Photodynamic Inhibition of Herpes Simplex Virus 1 Infection by Tricationic Amphiphilic Porphyrin with a Long Alkyl Chain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Viruses
2.2. Photosensitizer and Light Source
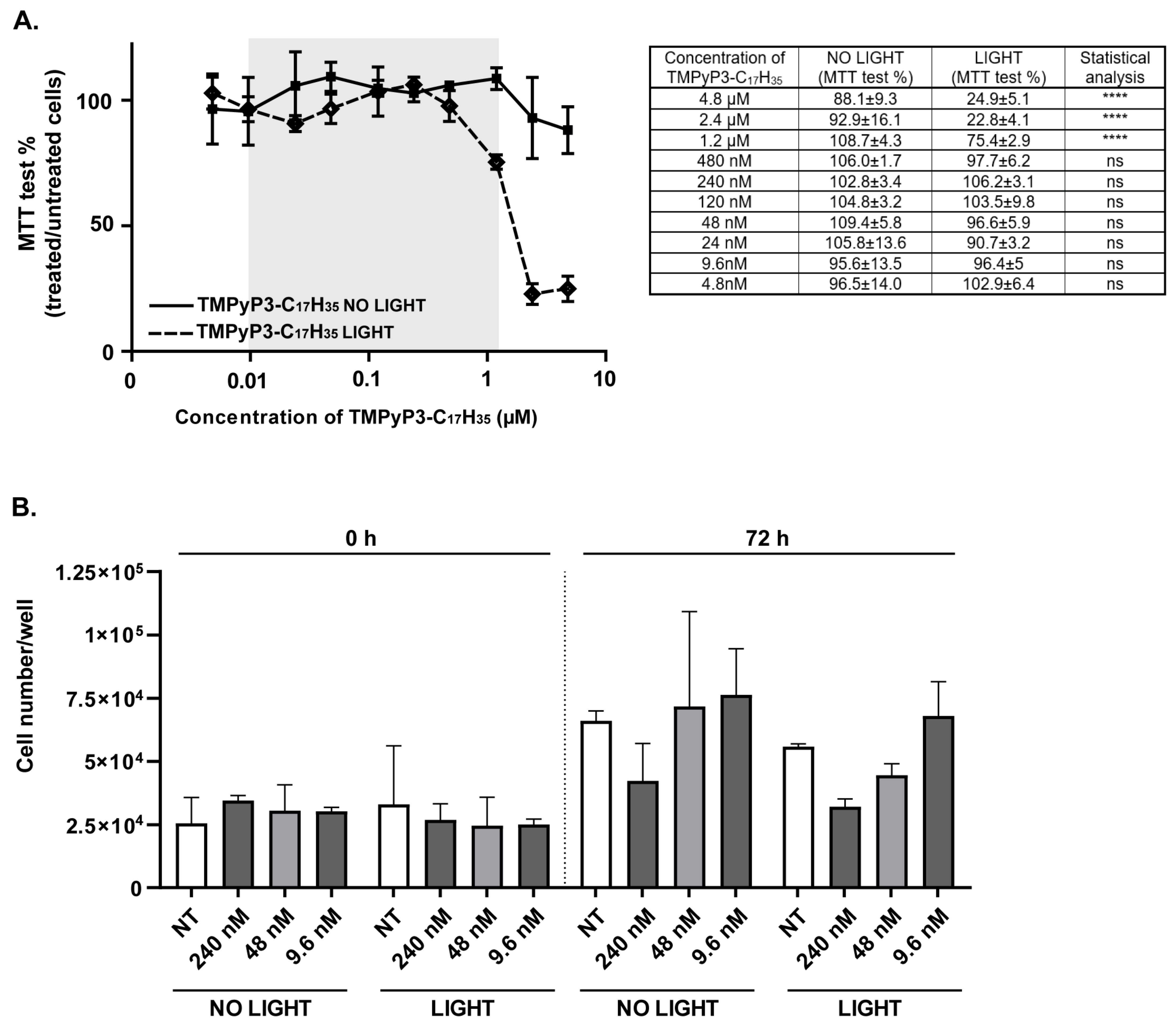
2.3. Cytotoxicity of TMPyP3-C17H35
2.4. Western Blot Analysis
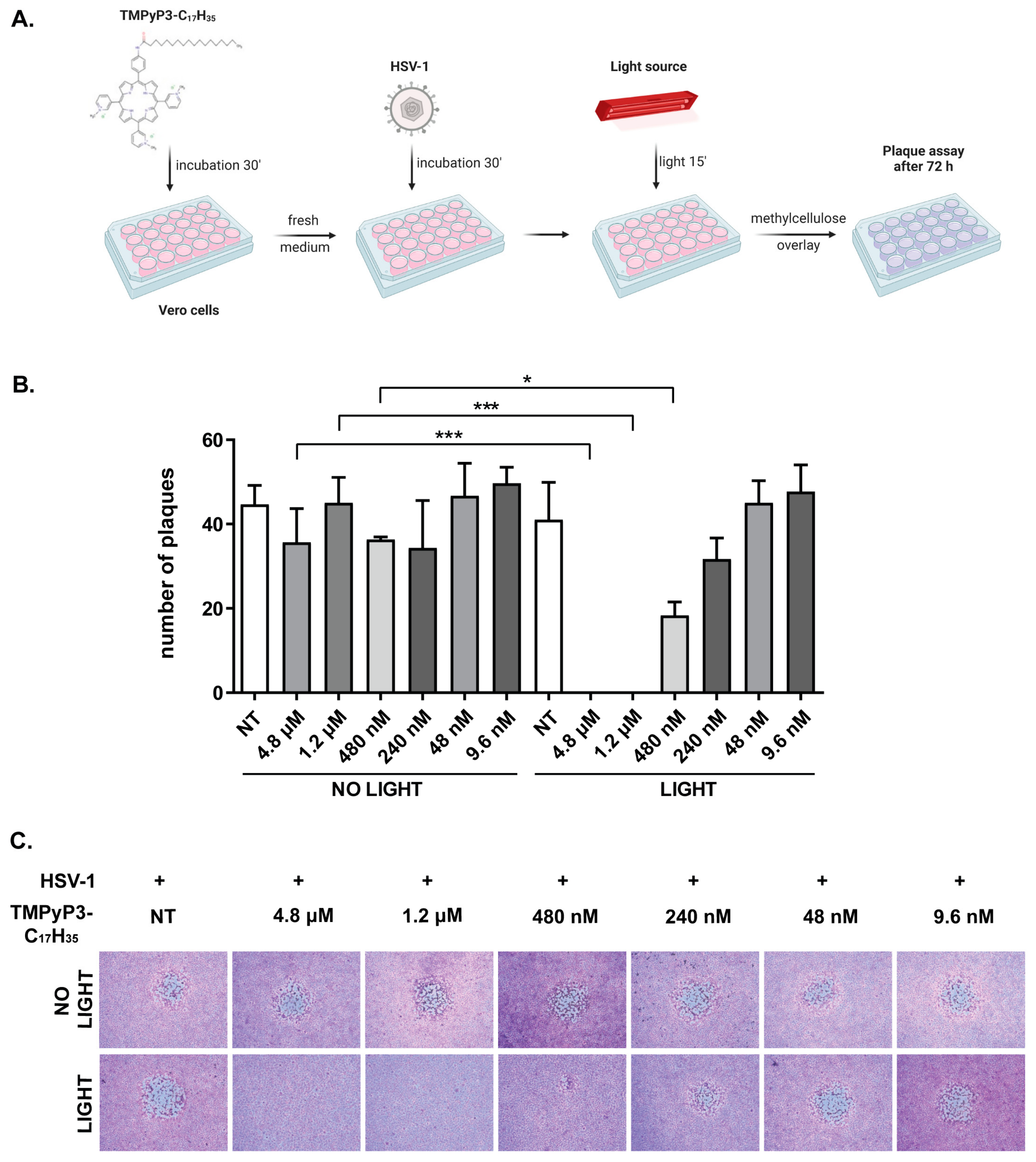
2.5. Photodynamic Inactivation of HSV-1
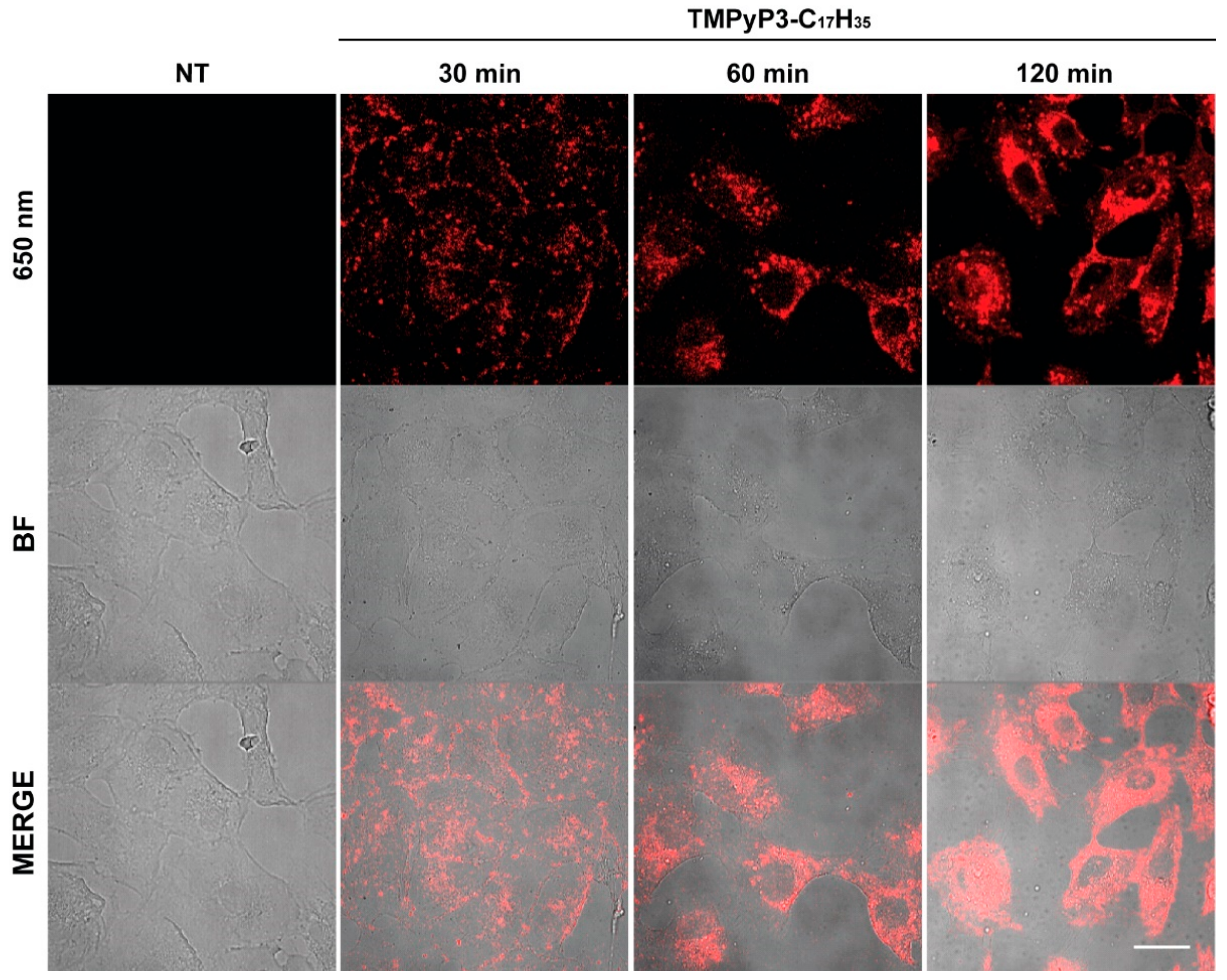
2.6. Microscopy
3. Results
3.1. Toxicity of TMPyP3-C17H35 in Vero Cells
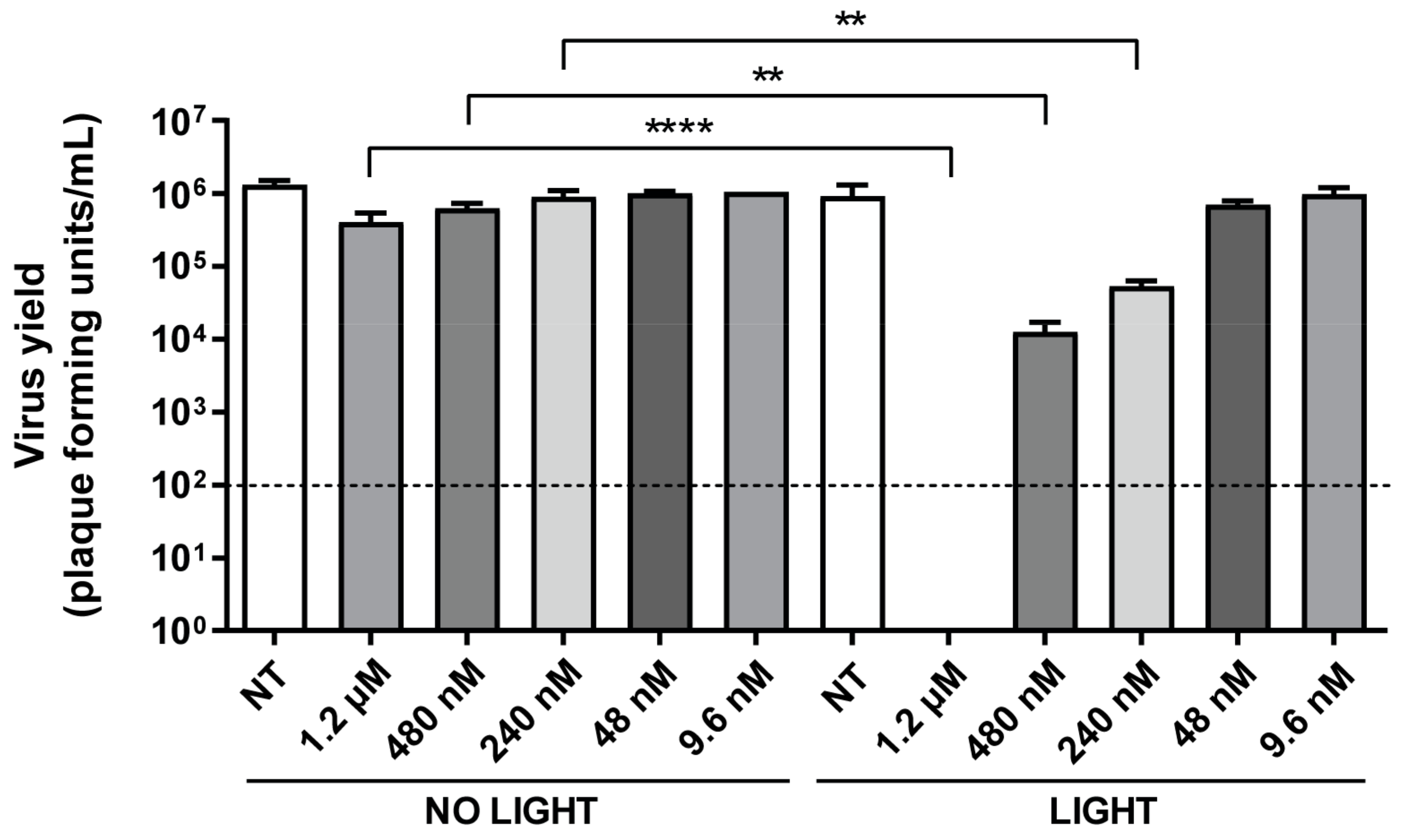
3.2. TMPyP3-C17H35 Inhibits Replication of HSV-1
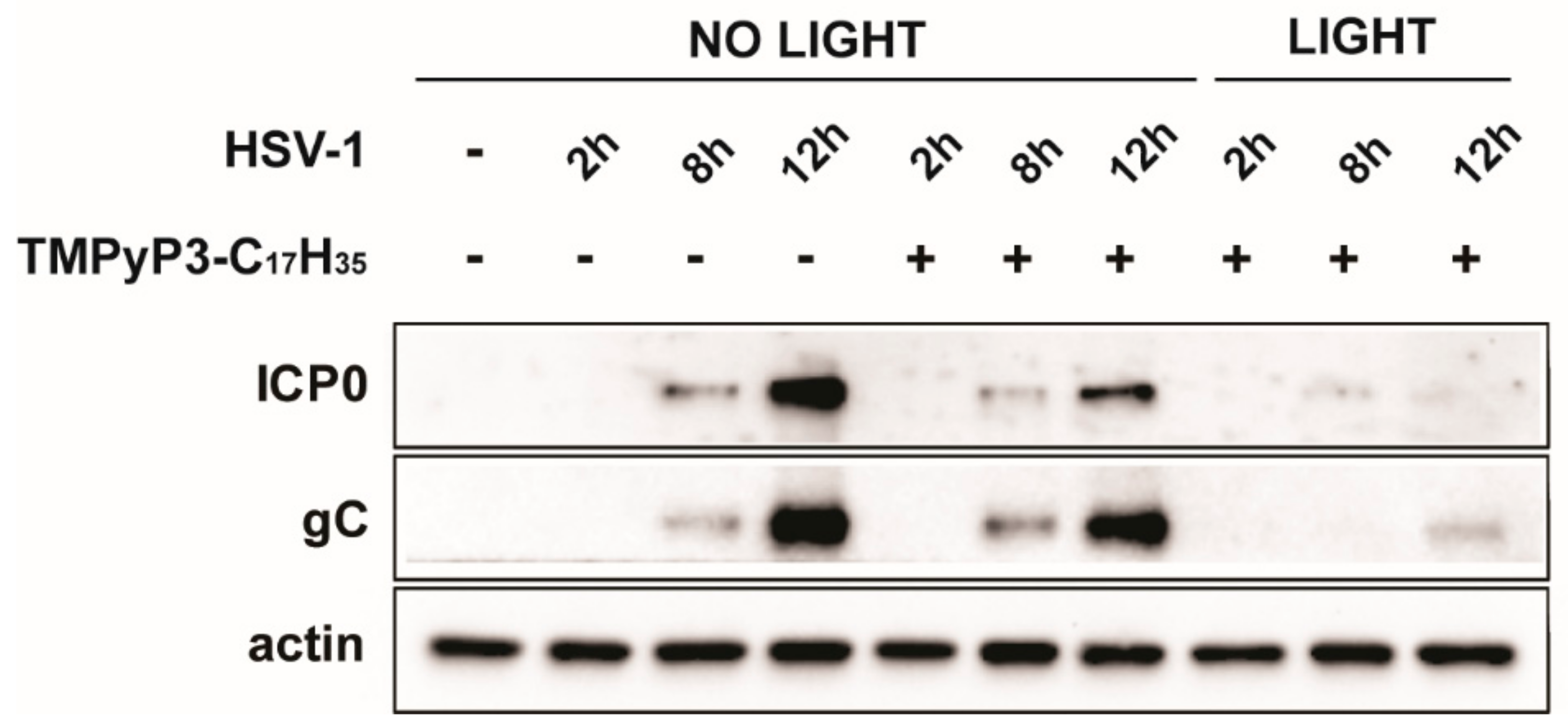
3.3. TMPyP3-C17H35 Reduces Levels of of Immediate Early Proteins
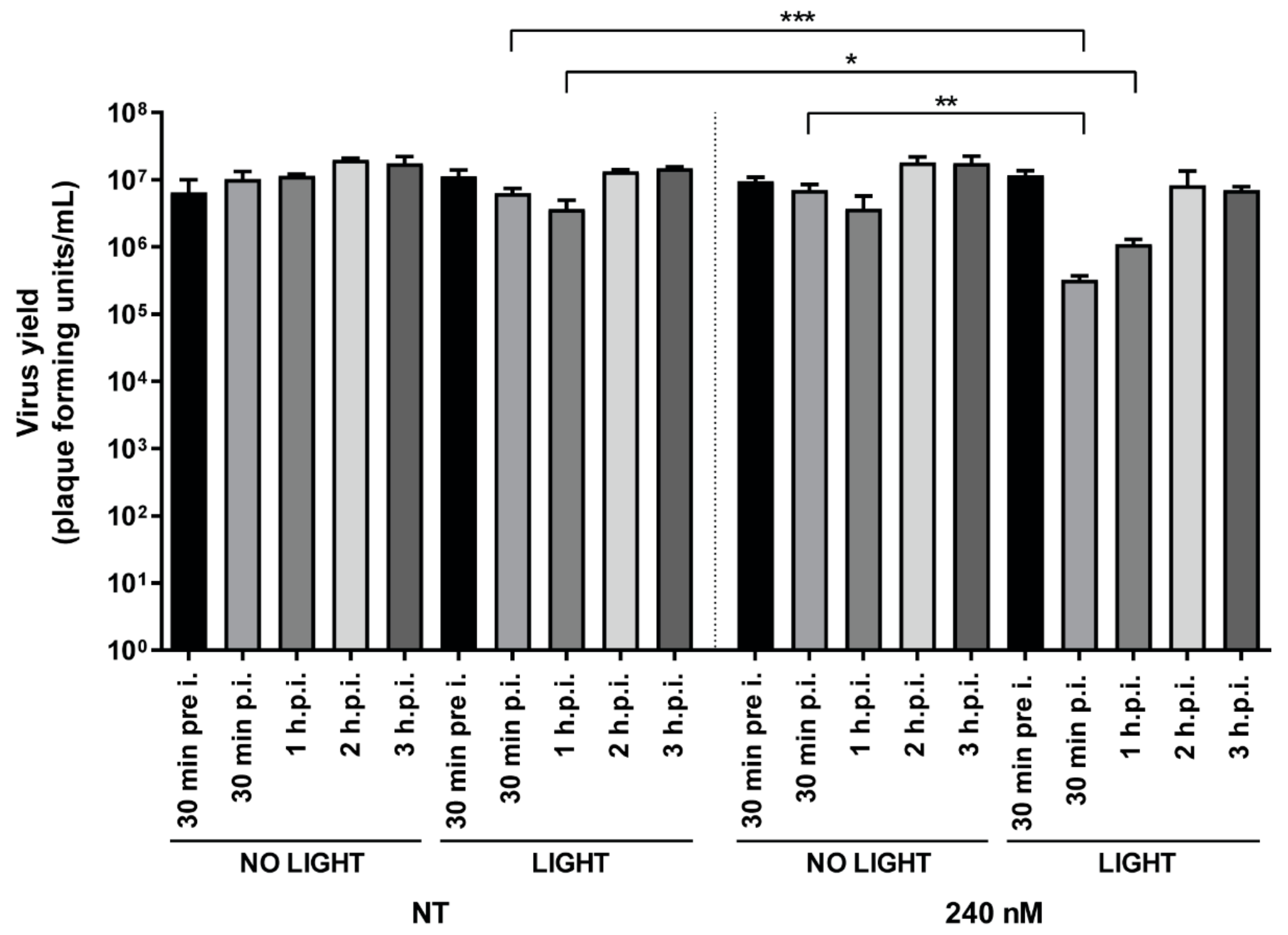
3.4. Addition of TMPyP3-C17H35 Early in Infection Inhibits HSV-1 Replication
3.5. TMPyP3-C17H35 Decreases HSV-1 Infectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malatesti, N.; Munitic, I.; Jurak, I. Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial and immunosuppressive agents. Biophys. Rev. 2017, 9, 149–168. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [Green Version]
- Thomas-Moore, B.A.; Del Valle, C.A.; Field, R.A.; Marin, M.J. Recent advances in nanoparticle-based targeting tactics for antibacterial photodynamic therapy. Photochem. Photobiol. Sci. 2022, 21, 1111–1131. [Google Scholar] [CrossRef]
- Hung, J.H.; Lee, C.N.; Hsu, H.W.; Ng, I.S.; Wu, C.J.; Yu, C.K.; Lee, N.Y.; Chang, Y.; Wong, T.W. Recent Advances in Photodynamic Therapy against Fungal Keratitis. Pharmaceutics 2021, 13, 2011. [Google Scholar] [CrossRef]
