Comparative HPLC–DAD–ESI-QTOF/MS/MS Analysis of Bioactive Phenolic Compounds Content in the Methanolic Extracts from Flowering Herbs of Monarda Species and Their Free Radical Scavenging and Antimicrobial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical Reagents
2.3. Microbiological Material
2.3.1. Preparation of Plant Extracts
2.3.2. HPLC–DAD–ESI-QTOF-MS/MS Analysis
2.3.3. Determination of Total Polyphenolic Content (TPC)
2.3.4. DPPH Radical Scavenging Activity
2.3.5. Antimicrobial Assay
3. Results
3.1. Chemical Analysis of Phenolic Compounds in Methanolic Extracts from Monarda Species
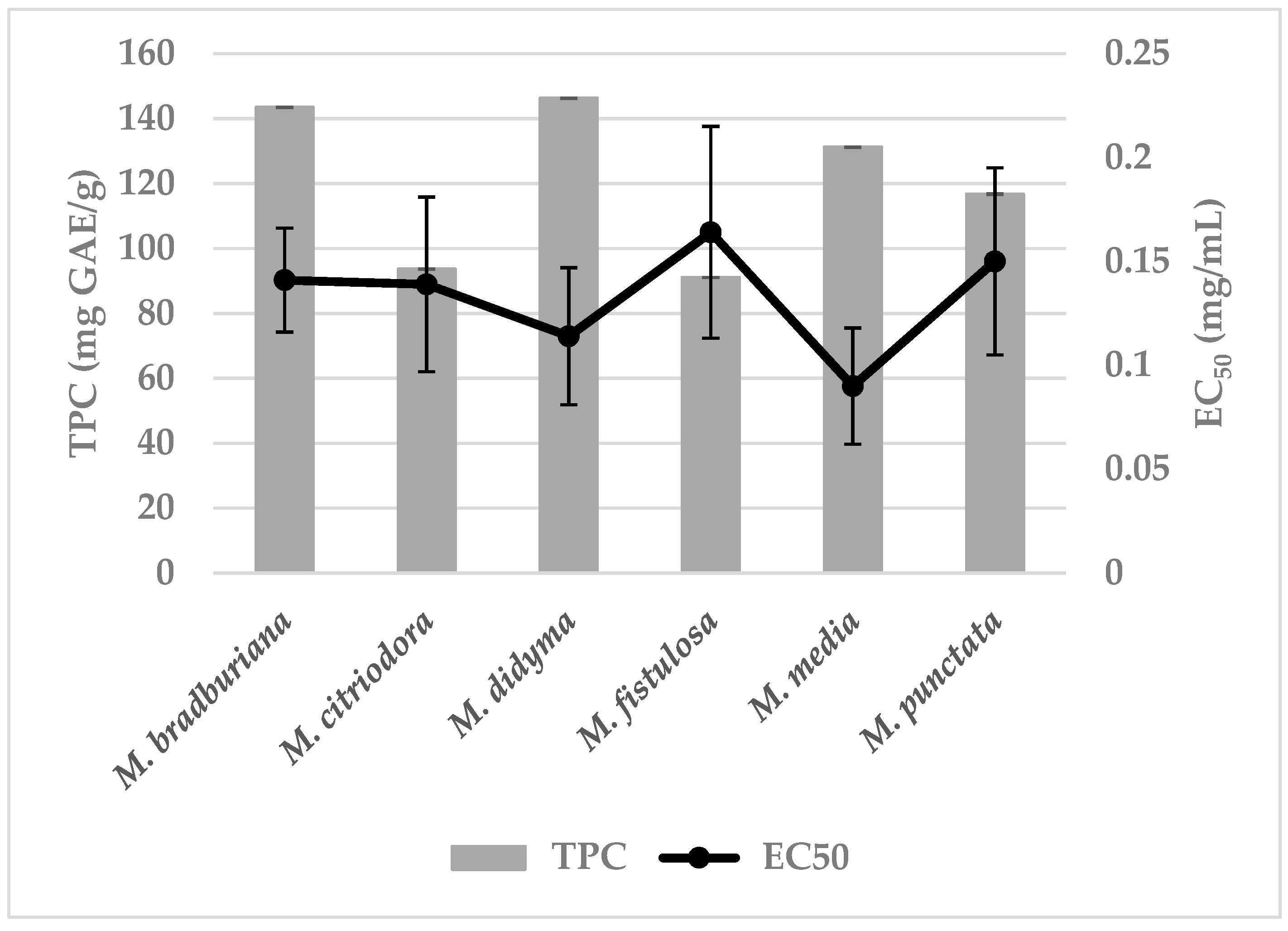
3.2. The Total Polyphenol Content (TPC) in the Methanolic Extracts from Monarda Species and Their Antioxidant Potential
3.3. The Antimicrobial Activity Assessment of Methanolic Extracts from Monarda Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Ricci, D.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antifungal and in vitro antioxidant properties of Monarda didyma L. essential oil. J. Essent. Oil Res. 2006, 18, 581–585. [Google Scholar] [CrossRef]
- Ricci, D.; Epifano, F.; Fraternale, D. The essential oil of Monarda didyma L. (Lamiaceae) exerts phytotoxic activity in vitro against various weed seeds. Molecules 2017, 22, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Stefanini, I.; Piccaglia, R.; Marotti, M.; Biavati, B. Characterization and biological activity of essential oils from fourteen Labiatae species. Acta Horticult. 2006, 723, 221–226. [Google Scholar] [CrossRef]
- Shanaida, M.; Jasicka-Misiak, I.; Makowicz, E.; Stanek, N.; Shanaida, V.; Wieczorek, P.P. Development of high-performance thin layer chromatography method for identification of phenolic compounds and quantification of rosmarinic acid content in some species of the Lamiaceae family. J. Pharm. Bioallied Sci. 2020, 12, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and pharmacological screening of a Monarda fistulosa L. dry extract based on a hydrodistilled residue by-product. Front. Pharmacol. 2021, 29, 563436. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.; Kiehne, P.; Maroko, J.; Kapsner, T.; Angerhofer, C.K. Seasonal variation in chemistry and biological activity of Monarda fistulosa L. Planta Med. 2013, 79, 11. [Google Scholar] [CrossRef]
