, C.; Swan, J.M.; Roberts, C.W.; Katsoyannis, P.G. The Synthesis of Oxytocin1. J. Am. Chem. Soc. 1954, 76, 3115–3121. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ghosh, D.; Williams, R.O. Just how prevalent are peptide therapeutic products? A critical review. Int. J. Pharm. 2020, 587, 119491. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Datta, S.P.; Smith, G.H.; Campbell, P.N.; Bentley, R.; McKenzie, H.A.; Jakoby, W.B. Oxford dictionary of biochemistry and molecular biology. Trends Biochem. Sci. 1998, 3, 228. [Google Scholar]
- Timmons, P.B.; Hewage, C.M. Biophysical study of the structure and dynamics of the antimicrobial peptide maximin 1. J. Pept. Sci. 2022, 28, e3370. [Google Scholar] [CrossRef] [PubMed]
- Rogne, P.; Fimland, G.; Nissen-Meyer, J.; Kristiansen, P.E. Three-dimensional structure of the two peptides that constitute the two-peptide bacteriocin lactociccin G. Biochim. Biophys. Acta 2008, 1784, 543–554. [Google Scholar] [CrossRef]
- Ohtake, S.; Kita, Y.; Payne, R.; Manning, M.; Arakawa, T. Structural characteristics of short peptides in solution. Protein Pept. Lett. 2013, 20, 1308–1323. [Google Scholar] [CrossRef]
- Bray, D. Protein molecules as computational elements in living cells. Nature 1995, 376, 307–312. [Google Scholar] [CrossRef]
- Khavinson, V.K.; Popovich, I.G.; Linkova, N.S.; Mironova, E.S.; Ilina, A.R. Peptide Regulation of Gene Expression: A Systematic Review. Molecules 2021, 26, 7053. [Google Scholar] [CrossRef]
- Vaudry, H.; Tonon, M.-C.; Vaudry, D. Editorial: Trends in Regulatory Peptides. Front. Endocrinol. 2018, 9, 125. [Google Scholar] [CrossRef]
- F.D.A. ANDAs for Certain Highly Purified Synthetic Peptide Drug Products That Refer to Listed Drugs of rDNA Origin: Guidance. 2021. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/andas-certain-highly-purified-synthetic-peptide-drug-products-refer-listed-drugs-rdna-origin (accessed on 4 January 2023).
- Malavolta, L.; Cabral, F.R. Peptides: Important tools for the treatment of central nervous system disorders. Neuropeptides 2011, 45, 309–316. [Google Scholar] [CrossRef]
- Forbes, J.; Krishnamurthy, K. Biochemistry, Peptide; StatPearls: Tampa, Florida, 2022. [Google Scholar]
- Papini, A.M. Peptide Chemistry Revolution. Chem. Today 2011, 29, 26–27. [Google Scholar]
- Jin, L.; Boyd, B.J.; White, P.J.; Pennington, M.W.; Norton, R.S.; Nicolazzo, J.A. Buccal mucosal delivery of a potent peptide leads to therapeutically-relevant plasma concentrations for the treatment of autoimmune diseases. J. Control. Release 2015, 199, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Baptista, P.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Combination of PLGA nanoparticles with mucoadhesive guar-gum films for buccal delivery of antihypertensive peptide. Int. J. Pharm. 2018, 547, 593–601. [Google Scholar] [CrossRef]
- Marciello, M.; Rossi, S.; Caramella, C.; Remuñán-López, C. Freeze-dried cylinders carrying chitosan nanoparticles for vaginal peptide delivery. Carbohydr. Polym. 2017, 170, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Shah, S.G.; Yadav, S.; Chugh, A. Novel corneal targeting cell penetrating peptide as an efficient nanocarrier with an effective antimicrobial activity. Eur. J. Pharm. Biopharm. 2021, 166, 216–226. [Google Scholar] [CrossRef]
- Fujiyama, T.; Oze, I.; Yagi, H.; Hashizume, H.; Matsuo, K.; Hino, R.; Kamo, R.; Imayama, S.; Hirakawa, S.; Ito, T.; et al. Induction of cytotoxic T cells as a novel independent survival factor in malignant melanoma with percutaneous peptide immunization. J. Dermatol. Sci. 2014, 75, 43–48. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Banerjee, G.B.; Mandal, A. Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. Int. J. Gynecol. Obstet. 2010, 109, 25–29. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; Khalmuratova, R.; Kang, S.; Lee, M.; Song, Y.; Park, J.-W.; Yu, J.; Shin, H.-W.; Lee, Y. α-Helical cell-penetrating peptide-mediated nasal delivery of resveratrol for inhibition of epithelial-to-mesenchymal transition. J. Control. Release 2020, 317, 181–194. [Google Scholar] [CrossRef]
- Dillon, C.; Hughes, H.; O’Reilly, N.J.; McLoughlin, P. Formulation and characterisation of dissolving microneedles for the transdermal delivery of therapeutic peptides. Int. J. Pharm. 2017, 526, 125–136. [Google Scholar] [CrossRef]
- Andrade, F.; das Neves, J.; Gener, P.; Schwartz, S.; Ferreira, D.; Oliva, M.; Sarmento, B. Biological assessment of self-assembled polymeric micelles for pulmonary administration of insulin. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Satterwhite, J.; Heathman, M.; Miller, P.D.; Marín, F.; Glass, E.V.; Dobnig, H. Pharmacokinetics of Teriparatide (rhPTH[1–34]) and Calcium Pharmacodynamics in Postmenopausal Women with Osteoporosis. Calcif. Tissue Int. 2010, 87, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Hawe, A.; Poole, R.; Romeijn, S.; Kasper, P.; van der Heijden, R.; Jiskoot, W. Towards Heat-stable Oxytocin Formulations: Analysis of Degradation Kinetics and Identification of Degradation Products. Pharm. Res. 2009, 26, 1679–1688. [Google Scholar] [CrossRef] [Green Version]
- WHO. Q1F Stability Guideline: Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical products. 2018. Available online: https://bit.ly/3lhmy2R (accessed on 4 January 2023).
- Hovgaard, L.; Frokjaer, S.; Van De Weert, M. Pharmaceutical Formulation Development of Peptides and Proteins, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- FDA. Lyophilization of Parenteral. 2014. Available online: https://bit.ly/3HTG0Lx (accessed on 4 January 2023).
