Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery
Abstract
1. Introduction
2. General Issues and Status Quo of Polymeric Micelles
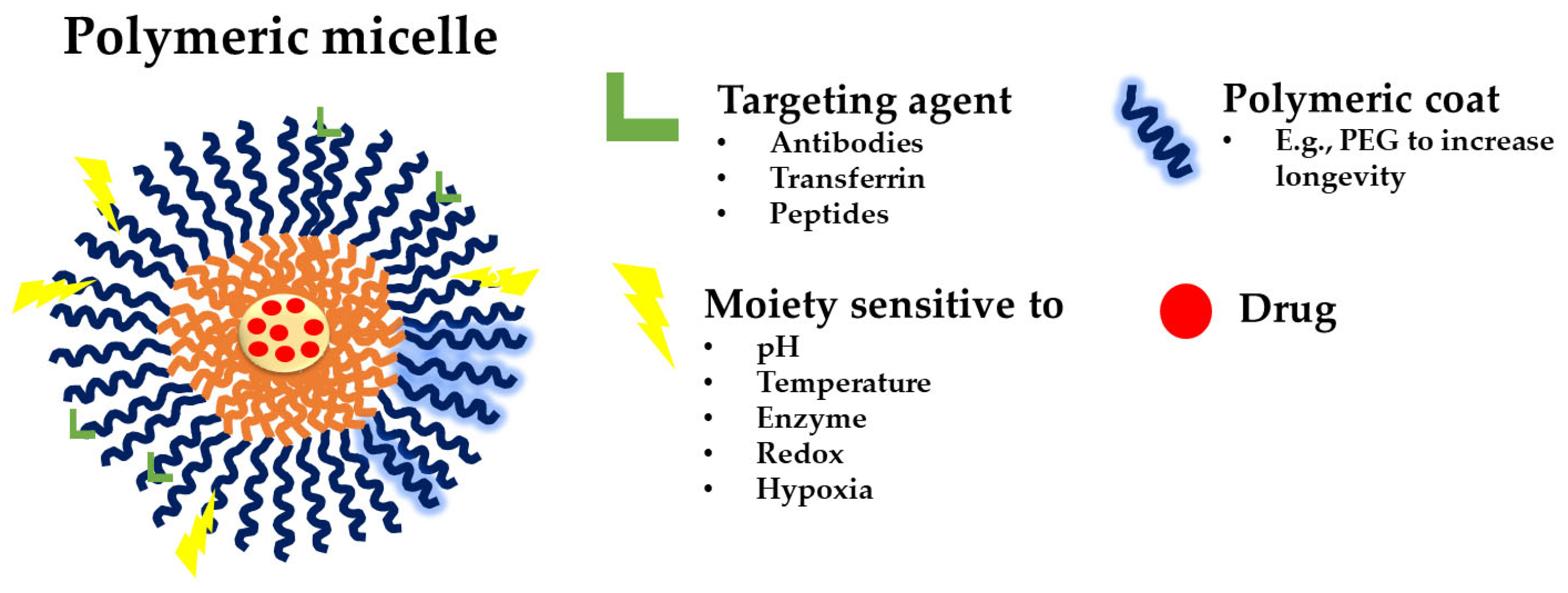
2.1. Polymeric Micelles and Micellar Structures
2.2. Critical Micelle Concentration
2.3. Preparation Methods
2.3.1. Direct Dissolution
2.3.2. Solvent Evaporation/Film Hydration
2.3.3. Oil in Water Emulsion
2.3.4. Dialysis
2.3.5. Other Preparation Methods
2.3.6. Functionalization Methods
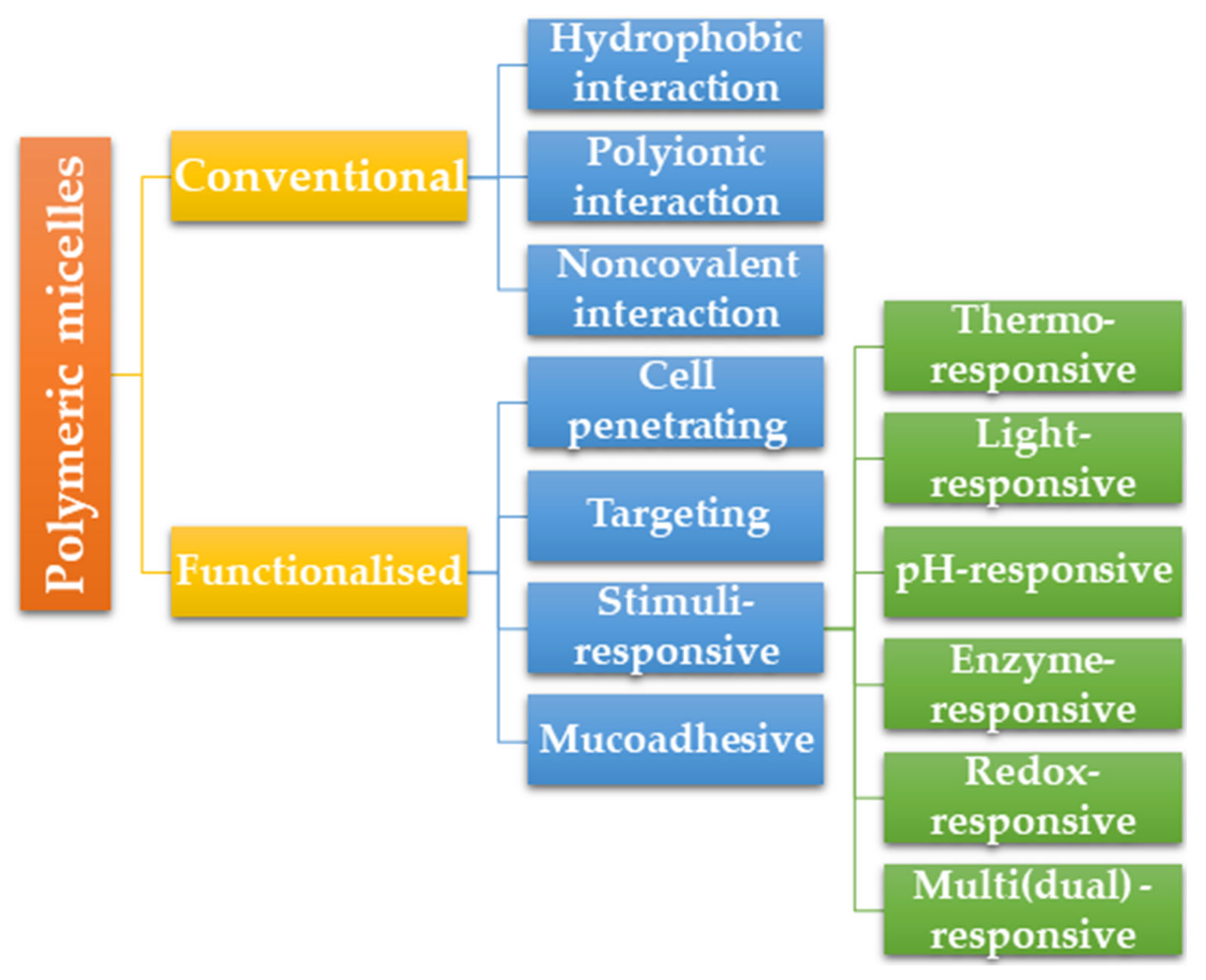
2.4. Polymeric Micelle Types
2.4.1. Conventional Polymeric Micelles
Polymeric Micelles Generated by Hydrophobic Contact
Polymeric Micelles Generated by Electrostatic Interactions
Polymeric Micelles Generated by Noncovalent Interaction
2.4.2. Functionalized Polymeric Micelles
Cell-Penetrating Polymeric Micelles
Targeting Polymeric Micelles
Active Targeting Polymeric Micelles
Passive Targeting Polymeric Micelles
2.4.3. Mucoadhesive and Mucus-Penetrating Polymeric Micelles
2.4.4. Stimuli-Responsive Polymeric Micelles
2.5. Biological Barriers and Polymeric Micelles for Efficient Anticancer Therapeutic Drug Delivery
2.5.1. Systemic Barriers
2.5.2. Organ-Level Barriers
2.5.3. Cellular-Level Barriers
3. Polymeric Micellar Systems with “Smart” Responsiveness
3.1. Internal Stimuli-Responsive Polymeric Micelles
3.1.1. pH
3.1.2. Temperature
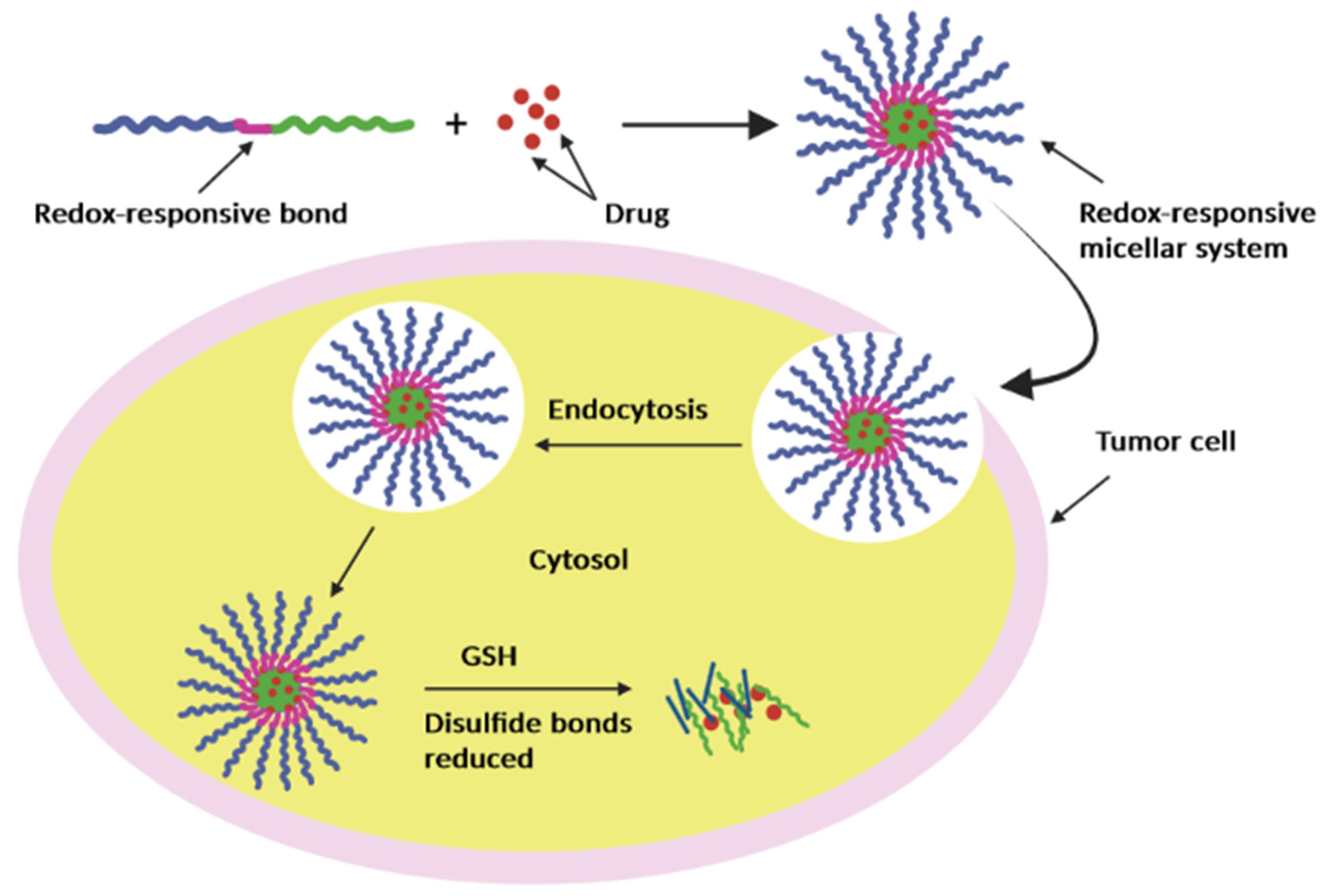
3.1.3. Redox
3.1.4. Enzyme
3.1.5. Hypoxia
3.1.6. ROS
3.1.7. Stimuli-Responsive Inversion of Macromolecules
3.2. External Stimuli-Responsive Polymeric Micelles
3.2.1. Light/Photo/NIR
3.2.2. Temperature
3.2.3. Ultrasound
3.2.4. Magnetic
3.3. Dual/Multi-Responsive Micellar Systems
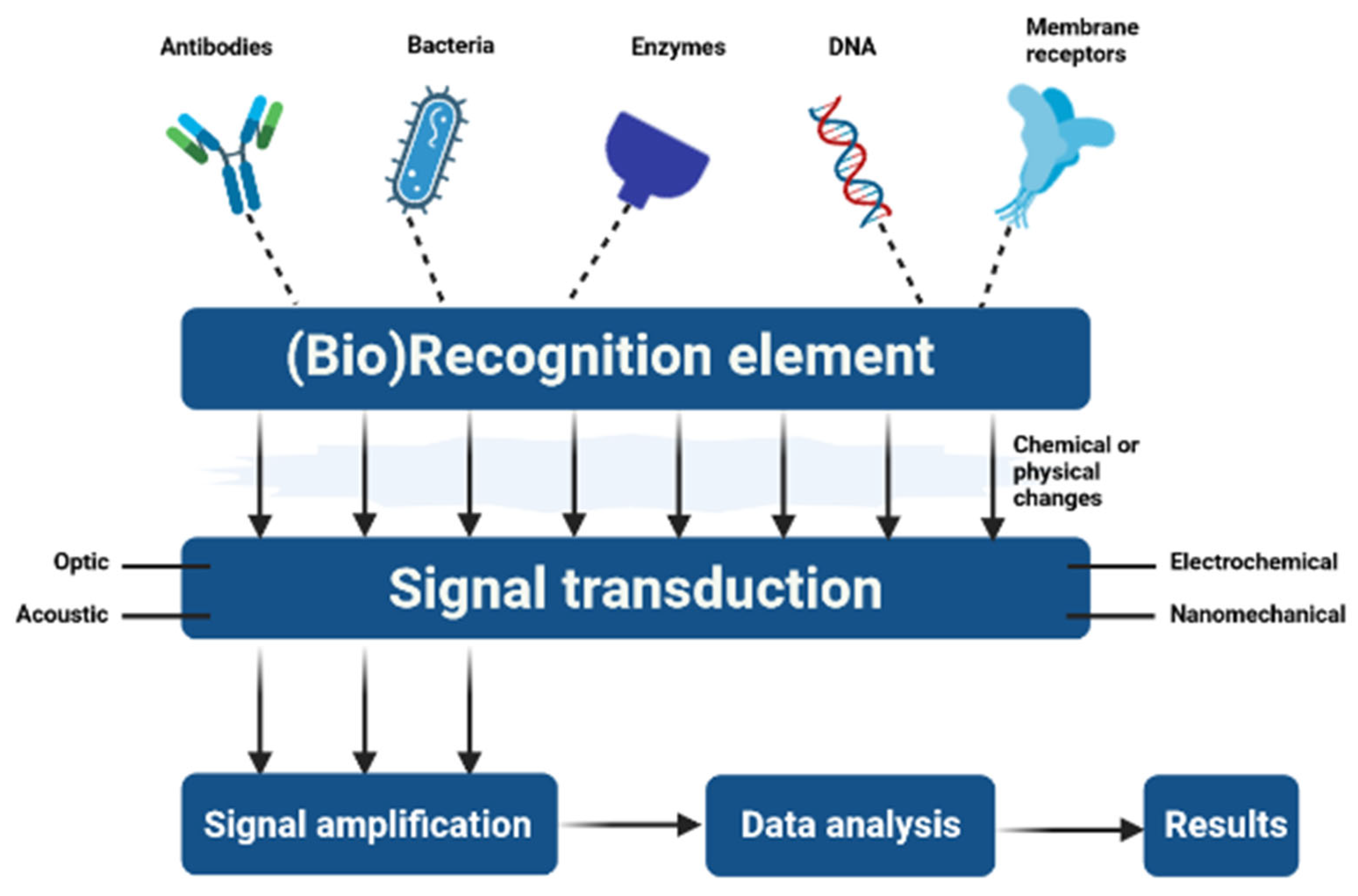
4. The Use of Polymeric Micellar Systems to Develop (Bio)Sensors
4.1. General Aspects of (Bio)Sensors
4.2. (Bio)Sensing Applications Based on Polymeric Micellar Systems
5. Clinical Status of Polymeric Micelles
6. Advantages and Drawbacks of Polymeric Micellar Systems
7. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Sig. Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Gala, U.H.; Miller, D.A.; Williams, R.O. Williams Harnessing the therapeutic potential of anticancer drugs through amorphous solid dispersions. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188319. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Li, S.; Zhu, X.; Wu, J.; Tian, H. Tumor-cell targeting polydiacetylene micelles encapsulated with an antitumor drug for the treatment of ovarian cancer. Chem. Commun. 2017, 53, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Nanotechnology based therapeutic application in cancer diagnosis and therapy. 3 Biotech 2019, 9, 415. [Google Scholar] [CrossRef]
- Avula, L.R.; Grodzinski, P. Nanotechnology-aided advancement in the combating of cancer metastasis. Cancer Metastasis Rev. 2022, 41, 383–404. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, J.; Li, Y.; Yang, C.; Hou, Y.; Tang, W.; McHugh, K.J.; Jing, L. Nanotechnology-enhanced immunotherapy for metastatic cancer. Innovation 2021, 2, 100174. [Google Scholar] [CrossRef]
- Şen, Ö.; Emanet, M.; Ciofani, G. Nanotechnology-Based Strategies to Evaluate and Counteract Cancer Metastasis and Neoangiogenesis. Adv. Healthc. Mater. 2021, 10, 2002163. [Google Scholar] [CrossRef]
- Shete, M.B.; Patil, T.S.; Deshpande, A.S.; Saraogi, G.; Vasdev, N.; Deshpande, M.; Rajpoot, K.; Tekade, R.K. Current trends in theranostic nanomedicines. J. Drug Deliv. Sci. Technol. 2022, 71, 103280. [Google Scholar] [CrossRef]