- Shanaida, M.; Hudz, N.; Korzeniowska, K.; Wieczorek, P. Antioxidant activity of essential oils obtained from aerial part of some Lamiaceae species. Int. J. Green Pharm. 2018, 12, 200–204. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 1, 402–414. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and quantitative analysis of polyphenols in Lamiaceae plants-a review. Plants 2018, 7, 25–55. [Google Scholar] [CrossRef] [Green Version]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Kondo, T.; Nakane, Y.; Tamura, H.; Goto, T. Structure of monardein, a bis-malonylated anthocyanin isolated from golden balm, Monarda didyma. Tetrahedron Lett. 1985, 26, 5879–5882. [Google Scholar] [CrossRef]
- Savickienė, N.; Dagilytė, A.; Barsteigienė, Z.; Kazlauskas, S.; Vaičiūnienė, J. Identification of flavonoids in the flowers and leaves of Monarda didyma L. Medicina 2002, 38, 1119–1122. [Google Scholar] [PubMed]
- Davies, A.J.; Mazza, G. Separation and characterization of anthocyanins of Monarda fistulosa by high performance liquid chromatograph. J. Agric. Food Chem. 1992, 40, 1341–1345. [Google Scholar] [CrossRef]
- Marshall, H.H.; Scora, R.W. A new chemical race of Monarda fistulosa (Labiatae). Can. J. Bot. 1972, 50, 1845–1849. [Google Scholar] [CrossRef]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 2020, 28, 691–721. [Google Scholar] [CrossRef]
- Brglez, M.E.; Knez, H.M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 11, 901–939. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Li, H.; Yang, T.; Li, F.-Y.; Yao, Y.; Sun, Z.-M. Antibacterial activity and mechanism of action of Monarda punctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int. J. Clin. Exp. Pathol. 2014, 7, 7389–7398. [Google Scholar]
- Wu, Q.; Gu, H.W.; Yin, X.L.; Zhou, H.H.; Chang, H.Y.; Shi, J.; Chen, Y.; Yan, X.F.; Zhi Liu, Z. Development of an HPLC-DAD method combined with chemometrics for differentiating geographical origins of chinese red wines on the basis of phenolic compounds. Food Anal. Methods 2021, 14, 1895–1907. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Kozyra, M.; Skalicka-Woźniak, K. Quantitative analysis of flavonoids and phenolic acids from inflorescences and aerial parts of selected Cirsium species using ASE method. Acta Pol. Pharm. 2014, 71, 877–881. [Google Scholar] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, E.; Berset, C.M. Use of free radical method to evaluate antioxidant activity. Lebensm.-Wiss. u.-Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dunn, J.; Wild, D. The Immunoassay Handbook, 4th ed.; Theory and Applications of Ligand Binding, ELISA and Related Techniques Chapter 3.6-Calibration Curve Fitting; Elsevier: Amsterdam, The Netherlands, 2013; pp. 323–336. [Google Scholar]