- Sharma, R.; Viana, S.M.; Ng, D.K.P.; Kolli, B.K.; Chang, K.P.; de Oliveira, C.I. Photodynamic inactivation of Leishmania braziliensis doubly sensitized with uroporphyrin and diamino-phthalocyanine activates effector functions of macrophages in vitro. Sci. Rep. 2020, 10, 17065. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.I.; Lu, L.M.; Chen, Y.; Lin, Y.K. Photodynamic therapy as an antifungal treatment. Exp. Ther. Med. 2016, 12, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.P.; Kolli, B.K.; New Light, G. New “light” for one-world approach toward safe and effective control of animal diseases and insect vectors from leishmaniac perspectives. Parasit. Vectors 2016, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, B.; Williams, B.; Rywkin, S.; Prince, A.M.; Pascual, D.; Geacintov, N.; Valinsky, J. Inactivation of viruses in blood with aluminum phthalocyanine derivatives. Transfusion 1991, 31, 102–108. [Google Scholar] [CrossRef]
- Smetana, Z.; Ben-Hur, E.; Mendelson, E.; Salzberg, S.; Wagner, P.; Malik, Z. Herpes simplex virus proteins are damaged following photodynamic inactivation with phthalocyanines. J. Photochem. Photobiol. B 1998, 44, 77–83. [Google Scholar] [CrossRef]
- Smetana, Z.; Mendelson, E.; Manor, J.; van Lier, J.E.; Ben-Hur, E.; Salzberg, S.; Malik, Z. Photodynamic inactivation of herpes viruses with phthalocyanine derivatives. J. Photochem. Photobiol. B 1994, 22, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Schnipper, L.E.; Lewin, A.A.; Swartz, M.; Crumpacker, C.S. Mechanisms of photodynamic inactivation of herpes simplex viruses: Comparison between methylene blue, light plus electricity, and hematoporhyrin plus light. J. Clin. Investig. 1980, 65, 432–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monjo, A.L.; Pringle, E.S.; Thornbury, M.; Duguay, B.A.; Monro, S.M.A.; Hetu, M.; Knight, D.; Cameron, C.G.; McFarland, S.A.; McCormick, C. Photodynamic Inactivation of Herpes Simplex Viruses. Viruses 2018, 10, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajmal, M. Effectiveness of photodynamic therapy as an adjunct to topical antiviral therapy in the treatment of herpes labialis: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2021, 34, 102302. [Google Scholar] [CrossRef]