- Yu, G.; Ning, Q.; Mo, Z.; Tang, S. Intelligent polymeric micelles for multidrug co-delivery and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1476–1487. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Yt, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Alfurhood, J.A.; Sun, H.; Kabb, C.P.; Tucker, B.S.; Matthews, J.H.; Luesch, H.; Sumerlin, B.S. Poly(N-(2-hydroxypropyl)methacrylamide)–valproic acid conjugates as block copolymer nanocarriers. Polym. Chem. 2017, 8, 4983–4987. [Google Scholar] [CrossRef]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K.; et al. Harnessing amphiphilic polymeric micelles for diagnostic and therapeutic applications: Breakthroughs and bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef]
- Majumder, N.; Das, N.G.; Das, S.K. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635. [Google Scholar] [CrossRef]
- Hevus, I.; Kohut, A.; Voronov, A. Interfacial micellar phase transfer using amphiphilic invertible polymers. Polym. Chem. 2011, 2, 2767–2770. [Google Scholar] [CrossRef]
- Kohut, A.; Hevus, I.; Voronov, S.; Voronov, A. Amphiphilic Invertible Polymers and Their Applications. In Industrial Applications for Intelligent Polymers and Coatings; Hosseini, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 399–415. [Google Scholar] [CrossRef]
- Gao, M.; Yang, Y.; Bergfel, A.; Huang, L.; Zheng, L.; Bowden, T.M. Self-assembly of cholesterol end-capped polymer micelles for controlled drug delivery. J. Nanobiotechnol. 2020, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yasugi, K.; Harada, A.; Nagasaki, Y.; Kataoka, K. Temperature-related change in the properties relevant to drug delivery of poly(ethylene glycol)–poly(d,l-lactide) block copolymer micelles in aqueous milieu. J. Control. Release 2002, 82, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Chokamonsirikun, A.; Phattaravorakarn, V.; Tiyaboonchai, W. Polymeric micelles for pulmonary drug delivery: A comprehensive review. J. Mater. Sci. 2021, 56, 2016–2036. [Google Scholar] [CrossRef]
- Bai, S.; Ma, X.; Zhang, T.; Gao, Y.-E.; Wang, Y.; Gao, Y.; Xu, Z. 12-Polymeric micelles as delivery systems. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 261–278. [Google Scholar] [CrossRef]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- Beibei, D.; Tiantang, F.; Jiafeng, L.; Li, G.; Qin, Z.; Wuyou, Y.; Hongyun, T.; Wenxin, W.; Zhongyong, F. PLLA-Grafted Gelatin Amphiphilic Copolymer and Its Self-Assembled Nano Carrier for Anticancer Drug Delivery. Macromol. Chem. Phys. 2019, 220, 1800528. [Google Scholar] [CrossRef]
- Toscanini, M.A.; Limeres, M.J.; Garrido, A.V.; Cagel, M.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A.; Cuestas, M.L. Polymeric micelles and nanomedicines: Shaping the future of next generation therapeutic strategies for infectious diseases. J. Drug Deliv. Sci. Technol. 2021, 66, 102927. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Aziz, Z.A.B.A.; Ahmad, A.; Mohd-Setapar, S.H.; Hassan, H.; Lokhat, D.; Kamal, M.A.; Ashraf, G.M. Recent Advances in Drug Delivery of Polymeric Nano-Micelles. Curr. Drug Metab. 2017, 18, 16–29. [Google Scholar] [CrossRef]
- Procházka, K.; Limpouchová, Z.; Štěpánek, M.; Šindelka, K.; Lísal, M. DPD Modelling of the Self- and Co-Assembly of Polymers and Polyelectrolytes in Aqueous Media: Impact on Polymer Science. Polymers 2022, 14, 404. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, S. Emerging trends in solution self-assembly of block copolymers. Polymer 2020, 207, 122914. [Google Scholar] [CrossRef]