- Łączkowski, K.Z.; Misiura, K.; Biernasiuk, A.; Malm, A. Synthesis and antimicrobial activities of (4,5,6,7-tetrahydro-1H-indazol- 2(3H)-yl)thiazole derivatives. Lett. Drug Design. Disc. 2014, 11, 960–967. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Marengo, A.; Maxia, A.; Sanna, C.; Bertea, C.M.; Bicchi, C.; Ballero, M.; Cagliero, C.; Rubiolo, P. Characterization of four wild edible Carduus species from the Mediterranean region via phytochemical and biomolecular analyses. Food Res. Int. 2017, 100, 822–831. [Google Scholar] [CrossRef]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Aksic, M.M.F.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž.L. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef]
- Gontar, Ł.; Gontar, A.; Geszprych, A.; Geszprych, J.; Przybył, J.; Buła, M.; Osińska, E. Chemical variability of lemon beebalm (Monarda citriodora Cerv. ex Lag.) during plant phenology. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100433. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Krasyuk, E.V.; Pupyrkina, K.A. Qualitative analysis and development of methods for quantitative determination of flavonoids in Monarda species introduced in the Republic of Bashkortostan. Med. Bull. Bashkortostan. 2016, 11, 73–77. [Google Scholar]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Zhumakanova, B.S.; Korona-Głowniak, I.; Skalicka-Wozniak, K.; Ludwiczuk, A.; Baj, T.; Wojtanowski, K.K.; Józefczyk, A.; Zhaparkulova, K.A.; Sakipova, Z.B.; Malm, A. Phytochemical fingerprinting and in vitro antimicrobial and antioxidant activity of the aerial parts of Thymus marschallianus Willd. and Thymus seravschanicus Klokov growing widely in Southern Kazakhstan. Molecules 2021, 26, 3193–3207. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. Cameroonian medicinal plants: Pharmacology and derived natural products. Front Pharmacol. 2010, 1, 123–142. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) models. Front. Microbiol. 2019, 10, 829–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Pandey, A. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750–162766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Adaszyńska, M.; Swarcewicz, M. Wybrane wtórne metabolity roślinne jako środki przeciwdrobnoustrojowe. Wiad. Chem. 2013, 67, 303–319. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microb. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Liu, S.; Song, F.; Guo, J.; Huang, C. Influence of naringenin on the biofilm formation of Streptococcus mutans. J. Dent. 2018, 76, 24–31. [Google Scholar] [CrossRef]