- Anil, S.; Yahia, M.E.; Alsarani, M.M.; Alolayani, B.M.; Alsadon, O.; Vellappally, S.; Hashem, M.; Fouad, H. Antimicrobial efficacy and topographical alterations of photodynamic therapy versus conventional antimicrobials on contaminated zirconia ceramic in vitro. Photodiagnosis Photodyn. Ther. 2022, 38, 102804. [Google Scholar] [CrossRef]
- Roizman, B.; Knipe, D.M.; Whitley, R.J. Fields Virology, 6th ed.; Knipe, D.M.H.P., Cohen, J.I., Griffin, D.E., Lamb, R.A., Martin, M.A., Racaniello, V.R., Roizman, B., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Whitley, R.J.; Roizman, B. Clinical Virology, 4th ed.; Richman, D.D., Whitley, R.J., Hayden, F.G., Eds.; ASM Press: Almere, The Netherlands, 2017. [Google Scholar]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef]
- Bacon, T.H.; Levin, M.J.; Leary, J.J.; Sarisky, R.T.; Sutton, D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003, 16, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Bohn-Wippert, K.; Schlattmann, P.; Zell, R.; Sauerbrei, A. Sequence Analysis of Herpes Simplex Virus 1 Thymidine Kinase and DNA Polymerase Genes from over 300 Clinical Isolates from 1973 to 2014 Finds Novel Mutations That May Be Relevant for Development of Antiviral Resistance. Antimicrob. Agents Chemother. 2015, 59, 4938–4945. [Google Scholar] [CrossRef] [Green Version]