- Lyubimov, I.; Beltran-Villegas, D.J.; Jayaraman, A. PRISM Theory Study of Amphiphilic Block Copolymer Solutions with Varying Copolymer Sequence and Composition. Macromolecules 2017, 50, 7419–7431. [Google Scholar] [CrossRef]
- Butt, A.M.; Amin, M.C.I.M.; Katas, H.; Sarisuta, N.; Witoonsaridsilp, W.; Benjakul, R. In vitro characterization of pluronic F127 and D-α-tocopheryl polyethylene glycol 1000 succinate mixed micelles as nanocarriers for targeted anticancer-drug delivery. J. Nanomater. 2012, 2012, 112. [Google Scholar] [CrossRef]
- Ahn, S.; Yoon, H.; Duan, C.; Li, W.; Kim, J.K. Core–Satellite Micelles by a Linear A1B1A2B2 Tetrablock Copolymer. Macromolecules 2022, 55, 1544–1551. [Google Scholar] [CrossRef]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Salatin, S.; Naderinia, A.; Jelvehgari, M. Novel Pentablock Copolymers as Thermosensitive Self-Assembling Micelles for Ocular Drug Delivery. Adv. Pharm. Bull. 2017, 7, 11–20. [Google Scholar] [CrossRef]
- Buwalda, S.; Al Samad, A.; El Jundi, A.; Bethry, A.; Bakkour, Y.; Coudane, J.; Nottelet, B. Stabilization of poly(ethylene glycol)-poly(ε-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties. J. Colloid Interface Sci. 2018, 514, 468–478. [Google Scholar] [CrossRef]
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Chapter 5-Polymeric Micelles for Drug Targeting and Delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Amin, M.C.I.M., Iyer, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 167–202. [Google Scholar] [CrossRef]
- Holder, S.J.; Sommerdijk, N.A.J.M. New micellar morphologies from amphiphilic block copolymers: Disks, toroids and bicontinuous micelles. Polym. Chem. 2011, 2, 1018–1028. [Google Scholar] [CrossRef]
- Son, I.; Lee, Y.; Baek, J.; Park, M.; Han, D.; Min, S.K.; Lee, D.; Kim, B.-S. pH-Responsive Amphiphilic Polyether Micelles with Superior Stability for Smart Drug Delivery. Biomacromolecules 2021, 22, 2043–2056. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Chu, Z.; Cai, L.; Shi, H.; Zhu, C.; Pan, D.; Pan, J.; Fei, X.; Lei, Y. Temperature-Responsive Multilayer Films of Micelle-Based Composites for Controlled Release of a Third-Generation EGFR Inhibitor. ACS Appl. Polym. Mater. 2020, 2, 741–750. [Google Scholar] [CrossRef]
- Yang, L.; Hou, X.; Zhang, Y.; Wang, D.; Liu, J.; Huang, F.; Liu, J. NIR-activated self-sensitized polymeric micelles for enhanced cancer chemo-photothermal therapy. J. Control. Release 2021, 339, 114–129. [Google Scholar] [CrossRef]
- Nagaich, U.; Deepak, P.; Sharma, A.; Gulati, N.; Chaudhary, A. Polymeric Micelles: Potential Drug Delivery Devices. Indones. J. Pharm. 2013, 24, 223–238. [Google Scholar]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 135–163. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.; Nawale, R.B.; Kulthe, S.S. Polymeric Micelles: General Considerations and their Applications. Indian J. Pharm. Educ. Res. 2011, 45, 128–138. [Google Scholar]
- Bera, A.; Trivedi, J.S.; Kumar, S.B.; Chandel, A.K.S.; Haldar, S.; Jewrajka, S.K. Anti-organic fouling and anti-biofouling poly(piperazineamide) thin film nanocomposite membranes for low pressure removal of heavy metal ions. J. Hazard. Mater. 2018, 343, 86–97. [Google Scholar] [CrossRef]
- Abedanzadeh, M.; Salmanpour, M.; Farjadian, F.; Mohammadi, S.; Tamaddon, A.M. Curcumin loaded polymeric micelles of variable hydrophobic lengths by RAFT polymerization: Preparation and in-vitro characterization. J. Drug Deliv. Sci. Technol. 2020, 58, 101793. [Google Scholar] [CrossRef]