- Curry, W.; Conway, S.; Goodfield, C.; Miller, K.; Mueller, R.L.; Polini, E. Reducing the Risk of Contamination of Sterile Parenteral Products via Ready-to-Use Closure Components. AAPS PharmSciTech 2010, 11, 1572–1579. [Google Scholar] [CrossRef] [Green Version]
- Zapadka, K.L.; Becher, F.J.; Dos Santos, A.L.G.; Jackson, S.E. Factors affecting the physical stability (aggregation) of peptide therapeutics. Interface Focus 2017, 7, 20170030. [Google Scholar] [CrossRef] [Green Version]
- Manning, M.C.; Chou, D.K.; Murphy, B.M.; Payne, R.W.; Katayama, D.S. Stability of Protein Pharmaceuticals: An Update. Pharm. Res. 2010, 27, 544–575. [Google Scholar] [CrossRef]
- Li, S.; Schöneich, C.; Borchardt, R.T. Chemical instability of protein pharmaceuticals: Mechanisms of oxidation and strategies for stabilization. Biotechnol. Bioeng. 1995, 48, 490–500. [Google Scholar] [CrossRef]
- Topp, E.M.; Zhang, L.; Zhao, H.; Payne, R.W.; Evans, G.J.; Manning, M.C. Chemical Instability in Peptide and Protein Pharmaceuticals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 41–67. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Finnman, J.; Flipo, M.; Galyean, R.; Schteingart, C.D. On the mechanism of degradation of oxytocin and its analogues in aqueous solution. Pept. Sci. 2013, 100, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.L.; Wong, R.L.; Zhang, Y.T.; Kao, Y.-H.; Wang, Y.J. Asparagine Deamidation Dependence on Buffer Type, pH, and Temperature. J. Pharm. Sci. 2013, 102, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Malm, M.; Madsen, I.; Kjellström, J. Development and stability of a heat-stable formulation of carbetocin for the prevention of postpartum haemorrhage for use in low and middle-income countries. J. Pept. Sci. 2018, 24, e3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avanti, C.; Oktaviani, N.A.; Hinrichs, W.L.; Frijlink, H.W.; Mulder, F.A. Aspartate buffer and divalent metal ions affect oxytocin in aqueous solution and protect it from degradation. Int. J. Pharm. 2013, 444, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Avanti, C.; Permentier, H.; Van Dam, A.; Poole, R.; Jiskoot, W.; Frijlink, H.W.; Hinrichs, W. A New Strategy to Stabilize Oxytocin in Aqueous Solutions: II. Suppression of Cysteine-Mediated Intermolecular Reactions by a Combination of Divalent Metal Ions and Citrate. Mol. Pharm. 2012, 9, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.J.; Liu, D.; Tran, V.P.; Wu, Z.; Jiang, K.; Zhu, H.; Zhang, J.; Gibbons, C.; Xue, B.; Shi, H.; et al. N-Linked Glycosylation Prevents Deamidation of Glycopeptide and Glycoprotein. ACS Chem. Biol. 2020, 15, 3197–3205. [Google Scholar] [CrossRef]
- Hagen, N.; Bizimana, T.; Kayumba, P.C.; Khuluza, F.; Heide, L. Stability of Oxytocin Preparations in Malawi and Rwanda: Stabilizing Effect of Chlorobutanol. Am. J. Trop. Med. Hyg. 2020, 103, 2129–2141. [Google Scholar] [CrossRef]
- Benet, A.; Halseth, T.; Kang, J.; Kim, A.; Ackermann, R.; Srinivasan, S.; Schwendeman, S.; Schwendeman, A. The Effects of pH and Excipients on Exenatide Stability in Solution. Pharmaceutics 2021, 13, 1263. [Google Scholar] [CrossRef]
- Beard, R.; Stucki, A.; Schmitt, M.; Py, G.; Grundschober, C.; Gee, A.D.; Tate, E.W. Building bridges for highly selective, potent and stable oxytocin and vasopressin analogs. Bioorg. Med. Chem. 2018, 26, 3039–3045. [Google Scholar] [CrossRef]
- Ghasemisarabbadieh, M.; Sigurdsson, S.J.; Dong, F.V.; Gizurarson, S.; Sveinbjörnsson, B.R. The effect of D-(+)-glucosamine, N-acetyl-D-glucosamine and tetraethylene glycol on the stability of oxytocin in aqueous solution. Die. Pharm. Int. J. Pharm. Sci. 2021, 76, 480–483. [Google Scholar] [CrossRef]
- Ghasemisarabbadieh, M.; Gizurarson, S.; Sveinbjörnsson, B.R. The effect of trehalose, antioxidants, and acetate buffer concentration on oxytocin stability. J. Pept. Sci. 2021, 27, e3324. [Google Scholar] [CrossRef]
- Neves-Petersen, M.T.; Klitgaard, S.; Pascher, T.; Skovsen, E.; Polivka, T.; Yartsev, A.; Sundström, V.; Petersen, S.B. Flash Photolysis of Cutinase: Identification and Decay Kinetics of Transient Intermediates Formed upon UV Excitation of Aromatic Residues. Biophys. J. 2009, 97, 211–226. [Google Scholar] [CrossRef] [Green Version]
- Neves-Petersen, M.T.; Jonson, P.H.; Petersen, S.B. Amino acid neighbours and detailed conformational analysis of cysteines in proteins. Protein Eng. Des. Sel. 1999, 12, 535–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creed, D. The photophysics and photochemistry of the near-uv absorbing amino Acids-I. tryptophan and its simple derivatives. Photochem. Photobiol. 1984, 39, 537–562. [Google Scholar] [CrossRef]
- Mozziconacci, O.; Schöneich, C. Photodegradation of Oxytocin and Thermal Stability of Photoproducts. J. Pharm. Sci. 2012, 101, 3331–3346. [Google Scholar] [CrossRef] [PubMed]
- Schöneich, C.; Williams, T.D. Cu(II)-Catalyzed Oxidation of β-Amyloid Peptide Targets His13 and His14 over His6: Detection of 2-Oxo-histidine by HPLC-MS/MS. Chem. Res. Toxicol. 2002, 15, 717–722. [Google Scholar] [CrossRef]
- Csire, G.; Turi, I.; Sóvágó, I.; Kárpáti, E.; Kállay, C. Complex formation processes and metal ion catalyzed oxidation of model peptides related to the metal binding site of the human prion protein. J. Inorg. Biochem. 2020, 203, 110927. [Google Scholar] [CrossRef]
- Law, S.L.; Huang, K.J.; Chou, V.H.Y. Stability of Desmopressin Loaded in Liposomes. J. Liposome Res. 2003, 13, 269–277. [Google Scholar] [CrossRef]
- Hawe, A.; Fries, W. Formulation Development for Hydrophobic Therapeutic Proteins. Pharm. Dev. Technol. 2007, 12, 223–237. [Google Scholar] [CrossRef]
- Ambrosio, E.; Podmore, A.; dos Santos, A.L.G.; Magarkar, A.; Bunker, A.; Caliceti, P.; Mastrotto, F.; van der Walle, C.F.; Salmaso, S. Control of Peptide Aggregation and Fibrillation by Physical PEGylation. Biomacromolecules 2018, 19, 3958–3969. [Google Scholar] [CrossRef]
- Bothe, J.R.; Andrews, A.; Smith, K.J.; Joyce, L.A.; Krishnamachari, Y.; Kashi, S. Peptide Oligomerization Memory Effects and Their Impact on the Physical Stability of the GLP-1 Agonist Liraglutide. Mol. Pharm. 2019, 16, 2153–2161. [Google Scholar] [CrossRef]
- Korang-Yeboah, M.; Ketcham, S.; Shih, M.; Ako-Adounvo, A.-M.; Zhang, J.; Bandaranayake, B.M.; Abbey-Berko, Y.; Faustino, P.; Ashraf, M. Effect of formulation and peptide folding on the fibrillar aggregation, gelation, and oxidation of a therapeutic peptide. Int. J. Pharm. 2021, 604, 120677. [Google Scholar] [CrossRef]