- Frobert, E.; Burrel, S.; Ducastelle-Lepretre, S.; Billaud, G.; Ader, F.; Casalegno, J.S.; Nave, V.; Boutolleau, D.; Michallet, M.; Lina, B.; et al. Resistance of herpes simplex viruses to acyclovir: An update from a ten-year survey in France. Antivir. Res. 2014, 111, 36–41. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Velzen, M.; van de Vijver, D.A.; van Loenen, F.B.; Osterhaus, A.D.; Remeijer, L.; Verjans, G.M. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J. Infect. Dis. 2013, 208, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, K.M.; Cunha, S.R.; Goncalves, F.; Escudeiro, G.S.; Steiner-Oliveira, C.; Horliana, A.C.R.T.; Eduardo, C.D. Photodynamic therapy and Acyclovir in the treatment of recurrent herpes labialis: A controlled randomized clinical trial. Photodiagnosis Photodyn. Ther. 2021, 33, 102093. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Im, S.K.; Park, H.Y. Recurrent herpes simplex keratitis after verteporfin photodynamic therapy for corneal neovascularization. Cornea 2010, 29, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Malatesti, N.; Harej, A.; Kraljevic Pavelic, S.; Loncaric, M.; Zorc, H.; Wittine, K.; Andjelkovic, U.; Josic, D. Synthesis, characterisation and in vitro investigation of photodynamic activity of 5-(4-octadecanamidophenyl)-10,15,20-tris(N-methylpyridinium-3-yl)porphyrin trichloride on HeLa cells using low light fluence rate. Photodiagnosis Photodyn. Ther. 2016, 15, 115–126. [Google Scholar] [CrossRef]
- Lesar, A.; Muskovic, M.; Begic, G.; Loncaric, M.; Tomic Linsak, D.; Malatesti, N.; Gobin, I. Cationic Porphyrins as Effective Agents in Photodynamic Inactivation of Opportunistic Plumbing Pathogen Legionella pneumophila. Int. J. Mol. Sci. 2020, 21, 5367. [Google Scholar] [CrossRef] [PubMed]
- Muskovic, M.; Cavar, I.; Lesar, A.; Loncaric, M.; Malatesti, N.; Gobin, I. Photodynamic Inactivation of Legionella pneumophila Biofilm Formation by Cationic Tetra- and Tripyridylporphyrins in Waters of Different Hardness. Int. J. Mol. Sci. 2021, 22, 9095. [Google Scholar] [CrossRef]