- De Luca, S.; Chen, F.; Seal, P.; Stenzel, M.H.; Smith, S.C. Binding and Release between Polymeric Carrier and Protein Drug: pH-Mediated Interplay of Coulomb Forces, Hydrogen Bonding, van der Waals Interactions, and Entropy. Biomacromolecules 2017, 18, 3665–3677. [Google Scholar] [CrossRef]
- Chen, F.; Stenzel, M.H.; Chen, F.; Stenzel, M.H. Polyion Complex Micelles for Protein Delivery*. Aust. J. Chem. 2018, 71, 768–780. [Google Scholar] [CrossRef]
- Nabiyan, A.; Max, J.B.; Schacher, F.H. Double hydrophilic copolymers-synthetic approaches, architectural variety, and current application fields. Chem. Soc. Rev. 2022, 51, 995–1044. [Google Scholar] [CrossRef]
- Huang, S.-J.; Wang, T.-H.; Chou, Y.-H.; Wang, H.-M.D.; Hsu, T.-C.; Yow, J.-L.; Tzang, B.-S.; Chiang, W.-H. Hybrid PEGylated chitosan/PLGA nanoparticles designed as pH-responsive vehicles to promote intracellular drug delivery and cancer chemotherapy. Int. J. Biol. Macromol. 2022, 210, 565–578. [Google Scholar] [CrossRef]
- Chen, W.; Liu, P. Dendritic polyurethane-based prodrug as unimolecular micelles for precise ultrasound-activated localized drug delivery. Mater. Today Chem. 2022, 24, 100819. [Google Scholar] [CrossRef]
- Kwiatkowski, A.L.; Molchanov, V.S.; Kuklin, A.I.; Chesnokov, Y.M.; Philippova, O.E. Salt-Induced Transformations of Hybrid Micelles Formed by Anionic Surfactant and Poly(4-vinylpyridine). Polymers 2022, 14, 5086. [Google Scholar] [CrossRef]
- Khursheed, R.; Paudel, K.R.; Gulati, M.; Vishwas, S.; Jha, N.K.; Hansbro, P.M.; Oliver, B.G.; Dua, K.; Singh, S.K. Expanding the arsenal against pulmonary diseases using surface-functionalized polymeric micelles: Breakthroughs and bottlenecks. Nanomedicine 2022, 17, 881–911. [Google Scholar] [CrossRef]
- Cai, Y.; Qi, J.; Lu, Y.; He, H.; Wu, W. The in vivo fate of polymeric micelles. Adv. Drug Deliv. Rev. 2022, 188, 114463. [Google Scholar] [CrossRef]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. WIREs Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Kapse, A.; Anup, N.; Patel, V.; Saraogi, G.K.; Mishra, D.K.; Tekade, R.K. Chapter 6-Polymeric micelles: A ray of hope among new drug delivery systems. In Drug Delivery Systems; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 235–289. [Google Scholar] [CrossRef]
- Zhang, H.; Mi, P. 12-Polymeric Micelles for Tumor Theranostics. In Theranostic Bionanomaterials; Cui, W., Zhao, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–302. [Google Scholar] [CrossRef]
- Farhoudi, L.; Kesharwani, P.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Polymeric nanomicelles of curcumin: Potential applications in cancer. Int. J. Pharm. 2022, 617, 121622. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, G.S. Applications of mannose-binding lectins and mannan glycoconjugates in nanomedicine. J. Nanopart. Res. 2022, 24, 228. [Google Scholar] [CrossRef]
- Jin, G.-W.; Rejinold, N.S.; Choy, J.-H. Multifunctional Polymeric Micelles for Cancer Therapy. Polymers 2022, 14, 4839. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, 1902604. [Google Scholar] [CrossRef]
- Shi, H.; van Steenbergen, M.J.; Lou, B.; Liu, Y.; Hennink, W.E.; Kok, R.J. Folate decorated polymeric micelles for targeted delivery of the kinase inhibitor dactolisib to cancer cells. Int. J. Pharm. 2020, 582, 119305. [Google Scholar] [CrossRef]