- Rastogi, N.; Mitra, K.; Kumar, D.; Roy, R. Metal Ions as Cofactors for Aggregation of Therapeutic Peptide Salmon Calcitonin. Inorg. Chem. 2012, 51, 5642–5650. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhang, R.; Li, X.; Wang, A.; Chen, D.; Sun, K.; Liu, W.; Li, Y. Stability of exenatide in poly(d,l-lactide-co-glycolide) solutions: A simplified investigation on the peptide degradation by the polymer. Eur. J. Pharm. Sci. 2013, 50, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Høgstedt, U.B.; Østergaard, J.; Weiss, T.; Sjögren, H.; van de Weert, M. Manipulating Aggregation Behavior of the Uncharged Peptide Carbetocin. J. Pharm. Sci. 2018, 107, 838–847. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, X.; Xu, W. Fibril Nucleation Kinetics of a Pharmaceutical Peptide: The Role of Conformation Stability, Formulation Factors, and Temperature Effect. Mol. Pharm. 2018, 15, 5591–5601. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.C.; Patel, K.; Borchardt, R.T. Stability of Protein Pharmaceuticals. Pharm. Res. J. Am Assoc. Pharm. Sci. 1989, 6, 903–918. [Google Scholar] [CrossRef]
- Hoitink, M.A.; Beijnen, J.H.; Boschma, M.U.S.; Bult, A.; Hop, E.; Nijholt, J.; Versluis, C.; Wiese, G.; Underberg, W.J.M. Identification of the Degradation Products of Gonadorelin and Three Analogues in Aqueous Solution. Anal. Chem. 1997, 69, 4972–4978. [Google Scholar] [CrossRef]
- Strickley, R.G.; Brandl, M.; Chan, K.W.; Straub, K.; Gu, L. High-Performance Liquid Chromatographic (HPLC) and HPLC-Mass Spectrometric (MS) Analysis of the Degradation of the Luteinizing Hormone-Releasing Hormone (LH-RH) Antagonist RS-26306 in Aqueous Solution. Pharm. Res. 1990, 7, 530–536. [Google Scholar] [CrossRef]
- Helm, V.J.; Müller, B.W. Stability of Gonadorelin and Triptorelin in Aqueous Solution. Pharm. Res. 1990, 7, 1253–1256. [Google Scholar] [CrossRef]
- Hoitink, M.A.; Beijnen, J.H.; Bult, A.; van der Houwen, O.A.; Nijholt, J.; Underberg, W.J. Degradation Kinetics of Gonadorelin in Aqueous Solution. J. Pharm. Sci. 1996, 85, 1053–1059. [Google Scholar] [CrossRef]
- Senderoff, R.I.; Kontor, K.M.; Kreilgaard, L.; Chang, J.J.; Patel, S.; Krakover, J.; Heffernan, J.K.; Snell, L.B.; Rosenberg, G.B. Consideration of Conformational Transitions And Racemization During Process Development of Recombinant Glucagon-Like Peptide-1. J. Pharm. Sci. 1998, 87, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Nabuchi, Y.; Fujiwara, E.; Kuboniwa, H.; Asoh, Y.; Ushio, H. The stability and degradation pathway of recombinant human par-athyroid hormone: Deamidation of asparaginyl residue and peptide bond cleavage at aspartyl and asparaginyl residues. Pharm. Res. 1997, 14, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Ashen, D.S.; Salmona, M.; Fariña, J.B.; Llabrés, M. Solid-state stability studies of cholecystokinin (CCK-4) peptide under nonisothermal conditions using thermal analysis, chromatography and mass spectrometry. Eur. J. Pharm. Sci. 2010, 39, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Woo, B.H.; Lee, J.T.; Moon, S.C.; Lee, K.C.; Deluca, P.P. Stability of Octastatin, a Somatostatin Analog Cyclic Octapeptide, in Aqueous Solution. Pharm. Dev. Technol. 1997, 2, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Bodmeier, R. Degradation Kinetics of Somatostatin in Aqueous Solution. Drug Dev. Ind. Pharm. 2003, 29, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.P.; Patel, K.; Borchardt, R.T. Chemical Pathways of Peptide Degradation. I. Deamidation of Adrenocorticotropic Hormone. Pharm. Res. 1990, 7, 593–599. [Google Scholar] [CrossRef]
- Geiger, T.; Clarke, S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 1987, 262, 785–794. [Google Scholar] [CrossRef]
- Reissner, K.J.; Aswad, D.W. Deamidation and isoaspartate formation in proteins: Unwanted alterations or surreptitious signals? Cell. Mol. Life Sci. 2003, 60, 1281–1295. [Google Scholar] [CrossRef]
- Kato, K.; Nakayoshi, T.; Kurimoto, E.; Oda, A. Mechanisms of Deamidation of Asparagine Residues and Effects of Main-Chain Conformation on Activation Energy. Int. J. Mol. Sci. 2020, 21, 7035. [Google Scholar] [CrossRef]
- Patel, K.; Borchardt, R.T. Chemical Pathways of Peptide Degradation. III. Effect of Primary Sequence on the Pathways of Deamidation of Asparaginyl Residues in Hexapeptides. Pharm. Res. 1990, 7, 787–793. [Google Scholar] [CrossRef]
- Patel, K.; Borchardt, R.T. Chemical Pathways of Peptide Degradation. II. Kinetics of Deamidation of an Asparaginyl Residue in a Model Hexapeptide. Pharm. Res. 1990, 7, 703–711. [Google Scholar] [CrossRef]
- Lee, K.C.; Lee, Y.J.; Song, H.M.; Chun, C.J.; De Luca, P.P. Degradation of Synthetic Salmon Calcitonin in Aqueous Solution. Pharm. Res. 1992, 9, 1521–1523. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, S.-L. Advances in simultaneous DSC–FTIR microspectroscopy for rapid solid-state chemical stability studies: Some dipeptide drugs as examples. Adv. Drug Deliv. Rev. 2012, 64, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.H.L. Peptide and Protein Drug Delivery; Marcel Dekker, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Robinson, N.E. Protein deamidation. Proc. Natl. Acad. Sci. USA 2002, 99, 5283–5288. [Google Scholar] [CrossRef] [Green Version]
- Chu, G.C.; Chelius, D.; Xiao, G.; Khor, H.K.; Coulibaly, S.; Bondarenko, P.V. Accumulation of Succinimide in a Recombinant Monoclonal Antibody in Mildly Acidic Buffers Under Elevated Temperatures. Pharm. Res. 2007, 24, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Sargaeva, N.P.; Goloborodko, A.A.; O’Connor, P.B.; Moskovets, E.; Gorshkov, M.V. Sequence-specific predictive chromatography to assist mass spectrometric analysis of asparagine deamidation and aspartate isomerization in peptides. Electrophoresis 2011, 32, 1962–1969. [Google Scholar] [CrossRef]
- Wakankar, A.A.; Borchardt, R.T. Formulation considerations for proteins susceptible to asparagine deamidation and aspartate isomerization. J. Pharm. Sci. 2006, 95, 2321–2336. [Google Scholar] [CrossRef]
- Oliyai, C.; Borchardt, R.T. Chemical Pathways of Peptide Degradation. IV. Pathways, Kinetics, and Mechanism of Degradation of an Aspartyl Residue in a Model Hexapeptide. Pharm. Res. 1993, 10, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Catak, S.; Monard, G.; Aviyente, V.; Ruiz-López, M.F. Deamidation of asparagine residues: Direct hydrolysis versus succin-imide-mediated deamidation mechanisms. J. Phys. Chem. A. 2009, 113, 1111–1120. [Google Scholar] [CrossRef]
- Solomons, T.W.G.; Fryhle, C. Organic Chemistry, 10th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Li, S.; Schöneich, C.; Borchardt, R.T. Chemical Pathways of Peptide Degradation. VIII. Oxidation of Methionine in Small Model Peptides by Prooxidant/Transition Metal Ion Systems: Influence of Selective Scavengers for Reactive Oxygen Intermediates. Pharm. Res. 1995, 12, 348–355. [Google Scholar] [CrossRef]
- Nauser, T.; Koppenol, W.H.; Schöneich, C. Reversible Hydrogen Transfer Reactions in Thiyl Radicals from Cysteine and Related Molecules: Absolute Kinetics and Equilibrium Constants Determined by Pulse Radiolysis. J. Phys. Chem. B 2012, 116, 5329–5341. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Fang, Y.; Chen, Y.; Liu, J. Reactions of Deprotonated Tyrosine and Tryptophan with Electronically Excited Singlet Molecular Oxygen (a1Δg): A Guided-Ion-Beam Scattering, Statistical Modeling, and Trajectory Study. J. Phys. Chem. B 2012, 116, 6369–6379. [Google Scholar] [CrossRef] [PubMed]