- Kozłowska, J.; Grela, E.; Baczyńska, D.; Grabowiecka, A.; Anioł, M. Novel o-alkyl derivatives of naringenin and their oximes with antimicrobial and anticancer activity. Molecules 2019, 24, 679–703. [Google Scholar] [CrossRef] [Green Version]
- Mundlia, J.; Ahuja, M.; Kumar, P.; Pillay, V. Improved antioxidant, antimicrobial and anticancer activity of naringenin on conjugation with pectin. 3 Biotech. 2019, 9, 312–333. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Kozłowska, J.; Krzyżek, P.; Anioł, M.; Seniuk, A.; Jermakow, K.; Dworniczek, E. Antimicrobial o-alkyl derivatives of naringenin and their oximes against multidrug-resistant bacteria. Molecules 2020, 25, 3642–3671. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Eumkeb, G.; Siriwong, S.; Thumanu, K. Synergistic activity of luteolin and amoxicillin combination against amoxicillin-resistant Escherichia coli and mode of action. J. Photochem. Photobiol. B. 2012, 117, 247–253. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sarjit, A.; Wang, Y.; Dykes, G.A. Antimicrobial activity of gallic acid against thermophilic Campylobacter is strain specific and associated with a loss of calcium ions. Food Microbiol. 2015, 46, 227–233. [Google Scholar] [CrossRef]
- Li, Z.J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.G.; Aibai, S. Antifungal activity of gallic acid in vitro and in vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef]
- Chao, C.Y.; Yin, M.C. Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 2009, 6, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R.; et al. Antimicrobial activity of ferulic acid against Cronobacter sakazakii and possible mechanism of action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar] [CrossRef]
- Ghosh, M.; Schepetkin, I.A.; Özek, G.; Özek, T.; Khlebnikov, A.I.; Damron, D.S.; Quinn, M.T. Essential oils from Monarda fistulosa: Chemical composition and activation of transient receptor potential A1 (TRPA1) channels. Molecules 2020, 25, 4873–4892. [Google Scholar] [CrossRef] [PubMed]

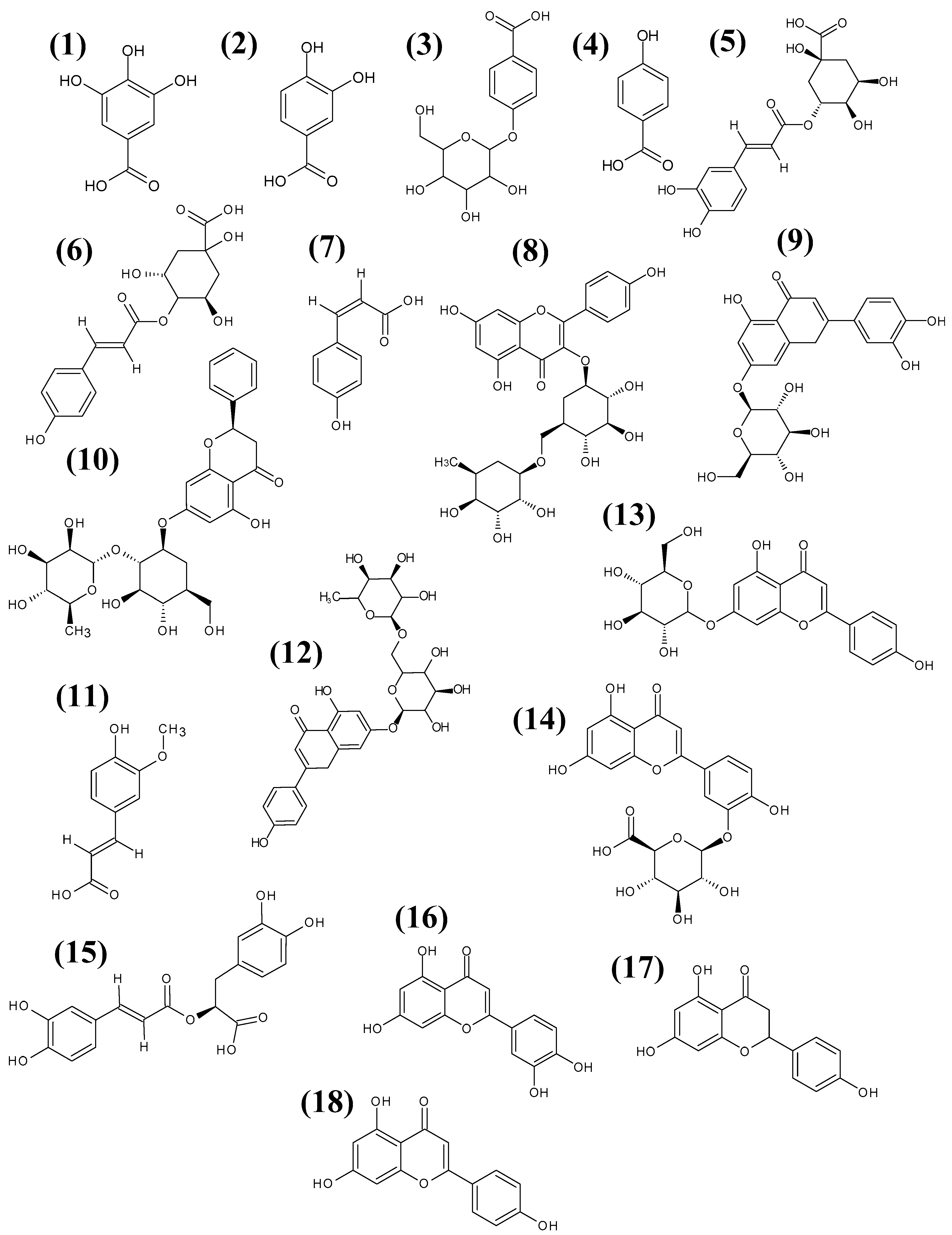
| No. | Rt [min] | Phenolic Compounds | Empirical Formula | [M − H]− (m/z) | Fragment Ions (m/z) | Diff (ppm) | M. b. | M. c. | M. d. | M. f. | M. m. | M. p. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.25 | gallic acid | C7H5O5− | 169.0100 | 125.0185 | −2.08 | + | + | + | + | + | − |