- Jurak, I.; Silverstein, L.B.; Sharma, M.; Coen, D.M. Herpes Simplex Virus Is Equipped with RNA- and Protein-Based Mechanisms To Repress Expression of ATRX, an Effector of Intrinsic Immunity. J. Virol. 2012, 86, 10093–10102. [Google Scholar] [CrossRef] [Green Version]
- Vedachalam, S.; Choi, B.H.; Pasunooti, K.K.; Ching, K.M.; Lee, K.; Yoon, H.S.; Liu, X.W. Glycosylated porphyrin derivatives and their photodynamic activity in cancer cells. Medchemcomm 2011, 2, 371–377. [Google Scholar] [CrossRef]
- McCormick, B.P.P.; Pansa, M.F.; Sanabria, L.N.M.; Carvalho, C.M.B.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Vittar, N.B.R.; Rivarola, V.A. Cationic porphyrin derivatives for application in photodynamic therapy of cancer. Laser Phys. 2014, 24, 45603. [Google Scholar] [CrossRef]
- Jelovica, M.; Grbcic, P.; Muskovic, M.; Sedic, M.; Pavelic, S.K.; Loncaric, M.; Malatesti, N. In Vitro Photodynamic Activity of N-Methylated and N-Oxidised Tripyridyl Porphyrins with Long Alkyl Chains and Their Inhibitory Activity in Sphingolipid Metabolism. Chemmedchem 2018, 13, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Giuntini, F.; Faustino, M.A.; Tome, J.P.; Neves, M.G.; Tome, A.C.; Silva, A.M.; Santana-Marques, M.G.; Ferrer-Correia, A.J.; Cavaleiro, J.A.; et al. Synthesis of cationic beta-vinyl substituted meso-tetraphenylporphyrins and their in vitro activity against herpes simplex virus type 1. Bioorg. Med. Chem. Lett. 2005, 15, 3333–3337. [Google Scholar] [CrossRef]