- Kállay, C.; Ősz, K.; Dávid, A.; Valastyán, Z.; Malandrinos, G.; Hadjiliadis, N.; Sóvágó, I. Zinc(ii) binding ability of tri-, tetra- and penta-peptides containing two or three histidyl residues. Dalton Trans. 2007, 36, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Merenyi, G.; Lind, J.; Jonsson, M. Autoxidation of closed-shell organics: An outer-sphere electron transfer. J. Am. Chem. Soc. 1993, 115, 4945–4946. [Google Scholar] [CrossRef]
- Lal, M.; Rao, R.; Fang, X.; Schuchmann, H.-P.; von Sonntag, C. Radical-Induced Oxidation of Dithiothreitol in Acidic Oxygenated Aqueous Solution: A Chain Reaction. J. Am. Chem. Soc. 1997, 119, 5735–5739. [Google Scholar] [CrossRef]
- Jomova, K.; Baros, S.; Valko, M. Redox active metal-induced oxidative stress in biological systems. Transit. Met. Chem. 2012, 37, 127–134. [Google Scholar] [CrossRef]
- Stadtman, E.R. Metal ion-catalyzed oxidation of proteins: Biochemical mechanism and biological consequences. Free. Radic. Biol. Med. 1990, 9, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Mozziconacci, O.; Ji, J.A.; Wang, Y.J.; Schöneich, C. Metal-Catalyzed Oxidation of Protein Methionine Residues in Human Parathyroid Hormone (1-34): Formation of Homocysteine and a Novel Methionine-Dependent Hydrolysis Reaction. Mol. Pharm. 2013, 10, 739–755. [Google Scholar] [CrossRef] [Green Version]
- Subelzu, N.; Schöneich, C. Near UV and Visible Light Induce Iron-Dependent Photodegradation Reactions in Pharmaceutical Buffers: Mechanistic and Product Studies. Mol. Pharm. 2020, 17, 4163–4179. [Google Scholar] [CrossRef]
- Subelzu, N.; Schöneich, C. Pharmaceutical Excipients Enhance Iron-Dependent Photo-Degradation in Pharmaceutical Buffers by near UV and Visible Light: Tyrosine Modification by Reactions of the Antioxidant Methionine in Citrate Buffer. Pharm. Res. 2021, 38, 915–930. [Google Scholar] [CrossRef]
- Zhang, Y.; Richards, D.S.; Grotemeyer, E.N.; Jackson, T.A.; Schöneich, C. Near-UV and Visible Light Degradation of Iron (III)-Containing Citrate Buffer: Formation of Carbon Dioxide Radical Anion via Fragmentation of a Sterically Hindered Alkoxyl Radical. Mol. Pharm. 2022, 19, 4026–4042. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, B.; Foley, S.; Cardey, B.; Fromm, M.; Enescu, M. Methionine oxidation by hydrogen peroxide in peptides and proteins: A theoretical and Raman spectroscopy study. J. Photochem. Photobiol. B Biol. 2018, 188, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Peskin, A.V.; Cox, A.G.; Nagy, P.; Morgan, P.E.; Hampton, M.B.; Davies, M.J.; Winterbourn, C.C. Removal of amino acid, peptide and protein hydroperoxides by reaction with peroxiredoxins 2 and 3. Biochem. J. 2010, 432, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khossravi, M.; Borchardt, R.T. Chemical pathways of peptide degradation: IX. Metal-catalyzed oxidation of histidine in model peptides. Pharm. Res. 1998, 15, 1096–1102. [Google Scholar] [CrossRef]
- Kishore, R.S.K.; Kiese, S.; Fischer, S.; Pappenberger, A.; Grauschopf, U.; Mahler, H.-C. The Degradation of Polysorbates 20 and 80 and its Potential Impact on the Stability of Biotherapeutics. Pharm. Res. 2011, 28, 1194–1210. [Google Scholar] [CrossRef]
- Galande, A.K.; Trent, J.O.; Spatola, A.F. Understanding base-assisted desulfurization using a variety of disulfide-bridged peptides. Biopolymers 2003, 71, 534–551. [Google Scholar] [CrossRef]
- Cohen, S.L.; Price, C.; Vlasak, J. β-Elimination and Peptide Bond Hydrolysis: Two Distinct Mechanisms of Human IgG1 Hinge Fragmentation upon Storage. J. Am. Chem. Soc. 2007, 129, 6976–6977. [Google Scholar] [CrossRef]
- Volkin, D.; Klibanov, A. Thermal destruction processes in proteins involving cystine residues. J. Biol. Chem. 1987, 262, 2945–2950. [Google Scholar] [CrossRef]
- Windisch, V.; Deluccia, F.; Duhau, L.; Herman, F.; Mencel, J.J.; Tang, S.-Y.; Vuilhorgne, M. Degradation Pathways of Salmon Calcitonin in Aqueous Solution. J. Pharm. Sci. 1997, 86, 359–364. [Google Scholar] [CrossRef]
- Benesch, R.E.; Benesch, R. The Mechanism of Disulfide Interchange in Acid Solution; Role of Sulfenium Ions. J. Am. Chem. Soc. 1958, 80, 1666–1669. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Nakagawa, H.; Tamura, A.; Koshiba, S.; Hoshijima, K.; Komada, M.; Ishikawa, T. Intramolecular Disulfide Bond Is a Critical Check Point Determining Degradative Fates of ATP-binding Cassette (ABC) Transporter ABCG2 Protein. J. Biol. Chem. 2007, 282, 27841–27846. [Google Scholar] [CrossRef] [Green Version]
- Fázio, M.A.; Oliveira, V.X.; Bulet, P.; Miranda, M.T.M.; Daffre, S.; Miranda, A. Structure-activity relationship studies of gomesin: Importance of the disulfide bridges for conformation, bioactivities, and serum stability. Pept. Sci. Orig. Res. Biomol. 2006, 84, 205–218. [Google Scholar] [CrossRef]
- Kourra, C.M.B.K.; Cramer, N. Converting disulfide bridges in native peptides to stable methylene thioacetals. Chem. Sci. 2016, 7, 7007–7012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malencik, D.A.; Anderson, S.R. Dityrosine Formation in Calmodulin: Conditions for Intermolecular Crosslinking. Biochemistry 1994, 33, 13363–13372. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.C.P.; DesLauriers, R.; Saitô, H.; Walter, R.; Garrigou-Lagrange, C.; McGregor, H.; Sarantakis, D. Carbon-13 nmr studies of peptide hormones and their components. Ann. N. Y. Acad. Sci. 1973, 222, 597–627. [Google Scholar] [CrossRef] [PubMed]
- Kamberi, M.; Chung, P.; Devas, R.; Li, L.; Li, Z.; Ma, X.; Fields, S.; Riley, C.M. Analysis of non-covalent aggregation of synthetic hPTH (1–34) by size-exclusion chromatography and the importance of suppression of non-specific interactions for a precise quantitation. J. Chromatogr. B 2004, 810, 151–155. [Google Scholar] [CrossRef]
- Wang, W. Protein aggregation and its inhibition in biopharmaceutics. Int. J. Pharm. 2005, 289, 1–30. [Google Scholar] [CrossRef]
- Powell, M.F.; Sanders, L.M.; Rogerson, A.; Si, V. Parenteral Peptide Formulations: Chemical and Physical Properties of Native Luteinizing Hormone-Releasing Hormone (LHRH) and Hydrophobic Analogues in Aqueous Solution. Pharm. Res. 1991, 8, 1258–1263. [Google Scholar] [CrossRef]