- Xu, X.-L.; Li, W.-S.; Wang, X.-J.; Du, Y.-L.; Kang, X.-Q.; Hu, J.-B.; Li, S.-J.; Ying, X.-Y.; You, J.; Du, Y.-Z. Endogenous sialic acid-engineered micelles: A multifunctional platform for on-demand methotrexate delivery and bone repair of rheumatoid arthritis. Nanoscale 2018, 10, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van Steenbergen, M.J.; Beztsinna, N.; Shi, Y.; Lammers, T.; van Nostrum, C.F.; Hennink, W.E. Biotin-decorated all-HPMA polymeric micelles for paclitaxel delivery. J. Control. Release 2020, 328, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.A. Chemical immobilization of antimicrobial peptides on biomaterial surfaces. Front. Biosci. 2016, 8, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T. Cell-Penetrating Peptide-Conjugated Polymer Micelle for pDNA/siRNA Delivery. In Cell-Penetrating Peptides; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 285–313. [Google Scholar] [CrossRef]
- De Lorenzi, F.; Rizzo, L.Y.; Daware, R.; Motta, A.; Baues, M.; Bartneck, M.; Vogt, M.; van Zandvoort, M.; Kaps, L.; Hu, Q.; et al. Profiling target engagement and cellular uptake of cRGD-decorated clinical-stage core-crosslinked polymeric micelles. Drug Deliv. Transl. Res. 2022. [CrossRef]
- Tian, L.; Pei, R.; Zhong, L.; Ji, Y.; Zhou, D.; Zhou, S. Enhanced targeting of 3D pancreatic cancer spheroids by aptamer-conjugated polymeric micelles with deep tumor penetration. Eur. J. Pharmacol. 2021, 894, 173814. [Google Scholar] [CrossRef]
- Akhter, M.H.; Rizwanullah, M.; Ahmad, J.; Ahsan, M.J.; Mujtaba, M.A.; Amin, S. Nanocarriers in advanced drug targeting: Setting novel paradigm in cancer therapeutics. Artif. Cells Nanomed. Biotechnol. 2018, 46, 873–884. [Google Scholar] [CrossRef]
- Suzuki, K.; Miura, Y.; Mochida, Y.; Miyazaki, T.; Toh, K.; Anraku, Y.; Melo, V.; Liu, X.; Ishii, T.; Nagano, O.; et al. Glucose transporter 1-mediated vascular translocation of nanomedicines enhances accumulation and efficacy in solid tumors. J. Control. Release 2019, 301, 28–41. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Tan, X.; Rao, R.; Ren, Y.; Liu, L.; Yang, X.; Liu, W. Multifunctional hybrid micelles with tunable active targeting and acid/phosphatase-stimulated drug release for enhanced tumor suppression. Biomaterials 2018, 157, 136–148. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Choudhary, M.I. Frontiers in Anti-Cancer Drug Discovery: Volume 11; Bentham Science Publishers: Sharjah, United Arab Emirates, 2020. [Google Scholar]
- Wu, D.; Si, M.; Xue, H.-Y.; Wong, H.-L. Nanomedicine applications in the treatment of breast cancer: Current state of the art. Int. J. Nanomed. 2017, 12, 5879–5892. [Google Scholar] [CrossRef]
- Sosnik, A.; Raskin, M.M. Polymeric micelles in mucosal drug delivery: Challenges towards clinical translation. Biotechnol. Adv. 2015, 33, 1380–1392. [Google Scholar] [CrossRef]
- Handa, M.; Singh, A.; Flora, S.J.S.; Shukla, R. Stimuli-responsive Polymeric Nanosystems for Therapeutic Applications. Curr. Pharm. Des. 2022, 28, 910–921. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; He, C.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z. Meta-Analysis of Nanoparticle Delivery to Tumors Using a Physiologically Based Pharmacokinetic Modeling and Simulation Approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef]
- Han, Y.; Wen, P.; Li, J.; Kataoka, K. Targeted nanomedicine in cisplatin-based cancer therapeutics. J. Control. Release 2022, 345, 709–720. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef]