- Tome, J.P.; Neves, M.G.; Tome, A.C.; Cavaleiro, J.A.; Mendonca, A.F.; Pegado, I.N.; Duarte, R.; Valdeira, M.L. Synthesis of glycoporphyrin derivatives and their antiviral activity against herpes simplex virus types 1 and 2. Bioorg. Med. Chem. 2005, 13, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Prasmickaite, L.; Hogset, A.; Selbo, P.K.; Engesaeter, B.O.; Hellum, M.; Berg, K. Photochemical disruption of endocytic vesicles before delivery of drugs: A new strategy for cancer therapy. Br. J. Cancer 2002, 86, 652–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicola, A.V.; McEvoy, A.M.; Straus, S.E. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and chinese hamster ovary cells. J. Virol. 2003, 77, 5324–5332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praena, B.; Bello-Morales, R.; Lopez-Guerrero, J.A. Hsv-1 Endocytic Entry into a Human Oligodendrocytic Cell Line Is Mediated by Clathrin and Dynamin but Not Caveolin. Viruses 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, G.; Pritchard, S.M.; Nicola, A.V. Herpes Simplex Virus Entry by a Nonconventional Endocytic Pathway. J. Virol. 2020, 94, e01910-20. [Google Scholar] [CrossRef]
- Lotufo, M.A.; Horliana, A.C.R.T.; Santana, T.; de Queiroz, A.C.; Gomes, A.O.; Motta, L.J.; Ferrari, R.A.M.; Fernandes, K.P.D.; Bussadori, S.K. Efficacy of photodynamic therapy on the treatment of herpes labialis: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 29, 101536. [Google Scholar] [CrossRef]
- Lago, A.D.N.; Furtado, G.S.; Ferreira, O.C.; Diniz, R.S.; Goncalves, L.M. Resolution of herpes simplex in the nose wing region using photodynamic therapy and photobiomodulation. Photodiagnosis Photodyn. Ther. 2018, 23, 237–239. [Google Scholar] [CrossRef]
- Carneiro, J.; Goncalves, A.; Zhou, Z.H.; Griffin, K.E.; Kaufman, N.E.M.; Vicente, M.D.H. Synthesis and in vitro PDT evaluation of new porphyrins containing meso-epoxymethylaryl cationic groups. Laser Surg. Med. 2018, 50, 566–575. [Google Scholar] [CrossRef]