- Tan, M.M.; Corley, C.A.; Stevenson, C.L. Effect of gelation on the chemical stability and conformation of leuprolide. Pharm. Res. 1998, 15, 1442–1448. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ohki, K.; Sakura, N. Hydrolytic Cleavage of Pyroglutamyl-Peptide Bond. I. The Susceptibility of Pyroglutamyl-Peptide Bond to Dilute Hydrochloric Acid. Chem. Pharm. Bull. 1995, 43, 2068–2074. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, C.A.; Ribeiro, G.B.; Knirsch, M.C.; Junior, A.P.; Penna, T.C.V. Influence of Pluronic® F68 on Ceftazidime Biological Activity in Parenteral Solutions. J. Pharm. Sci. 2011, 100, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.M.; E Piccinini, T.; Lammel, C.J.; Hadley, W.K.; Brooks, G.F. Effect of storage temperature and pH on the stability of antimicrobial agents in MIC trays. J. Clin. Microbiol. 1986, 23, 959–961. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Yalkowsky, S.H. Stabilization of eptifibatide by cosolvents. Int. J. Pharm. 2001, 218, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, R.; Mitra, A.K. Kinetics and Mechanism of Degradation of a Cyclic Hexapeptide (Somatostatin Analogue) in Aqueous Solution. Pharm. Res. 1992, 9, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Kempe, K.; Wilson, P.; Blindauer, C.A.; McIntosh, M.P.; Davis, T.P.; Whittaker, M.R.; Haddleton, D.M. Stability Enhancing N-Terminal PEGylation of Oxytocin Exploiting Different Polymer Architectures and Conjugation Approaches. Biomacromolecules 2016, 17, 2755–2766. [Google Scholar] [CrossRef] [Green Version]
- Stratton, L.P.; Kelly, R.; Rowe, J.; Shively, J.E.; Smith, D.; Carpenter, J.F.; Manning, M.C. Controlling deamidation rates in a model peptide: Effects of temperature, peptide concentration, and additives. J. Pharm. Sci. 2001, 90, 2141–2148. [Google Scholar] [CrossRef]
- Hall, S.; Tan, M.; Leonard, J.; Stevenson, C. Characterization and comparison of leuprolide degradation profiles in water and dimethyl sulfoxide. J. Pept. Res. 1999, 53, 432–441. [Google Scholar] [CrossRef]
- Wang, Y.; Lomakin, A.; Kanai, S.; Alex, R.; Benedek, G.B. Transformation of Oligomers of Lipidated Peptide Induced by Change in pH. Mol. Pharm. 2015, 12, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Seyferth, S.; Lee, G. Structural studies of EDTA-induced fibrillation of salmon calcitonin. Pharm. Res. 2003, 20, 73–80. [Google Scholar] [CrossRef]
- Kamberi, M.; Kim, Y.; Jun, B.; Riley, C. The effects of sucrose on stability of human brain natriuretic peptide [hBNP (1-32)] and human parathyroid hormone [hPTH (1-34)]. J. Pept. Res. 2005, 66, 348–356. [Google Scholar] [CrossRef]
- Guan, Z.; Yates, N.A.; Bakhtiar, R. Detection and characterization of methionine oxidation in peptides by collision-induced dissociation and electron capture dissociation. J. Am. Soc. Mass Spectrom. 2003, 14, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.P.; Ciccotosto, G.D.; Tew, D.J.; Fodero-Tavoletti, M.T.; Johanssen, T.; Masters, C.L.; Barnham, K.J.; Cappai, R. Concentration Dependent Cu2+ Induced Aggregation and Dityrosine Formation of the Alzheimer’s Disease Amyloid-β Peptide. Biochemistry 2007, 46, 2881–2891. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Nakagawa, A.; Hino, T.; Oka, H. Screening Assay for Metal-Catalyzed Oxidation Inhibitors Using Liquid Chromatography−Mass Spectrometry with an N-Terminal β-Amyloid Peptide. Anal. Chem. 2009, 81, 1819–1825. [Google Scholar] [CrossRef]
- Dunkelberger, E.B.; Buchanan, L.E.; Marek, P.; Cao, P.; Raleigh, D.P.; Zanni, M.T. Deamidation Accelerates Amyloid Formation and Alters Amylin Fiber Structure. J. Am. Chem. Soc. 2012, 134, 12658–12667. [Google Scholar] [CrossRef] [Green Version]
- Brazeau, G.A.; Cooper, B.; Svetic, K.A.; Smith, C.L.; Gupta, P. Current Perspectives on Pain upon Injection of Drugs. J. Pharm. Sci. 1998, 87, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Extemp.ie. Sterile Preparations—Parenterals. Available online: http://www.extemp.ie/general-methods/sterile-preparations/parenterals (accessed on 11 December 2021).
- Jorgensen, L.; Hostrup, S.; Moeller, E.H.; Grohganz, H. Recent trends in stabilising peptides and proteins in pharmaceutical formulation—Considerations in the choice of excipients. Expert Opin. Drug Deliv. 2009, 6, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.L.; Powell, M.F.; Shire, S.J. The development of stable protein formulations: A close look at protein aggregation, deamidation, and oxidation. Crit. Rev. Ther. Drug Carr. Syst. 1993, 10, 307–377. [Google Scholar]
- Wang, W.; Martin-Moe, S.; Pan, C.; Musza, L.; Wang, Y.J. Stabilization of a polypeptide in non-aqueous solvents. Int. J. Pharm. 2008, 351, 1–7. [Google Scholar] [CrossRef]
- Good, N.E.; Winget, G.D.; Winter, W.; Connolly, T.N.; Izawa, S.; Singh, R.M.M. Hydrogen Ion Buffers for Biological Research *. Biochemistry 1966, 5, 467–477. [Google Scholar] [CrossRef]
- Liu, F.; Lai, S.; Tong, H.; Lakey, P.S.J.; Shiraiwa, M.; Weller, M.G.; Pöschl, U.; Kampf, C.J. Release of free amino acids upon oxidation of peptides and proteins by hydroxyl radicals. Anal. Bioanal. Chem. 2017, 409, 2411–2420. [Google Scholar] [CrossRef] [Green Version]
- Avanti, C.; Amorij, J.-P.; Setyaningsih, D.; Hawe, A.; Jiskoot, W.; Visser, J.; Kedrov, A.; Driessen, A.J.M.; Hinrichs, W.L.J.; Frijlink, H.W. A New Strategy to Stabilize Oxytocin in Aqueous Solutions: I. The Effects of Divalent Metal Ions and Citrate Buffer. AAPS J. 2011, 13, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Avanti, C.; Hinrichs, W.L.; Casini, A.; Eissens, A.C.; Van Dam, A.; Kedrov, A.; Driessen, A.J.; Frijlink, H.W.; Permentier, H.P. The Formation of Oxytocin Dimers is Suppressed by the Zinc-Aspartate-Oxytocin Complex. J. Pharm. Sci. 2013, 102, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Ryu, K.-W.; Na, D.-H. Stability of Octreotide Acetate in Aqueous Solutions and PLGA Films. J. Korean Pharm. Sci. 2009, 39, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; D’Souza, A.J.; Schowen, R.L.; Borchardt, R.T.; Topp, E.M.; Laird, B.B. Effects of solution polarity and viscosity on peptide deamidation. J. Pept. Res. 2000, 56, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ramm, I.; Sanchez-Fernandez, A.; Choi, J.; Lang, C.; Fransson, J.; Schagerlöf, H.; Wahlgren, M.; Nilsson, L. The Impact of Glycerol on an Affibody Conformation and Its Correlation to Chemical Degradation. Pharmaceutics 2021, 13, 1853. [Google Scholar] [CrossRef]
- Parkins, D.A.; Lashmar, U.T. The formulation of biopharmaceutical products. Pharm. Sci. Technol. Today 2000, 3, 129–137. [Google Scholar] [CrossRef]
- Brennan, T.V.; Clarke, S. Spontaneous degradation of polypeptides at aspartyl and asparaginyl residues: Effects of the solvent dielectric. Protein Sci. 1993, 2, 331–338. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, A.J.M.; Schowen, R.L.; Borchardt, R.T.; Salsbury, J.S.; Munson, E.J.; Topp, E.M. Reaction of a Peptide with Polyvinylpyrrolidone in the Solid State. J. Pharm. Sci. 2003, 92, 585–593. [Google Scholar] [CrossRef]
- Hovgaard, L.; Frokjaer, S.; van de Weert, M. Pharmaceutical Formulation Development of Peptides and Proteins; Taylor & Francis: Milton Park, OX, USA, 1999; Available online: https://books.google.co.id/books?id=VVlLyQEACAAJ (accessed on 4 January 2023).