| 2 | 9.16 | protocatechuic acid * | C7H5O4− | 153.0217 | 109.0279 | −9.53 | + | + | + | + | + | + |
| 3 | 12.72 | hydroxybenzoic acid glucoside | C13H15O8− | 299.0784 | 137.0252 | −3.86 | + | + | + | + | + | − |
| 4 | 15.63 | p-OH-benzoic acid | C7H5O3− | 137.0259 | 108.0218 | −8.57 | + | + | + | + | + | + |
| 5 | 18.78 | chlorogenic acid | C16H17O9− | 353.0859 | 191.0532; 179.0339; 173.0405; 135.0454 | −7.61 | + | + | + | + | + | + |
| 6 | 20.47 | coumaryl-quinic acid * | C16H17O8− | 337.0962 | 191.0590; 173.0484; 163.0414 | −8.01 | + | + | + | + | + | + |
| 7 | 23.91 | p-coumaric acid | C9H7O3− | 163.0369 | 119.0480 | −8.12 | + | + | + | + | − | − |
| 8 | 27.40 | kaempherol-3-rutinoside | C27H29O15− | 593.1445 | 285.0403 | −7.25 | − | + | − | + | − | + |
| 9 | 28.84 | luteolin-7-glucoside | C21H19O11− | 447.0965 | 285.0555 | −5.39 | + | + | + | + | + | + |
| 10 | 28.96 | naringin | C27H31O14− | 579.1739 | 271.0586 | −7.88 | + | + | + | + | + | + |
| 11 | 29.41 | ferulic acid * | C10H9O4− | 193.0474 | 178.0205; 149.0590 | −11.69 | + | − | − | − | − | + |
| 12 | 29.49 | apigenin-7-rutinoside | C27H29O14− | 577.1597 | 269.0492 | −5.92 | + | + | + | + | + | + |
| 13 | 30.01 | apigenin-7-glucoside | C21 H19 O10− | 431.0970 | 269.0490 | 3.17 | − | − | + | + | − | + |
| 14 | 30.12 | luteolin-3-glucuronide * | C21H17O12− | 461.0725 | 285.0404 | 0.11 | + | + | + | + | + | + |
| 15 | 31.74 | rosmarinic acid | C18H15 O8− | 359.0756 | 197.0431; 161,0228 | −9.05 | + | + | + | + | + | + |
| 16 | 35.37 | luteolin * | C15H9O6− | 285.0430 | 267.7263 | −8.87 | + | + | + | + | + | + |
| 17 | 37.23 | naringenin | C15H11O5− | 271.0603 | 151.0088; 119.0276; 107.0412 | 3.30 | + | + | + | + | + | + |
| 18 | 37.47 | apigenin | C15H9O5− | 269.0469 | 117.0338 | −0.94 | + | + | + | + | + | + |
| Species | Zone of Growth Inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| M. bradburiana | M. citriodora | M. didyma | M. fistulosa | M. media | M. punctata | |
| Gram-positive bacteria | ||||||
| Staphylococcus aureus ATCC 25923 | +/− | 13 | 16 | +/− | 11 | – |
| Staphylococcus aureus ATCC 6538 | 10 | 12 | 15 | – | 8 | 8 |
| Staphylococcus aureus ATCC 29213 | – | 12 | 14 | – | 11 | – |
| Staphylococcus aureus ATCC 43300 | 9 | 14 | 15 | – | 11 | 11 |
| Staphylococcus epidermidis ATCC 12228 | – | – | 13 | – | 11 | 10 |
| Micrococcus luteus ATCC 10240 | 11 | 15 | 15 | – | 12 | 10 |