- Woodburn, K.W.; Vardaxis, N.J.; Hill, J.S.; Kaye, A.H.; Phillips, D.R. Subcellular-Localization of Porphyrins Using Confocal Laser Scanning Microscopy. Photochem. Photobiol. 1991, 54, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Far-Red Fluorescence Probe for Monitoring Singlet Oxygen during Photodynamic Therapy. J. Am. Chem. Soc. 2014, 136, 11707–11715. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, S.A.G.; Schwartz, K.R.; Aalders, M.C.G.; Dankert, J.B. Photodynamic inactivation of fibroblasts by a cationic porphyrin. Laser Med. Sci. 2005, 20, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Rapozzi, V.; Zorzet, S.; Zacchigna, M.; Della Pietra, E.; Cogoi, S.; Xodo, L.E. Anticancer activity of cationic porphyrins in melanoma tumour-bearing mice and mechanistic in vitro studies. Mol. Cancer 2014, 13, 75. [Google Scholar] [CrossRef] [Green Version]
- Zhdanova, K.A.; Savelyeva, I.O.; Ezhov, A.V.; Zhdanov, A.P.; Zhizhin, K.Y.; Mironov, A.F.; Bragina, N.A.; Babayants, A.A.; Frolova, I.S.; Filippova, N.I.; et al. Novel Cationic Meso-Arylporphyrins and Their Antiviral Activity against HSV-1. Pharmaceuticals 2021, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Weber, S.; Kaufmann, R. Genotoxic potential of porphyrin type photosensitizers with particular emphasis on 5-aminolevulinic acid: Implications for clinical photodynamic therapy. Free. Radic. Biol. Med. 2000, 28, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.; Grimaldi, E.; Perfetto, B.; Donnarumma, M.; De Filippis, A.; Donnarumma, G.; Tufano, M.A. 5-aminolaevulinic acid and photodynamic therapy reduce HSV-1 replication in HaCat cells through an apoptosis-independent mechanism. Photodermatol. Photoimmunol. Photomed. 2008, 24, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Elesela, S.; Lukacs, N.W. Role of Mitochondria in Viral Infections. Life 2021, 11, 232. [Google Scholar] [CrossRef]