- Li, R.; Topp, E.; Hageman, M. Effect of viscosity on the deamidation rate of a model Asn-hexapeptide. J. Pept. Res. 2002, 59, 211–220. [Google Scholar] [CrossRef]
- Thieme, V.; Jolly, N.; Madsen, A.N.; Bellmann-Sickert, K.; Schwartz, T.W.; Holst, B.; Cox, H.M.; Beck-Sickinger, A.G. High molecular weight PEGylation of human pancreatic polypeptide at position 22 improves stability and reduces food intake in mice. Br. J. Pharmacol. 2016, 173, 3208–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, J.C.M.; Carvalho, L.R.; de Souza, A.N.; Carneiro, G.; Magalhães, P.P.; Farias, L.M.; Guimarães, N.R.; Verly, R.M.; Resende, J.M.; de Lima, M.E. PEGylation of the antimicrobial peptide LyeTx I-b maintains structure-related biological properties and improves selectivity. Front. Mol. Biosci. 2022, 9, 1001508. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Stone, C.A. Allergy evaluation of messenger RNA vaccine reactions is crucial, with a specific role for polyethylene glycol testing. Ann. Allergy Asthma Immunol. 2022, 129, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Hatziantoniou, S.; Maltezou, H.C.; Tsakris, A.; Poland, G.A.; Anastassopoulou, C. Anaphylactic reactions to mRNA COVID-19 vaccines: A call for further study. Vaccine 2021, 39, 2605–2607. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C.; Adami, R.C.; Alsante, K.M.; Hong, J.; Landis, M.S.; Lombardo, F.; Roberts, C.J. Stabilization of Pharmaceuticals to Oxidative Degradation. Pharm. Dev. Technol. 2002, 7, 1–32. [Google Scholar] [CrossRef]
- Chu, J.-W.; Brooks, B.R.; Trout, B.L. Oxidation of Methionine Residues in Aqueous Solutions: Free Methionine and Methionine in Granulocyte Colony-Stimulating Factor. J. Am. Chem. Soc. 2004, 126, 16601–16607. [Google Scholar] [CrossRef]
- Landi, S.; Held, H.R. Effect of oxidation on the stability of tuberculin purified protein derivative (P.P.D.). Dev. Biol. Stand. 1986, 58, 545–552. [Google Scholar]
- Rahimi, M.; Mobedi, H.; Behnamghader, A. Aqueous stability of leuprolide acetate: Effect of temperature, dissolved oxygen, pH and complexation with β-cyclodextrin. Pharm. Dev. Technol. 2016, 21, 108–115. [Google Scholar] [CrossRef]
- Pagano, D.A.; Zeiger, E.; Stark, A.-A. Autoxidation and mutagenicity of sodium bisulfite. Mutat. Res. Mol. Mech. Mutagen. 1990, 228, 89–96. [Google Scholar] [CrossRef]
- Ingold, K.U. Peroxy radicals. Acc. Chem. Res. 1969, 2, 1–9. [Google Scholar] [CrossRef]
- Schöneich, C.; Zhao, F.; Wilson, G.S.; Borchardt, R.T. Iron-thiolate induced oxidation of methionine to methionine sulfoxide in small model peptides. Intramolecular catalysis by histidine. Biochim. Biophys. Acta Gen. Subj. 1993, 1158, 307–322. [Google Scholar] [CrossRef]
- Li, S.; Schöneich, C.; Wilson, G.S.; Borchardt, R.T. Chemical Pathways of Peptide Degradation. V. Ascorbic Acid Promotes Rather than Inhibits the Oxidation of Methionine to Methionine Sulfoxide in Small Model Peptides. Pharm. Res. 1993, 10, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Moskovitz, J.; Stadtman, E.R. Oxidation of Methionine in Proteins: Roles in Antioxidant Defense and Cellular Regulation. IUBMB Life 2000, 50, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.K.; Volkin, D.B.; Dabora, J.M.; Thompson, K.C.; Bruner, M.W.; Gress, J.O.; Matuszewska, B.; Keogan, M.; Bondi, J.V.; Middaugh, C.R. Formulation Design of Acidic Fibroblast Growth Factor. Pharm. Res. 1993, 10, 649–659. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, J.; Schöneich, C. Effects of Polyaminocarboxylate Metal Chelators on Iron-thiolate Induced Oxidation of Methionine- and Histidine-Containing Peptides. Pharm. Res. 1996, 13, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.D.; Koppenol, W.H. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c. J. Biol. Chem. 1986, 261, 6730–6733. [Google Scholar] [CrossRef]
- Torosantucci, R.; Weinbuch, D.; Klem, R.; Jiskoot, W. Triethylenetetramine prevents insulin aggregation and fragmentation during copper catalyzed oxidation. Eur. J. Pharm. Biopharm. 2013, 84, 464–471. [Google Scholar] [CrossRef]
- Li, S.; Patapoff, T.W.; Nguyen, T.H.; Borchardt, R.T. Inhibitory Effect of Sugars and Polyols on the Metal-Catalyzed Oxidation of Human Relaxin. J. Pharm. Sci. 1996, 85, 868–872. [Google Scholar] [CrossRef]
- Obiols, B.P.; Farres, G.J.; Rodriguez, F.J.C.; Fernandez, S.P.; Cabado, J.B. Stable Pharmaceutical Formulation for Intravenous or In-Tramuscular Administration of Active Peptide Compound. 2003. Available online: https://patentimages.storage.googleapis.com/e9/8d/6c/1e6ec5ec4ba9b2/US6521599.pdf (accessed on 4 January 2023).