| Bacillus subtilis ATCC 6633 | 20 | 31 | 35 | 16 | 30 | 29 |
| Bacillus cereus ATCC 10876 | 14 | 17 | 17 | 13 | 14 | 14 |
| Enterococcus faecalis ATCC 29212 | – | 11 | 11 | – | 12 | +/− |
| Gram-negative bacteria | ||||||
| Bordetella bronchiseptica ATCC 4617 | 13 | 18 | 19 | 13 | 19 | 16 |
| Klebsiella pneumoniae ATCC 13883 | +/− | – | 13 | – | 12 | +/− |
| Proteus mirabilis ATCC 12453 | – | – | 11 | – | 10 | – |
| Salmonella Typhimurium ATCC 14028 | 10 | 11 | 13 | – | 12 | 11 |
| Escherichia coli ATCC 25922 | – | – | +/− | – | 10 | +/− |
| Pseudomonas aeruginosa ATCC 27853 | 13 | 13 | 13 | 13 | 14 | 12 |
| Fungi | ||||||
| Candida albicans ATCC 2091 | 13 | 12 | 12 | 13 | 12 | 13 |
| Candida albicans ATCC 10231 | +/− | +/− | 16 | +/− | 16 | 14 |
| Candida parapsilosis ATCC 22019 | +/− | +/− | +/− | +/− | +/− | +/− |
| Candida glabrata ATCC 90030 | – | – | – | – | – | – |
| Candida krusei ATCC 14243 | 11 | 12 | 15 | 10 | 14 | 15 |
| Species | M. bradburiana | M. citriodora | M. didyma | M. fistulosa | M. media | M. punctata | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. aureus ATCC 25923 | 0.63 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 | 0.63 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 |
| S. aureus ATCC 6538 | 0.63 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.63 | 0.63 |
| S. aureus ATCC 29213 | 0.63 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 | 0.63 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 |
| S. aureus ATCC 43300 | 0.63 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 |
| S. epidermidis ATCC 12228 | 0.31 | 0.31 | 0.15 | 0.15 | 0.15 | 0.15 | 0.31 | 0.63 | 0.15 | 0.31 | 0.31 | 0.31 |
| M. luteus ATCC 10240 | 0.63 | 0.63 | 0.31 | 0.31 | 0.07 | 0.15 | 0.63 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 |
| B. subtilis ATCC 6633 | 0.63 | 0.63 | 0.31 | 0.31 | 0.31 | 0.31 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 |
| B. cereus ATCC 10876 | 0.63 | 0.63 | 0.63 | 0.63 | 0.31 | 0.31 | 1.25 | 1.25 | 0.63 | 0.63 | 1.25 | 1.25 |
| E. faecalis ATCC 29212 | 1.25 | 1.25 | 0.63 | 0.63 | 0.31 | 0.63 | 1.25 | 1.25 | 0.63 | 0.63 | 0.63 | 0.63 |
| Species | M. bradburiana | M. citriodora | M. didyma | M. fistulosa | M. media | M. punctata | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| B. bronchiseptica ATCC 4617 | 1.25 | 1.25 | 0.63 | 0.63 | 2.5 | 2.5 | 1.25 | 1.25 | 2.5 | 2.5 | 1.25 | 1.25 |
| K. pneumoniae ATCC 13883 | 5 | 5 | 10 | 10 | 2.5 | 2.5 | 10 | 10 | 2.5 | 2.5 | 10 | 10 |