- Conrado, P.C.V.; Sakita, K.M.; Arita, G.S.; Galinari, C.B.; Goncalves, R.S.; Lopes, L.D.G.; Lonardoni, M.V.C.; Teixeira, J.J.V.; Bonfim-Mendonca, P.S.; Kioshima, E.S. A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2. Photodiagnosis Photodyn. Ther. 2021, 34, 102221. [Google Scholar] [CrossRef]
- Fossarello, M.; Peiretti, E.; Zucca, I.; Serra, A. Photodynamic therapy of corneal neovascularization with verteporfin. Cornea 2003, 22, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Feng, J.; Song, X.; Xiang, W. Efficacy of photodynamic therapy for warts induced by human papilloma virus infection: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2022, 39, 102913. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurak, I.; Cokarić Brdovčak, M.; Djaković, L.; Bertović, I.; Knežević, K.; Lončarić, M.; Jurak Begonja, A.; Malatesti, N. Photodynamic Inhibition of Herpes Simplex Virus 1 Infection by Tricationic Amphiphilic Porphyrin with a Long Alkyl Chain. Pharmaceutics 2023, 15, 956. https://doi.org/10.3390/pharmaceutics15030956
Jurak I, Cokarić Brdovčak M, Djaković L, Bertović I, Knežević K, Lončarić M, Jurak Begonja A, Malatesti N. Photodynamic Inhibition of Herpes Simplex Virus 1 Infection by Tricationic Amphiphilic Porphyrin with a Long Alkyl Chain. Pharmaceutics. 2023; 15(3):956. https://doi.org/10.3390/pharmaceutics15030956
Chicago/Turabian StyleJurak, Igor, Maja Cokarić Brdovčak, Lara Djaković, Ivana Bertović, Klaudia Knežević, Martin Lončarić, Antonija Jurak Begonja, and Nela Malatesti. 2023. "Photodynamic Inhibition of Herpes Simplex Virus 1 Infection by Tricationic Amphiphilic Porphyrin with a Long Alkyl Chain" Pharmaceutics 15, no. 3: 956. https://doi.org/10.3390/pharmaceutics15030956