- Zheng, K.; Middaugh, C.; Siahaan, T.J. Evaluation of the physical stability of the EC5 domain of E-cadherin: Effects of pH, temperature, ionic strength, and disulfide bonds. J. Pharm. Sci. 2009, 98, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Bursakov, S.; Carneiro, C.; Almendra, M.; Duarte, R.; Caldeira, J.; Moura, I.; Moura, J.J.G. Enzymatic Properties and Effect of Ionic Strength on Periplasmic Nitrate Reductase (NAP) fromDesulfovibrio desulfuricansATCC 27774. Biochem. Biophys. Res. Commun. 1997, 239, 816–822. [Google Scholar] [CrossRef]
- Tyler-Cross, R.; Schirch, V. Effects of amino acid sequence, buffers, and ionic strength on the rate and mechanism of deami-dation of asparagine residues in small peptides. J. Biol. Chem. 1991, 266, 22549–22556. [Google Scholar] [CrossRef]
- Campos-Ramírez, A.; Márquez, M.; Quintanar, L.; Rojas-Ochoa, L.F. Effect of ionic strength on the aggregation kinetics of the amidated amyloid beta peptide Aβ (1-40) in aqueous solutions. Biophys. Chem. 2017, 228, 98–107. [Google Scholar] [CrossRef]
- Keyes, E.D.; Kauser, K.; Warner, K.S.; Roberts, A.G. Photosensitized Oxidative Dimerization at Tyrosine by a Water-Soluble 4-Amino-1, 8-naphthalimide. Chembiochem 2021, 22, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Lentzen, G.; Schwarz, T. Extremolytes: Natural compounds from extremophiles for versatile applications. Appl. Microbiol. Biotechnol. 2006, 72, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Galinski, E.A.; Stein, M.; Amendt, B.; Kinder, M. The Kosmotropic (Structure-Forming) Effect of Compensatory Solutes. Comp. Biochem. Physiol. Part A Physiol. 1997, 117, 357–365. [Google Scholar] [CrossRef]
- Bolen, D.W.; Rose, G.D. Structure and Energetics of the Hydrogen-Bonded Backbone in Protein Folding. Annu. Rev. Biochem. 2008, 77, 339–362. [Google Scholar] [CrossRef] [Green Version]
- Avanti, C.; Saluja, V.; Van Streun, E.L.P.; Frijlink, H.W.; Hinrichs, W. Stability of Lysozyme in Aqueous Extremolyte Solutions during Heat Shock and Accelerated Thermal Conditions. PLoS ONE 2014, 9, e86244. [Google Scholar] [CrossRef]
- Panuszko, A.; Bruździak, P.; Kaczkowska, E.; Stangret, J. General Mechanism of Osmolytes’ Influence on Protein Stability Irrespective of the Type of Osmolyte Cosolvent. J. Phys. Chem. B 2016, 120, 11159–11169. [Google Scholar] [CrossRef]
- Maclagan, R.G.A.R.; Malardier-Jugroot, C.; Whitehead, M.A.; Lever, M. Theoretical Studies of the Interaction of Water with Compensatory and Noncompensatory Solutes for Proteins. J. Phys. Chem. A 2004, 108, 2514–2519. [Google Scholar] [CrossRef]
- Kuhlmann, A.U.; Hoffmann, T.; Bursy, J.; Jebbar, M.; Bremer, E. Ectoine and hydroxyectoine as protectants against osmotic and cold stress: Uptake through the SigB-controlled betaine-choline-carnitine transporter-type carrier EctT from Virgibacillus pan-tothenticus. J. Bacteriol. 2011, 193, 4699–4708. [Google Scholar] [CrossRef] [Green Version]
- Hédoux, A.; Paccou, L.; Guinet, Y. Relationship between β-relaxation and structural stability of lysozyme: Microscopic insight on thermostabilization mechanism by trehalose from Raman spectroscopy experiments. J. Chem. Phys. 2014, 140, 225102. [Google Scholar] [CrossRef]
- Adamczak, B.; Kogut, M.; Czub, J. Effect of osmolytes on the thermal stability of proteins: Replica exchange simulations of Trp-cage in urea and betaine solutions. Phys. Chem. 2018, 20, 11174–11182. [Google Scholar] [CrossRef]
- Pais, T.M.; Lamosa, P.; Matzapetakis, M.; Turner, D.L.; Santos, H. Mannosylglycerate stabilizes staphylococcal nuclease with restriction of slow β-sheet motions. Protein Sci. 2012, 21, 1126–1137. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Kanapathipillai, M.; Lentzen, G.; Park, C.B. Inhibition of β-amyloid peptide aggregation and neurotoxicity by α-d-mannosylglycerate, a natural extremolyte. Peptides 2008, 29, 578–584. [Google Scholar] [CrossRef]
- Ji, J.A.; Zhang, B.; Cheng, W.; Wang, Y.J. Methionine, tryptophan, and histidine oxidation in a model protein, PTH: Mechanisms and stabilization. J. Pharm. Sci. 2009, 98, 4485–4500. [Google Scholar] [CrossRef]
- Drayton, M.; Alford, M.A.; Pletzer, D.; Haney, E.F.; Machado, Y.; Luo, H.D.; Overall, C.M.; Kizhakkedathu, J.N.; Hancock, R.E.; Straus, S.K. Enzymatically releasable polyethylene glycol—Host defense peptide conjugates with improved activity and biocompatibility. J. Control. Release 2021, 339, 220–231. [Google Scholar] [CrossRef]
- Harrison, E.; Nicol, J.R.; Macias–Montero, M.; Burke, G.A.; Coulter, J.A.; Meenan, B.J.; Dixon, D. A comparison of gold nanoparticle surface co-functionalization approaches using Polyethylene Glycol (PEG) and the effect on stability, non-specific protein adsorption and internalization. Mater. Sci. Eng. C 2016, 62, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Prestrelski, S.J.; Kenney, W.C.; Carpenter, J.F. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 2001, 46, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Tomita, S.; Hamada, H.; Shiraki, K. Amidated amino acids are prominent additives for preventing heat-induced aggregation of lysozyme. J. Biosci. Bioeng. 2007, 103, 440–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, R.; Andrade, J. Minimizing the Aggregation of Neutral Insulin Solutions. J. Pharm. Sci. 1983, 72, 1472–1473. [Google Scholar] [CrossRef]
- Chou, D.K.; Krishnamurthy, R.; Randolph, T.W.; Carpenter, J.F.; Manning, M.C. Effects of Tween 20® and Tween 80® on the Stability of Albutropin During Agitation. J. Pharm. Sci. 2005, 94, 1368–1381. [Google Scholar] [CrossRef]
- Lahlou, A.; Blanchet, B.; Carvalho, M.; Paul, M.; Astier, A. Mechanically-induced aggregation of the monoclonal antibody cetuximab. Ann. Pharm. Fr. 2009, 67, 340–352. [Google Scholar] [CrossRef]
- Foster, T.; Dormish, J.J.; Narahari, U.; Meyer, J.D.; Vrkljan, M.; Henkin, J.; Porter, M.; Staack, H.; Carpenter, J.; Manning, M. Thermal stability of low molecular weight urokinase during heat treatment. III. Effect of salts, sugars and Tween 80. Int. J. Pharm. 1996, 134, 193–201. [Google Scholar] [CrossRef]
- Vrkljan, M.; Foster, T.M.; Powers, M.E.; Henkin, J.; Porter, W.R.; Staack, H.; Carpenter, J.F.; Manning, M.C. Thermal Stability of Low Molecular Weight Urokinase During Heat Treatment. II. Effect of Polymeric Additives. Pharm. Res. 1994, 11, 1004–1008. [Google Scholar] [CrossRef]
- Ristroph, K.D.; Prud’Homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, K.; Jaśkiewicz, M.; Neubauer, D.; Migoń, D.; Kamysz, W. The Role of Counter-Ions in Peptides—An Overview. Pharmaceuticals 2020, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef]
- Ismail, R.; Phan, T.N.Q.; Laffleur, F.; Csóka, I.; Bernkop-Schnürch, A. Hydrophobic ion pairing of a GLP-1 analogue for incorporating into lipid nanocarriers designed for oral delivery. Eur. J. Pharm. Biopharm. 2020, 152, 10–17. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Mandal, A.; Gokulgandhi, M.; Patel, S.; Mitra, A.K. Reversible hydrophobic ion-paring complex strategy to minimize acylation of octreotide during long-term delivery from PLGA microparticles. Int. J. Pharm. 2015, 489, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Jörgensen, A.M.; Knoll, P.; Haddadzadegan, S.; Fabian, H.; Hupfauf, A.; Gust, R.; Jörgensen, R.G.; Bernkop-Schnürch, A. Biodegradable arginine based steroid-surfactants: Cationic green agents for hydrophobic ion-pairing. Int. J. Pharm. 2023, 630, 122438. [Google Scholar] [CrossRef]