- Das, A.; Pathak, M.P.; Patowary, P.; Das, S. Introduction to Lung Physiology from a Drug Delivery Perspective. In Handbook of Lung Targeted Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Myerson, J.W.; Braender, B.; Mcpherson, O.; Glassman, P.M.; Kiseleva, R.Y.; Shuvaev, V.V.; Marcos-Contreras, O.; Grady, M.E.; Lee, H.S.; Greineder, C.F.; et al. Flexible Nanoparticles Reach Sterically Obscured Endothelial Targets Inaccessible to Rigid Nanoparticles. Adv. Mater. 2018, 30, 1802373. [Google Scholar] [CrossRef]
- Wong, P.-P.; Bodrug, N.; Hodivala-Dilke, K.M. Exploring Novel Methods for Modulating Tumor Blood Vessels in Cancer Treatment. Curr. Biol. 2016, 26, R1161–R1166. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagn. Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef]
- Ho, Y.T.; Kamm, R.D.; Kah, J.C.Y. Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis. Nanoscale 2018, 10, 12386–12397. [Google Scholar] [CrossRef]
- Gonda, A.; Zhao, N.; Shah, J.V.; Calvelli, H.R.; Kantamneni, H.; Francis, N.L.; Ganapathy, V. Engineering Tumor-Targeting Nanoparticles as Vehicles for Precision Nanomedicine. Med One 2019, 4, e190021. [Google Scholar] [CrossRef] [PubMed]
- Dilliard, S.A.; Siegwart, D.J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Oller-Salvia, B.; Sánchez-Navarro, M.; Giralt, E.; Teixidó, M. Blood–brain barrier shuttle peptides: An emerging paradigm for brain delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. [Google Scholar] [CrossRef] [PubMed]
- Dingezweni, S. The blood–brain barrier. South. Afr. J. Anaesth. Analg. 2020, 26, S32–S34. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Gupta, T.; Batheja, S.; Goyal, A.K.; Gupta, U. Surface Engineered Dendrimers: A Potential Nanocarrier for the Effective Management of Glioblastoma Multiforme. Curr. Drug Metab. 2022, 23, 708–722. [Google Scholar] [CrossRef]
- Durand, M.; Lelievre, E.; Chateau, A.; Berquand, A.; Laurent, G.; Carl, P.; Roux, S.; Chazee, L.; Bazzi, R.; Eghiaian, F.; et al. The detrimental invasiveness of glioma cells controlled by gadolinium chelate-coated gold nanoparticles. Nanoscale 2021, 13, 9236–9251. [Google Scholar] [CrossRef]
- Meng, L.; Chu, X.; Xing, H.; Liu, X.; Xin, X.; Chen, L.; Jin, M.; Guan, Y.; Huang, W.; Gao, Z. Improving glioblastoma therapeutic outcomes via doxorubicin-loaded nanomicelles modified with borneol. Int. J. Pharm. 2019, 567, 118485. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, J.; Xiao, H.; Wu, T.; Shuai, X. Codelivery of temozolomide and siRNA with polymeric nanocarrier for effective glioma treatment. Int. J. Nanomed. 2018, 13, 3467–3480. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Stroeve, P.; Mahmoudi, M. Drug Delivery Systems; World Scientific: Singapore, 2017. [Google Scholar]
- Zheng, N.; Li, J.; Xu, C.; Xu, L.; Li, S.; Xu, L. Mesoporous silica nanorods for improved oral drug absorption. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1132–1140. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Xie, J.; Becton, M.; Wang, X. Interplay of Nanoparticle Rigidity and Its Translocation Ability through Cell Membrane. J. Phys. Chem. B 2019, 123, 8923–8930. [Google Scholar] [CrossRef]
- Hocking, K.M.; Evans, B.C.; Komalavilas, P.; Cheung-Flynn, J.; Duvall, C.L.; Brophy, C.M. Nanotechnology Enabled Modulation of Signaling Pathways Affects Physiologic Responses in Intact Vascular Tissue. Tissue Eng. Part A 2019, 25, 416–426. [Google Scholar] [CrossRef]
- Lunnoo, T.; Assawakhajornsak, J.; Puangmali, T. In Silico Study of Gold Nanoparticle Uptake into a Mammalian Cell: Interplay of Size, Shape, Surface Charge, and Aggregation. J. Phys. Chem. C 2019, 123, 3801–3810. [Google Scholar] [CrossRef]