| P. mirabilis ATCC 12453 | 10 | 10 | 10 | 10 | 5 | 5 | 10 | 10 | 5 | 5 | 10 | 10 |
| S. Typhimurium ATCC 14028 | 10 | 10 | 10 | 10 | 5 | 5 | 10 | 10 | 5 | 5 | 10 | 10 |
| E. coli ATCC 25922 | 10 | 10 | 10 | 10 | 5 | 5 | 10 | 10 | 10 | 10 | 10 | 10 |
| P. aeruginosa ATCC 27853 | 10 | 10 | 5 | 5 | 5 | 5 | 10 | 10 | 5 | 5 | 10 | 10 |
| Species | M. bradburiana | M. citriodora | M. didyma | M. fistulosa | M. media | M. punctata | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| C. albicans ATCC 2091 | 2.5 | 5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 5 | 2.5 | 5 | 5 | 5 |
| C. albicans ATCC 10231 | 2.5 | 5 | 1.25 | 2.5 | 1.25 | 2.5 | 2.5 | 5 | 1.25 | 2.5 | 1.25 | 2.5 |
| C. parapsilosis ATCC 22019 | 5 | 5 | 2.5 | 5 | 2.5 | 5 | 5 | 5 | 2.5 | 5 | 2.5 | 5 |
| C. glabrata ATCC 90030 | 5 | 10 | 2.5 | 2.5 | 2.5 | 5 | 5 | 10 | 5 | 5 | 5 | 5 |
| C. krusei ATCC 14243 | 5 | 5 | 2.5 | 2.5 | 1.25 | 2.5 | 5 | 5 | 2.5 | 5 | 2.5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozyra, M.; Biernasiuk, A.; Wiktor, M.; Kukula-Koch, W.; Malm, A. Comparative HPLC–DAD–ESI-QTOF/MS/MS Analysis of Bioactive Phenolic Compounds Content in the Methanolic Extracts from Flowering Herbs of Monarda Species and Their Free Radical Scavenging and Antimicrobial Activities. Pharmaceutics 2023, 15, 964. https://doi.org/10.3390/pharmaceutics15030964
Kozyra M, Biernasiuk A, Wiktor M, Kukula-Koch W, Malm A. Comparative HPLC–DAD–ESI-QTOF/MS/MS Analysis of Bioactive Phenolic Compounds Content in the Methanolic Extracts from Flowering Herbs of Monarda Species and Their Free Radical Scavenging and Antimicrobial Activities. Pharmaceutics. 2023; 15(3):964. https://doi.org/10.3390/pharmaceutics15030964
Chicago/Turabian StyleKozyra, Małgorzata, Anna Biernasiuk, Magdalena Wiktor, Wirginia Kukula-Koch, and Anna Malm. 2023. "Comparative HPLC–DAD–ESI-QTOF/MS/MS Analysis of Bioactive Phenolic Compounds Content in the Methanolic Extracts from Flowering Herbs of Monarda Species and Their Free Radical Scavenging and Antimicrobial Activities" Pharmaceutics 15, no. 3: 964. https://doi.org/10.3390/pharmaceutics15030964
APA StyleKozyra, M., Biernasiuk, A., Wiktor, M., Kukula-Koch, W., & Malm, A. (2023). Comparative HPLC–DAD–ESI-QTOF/MS/MS Analysis of Bioactive Phenolic Compounds Content in the Methanolic Extracts from Flowering Herbs of Monarda Species and Their Free Radical Scavenging and Antimicrobial Activities. Pharmaceutics, 15(3), 964. https://doi.org/10.3390/pharmaceutics15030964