| Peptide | Year of Approval | Indications | Dosage Form | Administration Route |
|---|---|---|---|---|
| Setmelanotide | 2020 | Chronic weight management | Liq. Inj | Subcutaneous |
| 64Cu-Dotatate | 2020 | Radiopharmaceutical | Liq. Inj | Intravenous |
| Vosoritide | 2021 | Pediatric bone growth | Powder for Inj | Subcutaneous |
| Difelikefalin | 2021 | Pruritus with chronic kidney disease | Liq, Inj | Intravenous |
| Melphalan flufenamide | 2021 | Relapsed or refractory multiple myeloma | Powder for Inj | Intravenous |
| Voclosporin | 2021 | Lupus nephritis | Capsule | Oral |
| Piflufolastat F 18 | 2021 | Radiopharmaceutical | Liq. Inj | Intravenous |
| Pegcetacoplan | 2021 | Paroxysmal noctural hemoglobinuria | Liq. Inj | Subcutaneous |
| Dasiglucagon | 2021 | Hypoglycemia | Liq. Inj | Subcutaneous |
| Tirzepatide | 2022 | Type 2 diabetes | Liq. Inj | Subcutaneous |
| Terlipressin | 2022 | Hepatorenal syndrome | Powder for Inj | Intravenous |
| Peptide | Target Receptor | Indication(s) for Investigation | Clinical Trial Phase |
|---|---|---|---|
| TT-232 BPI-3016 NBI-6024 Many more | Somatostatin GLP-1 TCR | Renal cell adenocarcinoma Type 2 diabetes Type 1 diabetes | I |
| Angiotensin 1–7 Bombesin Cenderitide Deslorelin Gastric inhibitory polypeptide MK-3207 Olcegepant Pancreatic polypeptide Peptide YY (3–36) Somatoprim Thyrotropin | AT 2 Bombesin NPRA and NPRB GnRH GIPr CGRP CGRP Neuropeptide Y4 Neuropeptide Y2 Somatostatin TSH | Miscellaneous Peripheral Blood Cell Abnormalities Prostate cancer Heart failure Puberty; precocious Type 2 diabetes Migraine Migraine disorders Type 1 diabetes Metabolic disease; obesity Acromegaly Benign nontoxic and toxic goiter; goiter; nodular | II |
| Albusomatropin Anamorelin G17DT Insulin peglispro Selepressin Somapacitan Taspoglutide Tirzepatide Ularitide Vapreotide Vosoritide Zoptarelin doxorubicin | GHR GHSRCCK-2 IR V1A GHR GLP-1 GIP and GLP-1 NPR Somatostatin 2 and 5 NPR-B LHRH | Growth hormone deficiency Cachexia; lung cancer non-small cell cancer Various forms of cancer Type 1 and 2 diabetes Shock, septic Adult growth hormone deficiency Type 2 diabetes Type 2 diabetes Decompensated heart failure Gastri varices; esophageal haemorrhage; portal hypertension; esophageal varices Achondroplasia Endometrial cancer; prostate cancer | III |
| Avexitide Calcitonin gene-related peptide Corticorelin Leptin Thymalfasin | GLP-1 CGRP-R CRF-1 LEP-R TLR | Hypoglycemia Migraine Brain neoplasms; brain swelling Obesity; lipodystrophy Liver cirrhosis, sepsis | IV |
| Degradation Pathway | Critical Parameters | The Amino Acid Residue(s) Involved | References |
|---|---|---|---|
| Chemical Instability | |||
| Hydrolysis | pH Temperature | Trp Ser Asn-Pro Asn-Tyr | [26,35,36,37] |
| Deamidation | pH Temperature | Asn Gln | [35,36,38,39,40,41,42] |
| β-elimination | Thermal stress pH | Cys-Cys | [35,37,43,44] |
| Oxidation | pH Temperature Oxygen | Trp Met Cys Tyr His | [36,37,39,44,45] |
| Light-induced oxidation | Light | Trp | [46,47,48,49] |
| Metal induced oxidation | Metal ions (copper, iron) | His Cys Arg Pro Met | [50,51] |
| Disulfide exchange | pH Oxygen Metal ions | Cys-Cys | [38,52] |
| Physical Instability | |||
| Adsorption | Container | His Arg | [53] |
| Aggregation | Stress condition Concentration pH | Cys-Cys Tyr-Tyr | [39,40,42,43,54,55,56,57,58,59,60] |
| Peptide | Number of A.A. | Degradation Pathway | Stabilization Strategy | A.A. Residue(s) Involved | References |
|---|---|---|---|---|---|
| Thyrotropin-releasing hormones (T.R.H.) | 3 | Hydrolysis | pH 6.5 | Glu | [119] |
| Ceftazidime | 5 | Hydrolysis | Pluronic® F68 pH 4.5–6.5 | Glu | [120,121] |
| Eptifibatide | 6 | Hydrolysis Isomerization Deamidation Oxidation Dimerization | pH 5.7 Co-solvent 0.025 M citrate buffer | Asp Cys-Cys | [122] |
| Octreotide | 8 | Hydrolysis Disulfide exchange | Air exclusion Buffer pH close to 4 | Tyr Trp | [69,123] |
| Oxytocin | 9 | Oxidation β-elimination Deamidation Hydrolysis Dimerization Light-induced oxidation | Antioxidant pH 4.5 Acetate/Citrate/Aspartate buffer Divalent metal ions Protect from lightPEGylation Cyclization | Tyr Cys Cys-Cys | [35,38,39,43,44,45,124] |
| Desmopressin | 9 | Oxidation Deamidation Disulfide exchange β-elimination Racemization | Surfactants Polyols Buffer Divalent metal ions Phosphate buffer (pH 4.5–5.5) | Asn Gln Cys Tyr | [32,52,125] |
| Leuprolide | 10 | Hydrolysis Isomerization β-elimination Oxidation Aggregation | pH 3–5 Acetate buffer Co-solvent (DMSO) | Ser Trp | [126] |
| Goserelin | 10 | Hydrolysis Debutylation Epimerization | pH 3–5 Acetate buffer Co-solvent | Ser | [62] |
| Gonadorelin | 10 | Hydrolysis Deamidation Epimerization | pH 3–5 Acetate buffer Co-solvent | Ser | [62,64] |
| Triptorelin | 10 | Hydrolysis Deamidation Epimerization | pH 3–5 Acetate buffer Co-solvent | Ser | [62,64] |
| Somatostatin and analogs | 14 | Hydrolysis Disulfide exchange | pH 4–5 Acetate buffer NaCl | Trp-Tyr Trp-Lys Cys-Cys | [69,123] |
| Liraglutide | 30 | Aggregation Oligomerization | pH > 6.9 | - | [55,127] |
| Salmon Calcitonin | 32 | Deamidation Dimerization Aggregation Hydrolysis Disulfide exchange | pH 3–4 Citrate buffer Phosphate buffer | Asn Gln Cys-Cys Cys-Ser | [57,108,128] |
| Human Brain Natriuretic Peptide [hBNP(1–32)] | 32 | Aggregation Deamidation Oxidation | Sucrose Air exclusion | Met Asn | [129] |
| Human Parathyroid Hormone [hPTH(1–34)] | 34 | Oxidation Deamidation Aggregation Cleavage Asp residue | Sucrose Co-solvent Air exclusion | Asp Asn | [49,115] |
| Adenocortico-tropin hormone (ACTH) | 39 | Hydrolysis Deamidation | pH 3.0–5.0 Acetate buffer | Asn Met | [71,130] |
| Amyloid-β (Aβ) peptides | 36–43 | Metal-catalyzed oxidation Deamidation Dimerization Aggregation | Chelating agents Polyols | His Cys Arg Pro Met | [131,132,133] |
| Exenatide | 39 | Aggregation Oxidation Deamidation | pH 4.5 Polyols | Gly Met Asp Trp | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugrahadi, P.P.; Hinrichs, W.L.J.; Frijlink, H.W.; Schöneich, C.; Avanti, C. Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review. Pharmaceutics 2023, 15, 935. https://doi.org/10.3390/pharmaceutics15030935
Nugrahadi PP, Hinrichs WLJ, Frijlink HW, Schöneich C, Avanti C. Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review. Pharmaceutics. 2023; 15(3):935. https://doi.org/10.3390/pharmaceutics15030935
Chicago/Turabian StyleNugrahadi, Primawan Putra, Wouter L. J. Hinrichs, Henderik W. Frijlink, Christian Schöneich, and Christina Avanti. 2023. "Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review" Pharmaceutics 15, no. 3: 935. https://doi.org/10.3390/pharmaceutics15030935
APA StyleNugrahadi, P. P., Hinrichs, W. L. J., Frijlink, H. W., Schöneich, C., & Avanti, C. (2023). Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review. Pharmaceutics, 15(3), 935. https://doi.org/10.3390/pharmaceutics15030935