- Dzuricky, M.; Xiong, S.; Weber, P.; Chilkoti, A. Avidity and Cell Uptake of Integrin-Targeting Polypeptide Micelles is Strongly Shape-Dependent. Nano Lett. 2019, 19, 6124–6132. [Google Scholar] [CrossRef]
- Sarisozen, C.; Joshi, U.; Mendes, L.P.; Torchilin, V.P. 10-Stimuli-responsive polymeric micelles for extracellular and intracellular drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 269–304. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.J.; Wang, Y.; Chen, B.Z.; Guo, X.D.; Zhang, C.Y. Dual redox/pH-responsive hybrid polymer-lipid composites: Synthesis, preparation, characterization and application in drug delivery with enhanced therapeutic efficacy. Chem. Eng. J. 2018, 341, 450–461. [Google Scholar] [CrossRef]
- Qiu, N.; Du, X.; Ji, J.; Zhai, G. A review of stimuli-responsive polymeric micelles for tumor-targeted delivery of curcumin. Drug Dev. Ind. Pharm. 2021, 47, 839–856. [Google Scholar] [CrossRef]
- Luo, Y.; Yin, X.; Yin, X.; Chen, A.; Zhao, L.; Zhang, G.; Liao, W.; Huang, X.; Li, J.; Zhang, C.Y. Dual pH/Redox-Responsive Mixed Polymeric Micelles for Anticancer Drug Delivery and Controlled Release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14, 1860. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, K.; Liu, R.; Guo, X.; He, B.; Yan, J.; Ren, J. pH-sensitive polymeric micelles assembled by stereocomplexation between PLLA-b-PLys and PDLA-b-mPEG for drug delivery. J. Mater. Chem. B 2019, 7, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhao, L.; Li, J.; Song, R.; Wang, Y.; Yu, K.; Hou, X.; Qiao, P.; Zong, L.; Chang, S. A polymeric micelle with an endosomal pH-sensitivity for intracellular delivery and enhanced antitumor efficacy of hydroxycamptothecin. Acta Biomater. 2019, 88, 357–369. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, D.; Chen, F.; Chen, Y.; Luo, X. Tracing Difference: In Vitro and in Vivo Antitumor Property Comparison of pH-Sensitive Biomimetic Phosphorylcholine Micelles with Insensitive Micelles. ACS Biomater. Sci. Eng. 2019, 5, 2258–2270. [Google Scholar] [CrossRef]
- Emami, J.; Kazemi, M.; Hasanzadeh, F.; Minaiyan, M.; Mirian, M.; Lavasanifar, A. Novel pH-triggered biocompatible polymeric micelles based on heparin–α-tocopherol conjugate for intracellular delivery of docetaxel in breast cancer. Pharm. Dev. Technol. 2020, 25, 492–509. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Cao, F.; Peng, D.; Chen, W.; Zhang, C. Amphiphilic Block Copolymer Poly (Acrylic Acid)-B-Polycaprolactone as a Novel pH-sensitive Nanocarrier for Anti-Cancer Drugs Delivery: In-vitro and In-vivo Evaluation. Polymers 2019, 11, 820. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Abujamous, L. pH-sensitive polymeric nanoparticles of quercetin as a potential colon cancer-targeted nanomedicine. J. Drug Deliv. Sci. Technol. 2019, 52, 670–676. [Google Scholar] [CrossRef]
- Ameli, H.; Alizadeh, N. Targeted delivery of capecitabine to colon cancer cells using nano polymeric micelles based on beta cyclodextrin. RSC Adv. 2022, 12, 4681–4691. [Google Scholar] [CrossRef]
- Gao, Q.-Q.; Zhang, C.-M.; Zhang, E.-X.; Chen, H.-Y.; Zhen, Y.-H.; Zhang, S.-B. Zwitterionic pH-responsive hyaluronic acid polymer micelles for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2019, 178, 412–420. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Xu, X.; Chen, Z.; Ma, L.; Wang, Y.; Guo, X.; Li, J.; Wang, X. Construction of pH-sensitive targeted micelle system co-delivery with curcumin and dasatinib and evaluation of anti-liver cancer. Drug Deliv. 2022, 29, 792–806. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Li, Z.; Feng, H.; Jin, L.; Zhang, Y.; Tian, X.; Li, J. Polymeric micelle with pH-induced variable size and doxorubicin and siRNA co-delivery for synergistic cancer therapy. Appl. Nanosci. 2020, 10, 1903–1913. [Google Scholar] [CrossRef]
- Ghasemi, S.; Ahmadi, L.; Farjadian, F. Thermo-responsive PNIPAAm-b-PLA amphiphilic block copolymer micelle as nanoplatform for docetaxel drug release. J. Mater. Sci. 2022, 57, 17433–17447. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Sun, C.; Lu, J.; Wang, J.; Hao, P.; Li, C.; Qi, L.; Yang, L.; Bin He, B.; Zhong, Z.; Na Hao, N. Redox-sensitive polymeric micelles with aggregation-induced emission for bioimaging and delivery of anticancer drugs. J. Nanobiotechnol. 2021, 19, 14. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, S.; He, Y.; Deng, L. A redox prodrug micelle co-delivering camptothecin and curcumin for synergetic B16 melanoma cells inhibition. Chem. Eng. J. 2019, 362, 877–886. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, H.; Shen, Y.; Yu, Q.; Gan, Z. Shell-Sheddable Poly(N-2-hydroxypropyl methacrylamide) Polymeric Micelles for Dual-Sensitive Release of Doxorubicin. Macromol. Rapid Commun. 2018, 39, 1800139. [Google Scholar] [CrossRef]
- Meng, X.; Gao, M.; Deng, J.; Lu, D.; Fan, A.; Ding, D.; Kong, D.; Wang, Z.; Zhao, Y. Self-immolative micellar drug delivery: The linker matters. Nano Res. 2018, 11, 6177–6189. [Google Scholar] [CrossRef]
- Gulfam, M.; Matini, T.; Monteiro, P.F.; Riva, R.; Collins, H.; Spriggs, K.; Howdle, S.M.; Jérôme, C.; Alexander, C. Bioreducible cross-linked core polymer micelles enhance in vitro activity of methotrexate in breast cancer cells. Biomater. Sci. 2017, 5, 532–550. [Google Scholar] [CrossRef]
- Chai, Z.; Teng, C.; Yang, L.; Ren, L.; Yuan, Z.; Xu, S.; Cheng, M.; Wang, Y.; Yan, Z.; Qin, C.; et al. Doxorubicin delivered by redox-responsive Hyaluronic Acid–Ibuprofen prodrug micelles for treatment of metastatic breast cancer. Carbohydr. Polym. 2020, 245, 116527. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zheng, Q.; Dai, J.; Liu, J.; Yin, J.; Xu, X.; Chen, A.; Ren, L. Reduction-sensitive mixed micelles based on mPEG-SS-PzLL /TPGS to enhance anticancer efficiency of doxorubicin. React. Funct. Polym. 2022, 174, 105242. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Yang, M.; Pan, X.; Hu, J. Docetaxel-loaded redox-sensitive nanoparticles self-assembling from poly(caprolactone) conjugates with disulfide-linked poly(ethylene glycol). J. Biomater. Sci. Polym. Ed. 2022, 33, 2185–2201. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Twizeyimana, E.; Lu, N.; Ke, W.; Mukerabigwi, J.F.; Mohammed, F.; Japir, A.A.-W.M.M.; Ge, Z. Reduction-Responsive Polymer Prodrug Micelles with Enhanced Endosomal Escape Capability for Efficient Intracellular Translocation and Drug Release. ACS Appl. Bio Mater. 2019, 2, 5099–5109. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Deka, S.R.; Tiwari, K.; Sharma, A.K.; Kumar, P. Multi-Stimuli Responsive Self-Assembled Nanostructures Useful for Colon Drug Delivery. IEEE Trans. NanoBiosci. 2017, 16, 764–772. [Google Scholar] [CrossRef]
- Sekhar, K.P.C.; Adicherla, H.; Nayak, R.R. Impact of Glycolipid Hydrophobic Chain Length and Headgroup Size on Self-Assembly and Hydrophobic Guest Release. Langmuir 2018, 34, 8875–8886. [Google Scholar] [CrossRef]
- Li, N.; Cai, H.; Jiang, L.; Hu, J.; Bains, A.; Hu, J.; Gong, Q.; Luo, K.; Gu, Z. Enzyme-Sensitive and Amphiphilic PEGylated Dendrimer-Paclitaxel Prodrug-Based Nanoparticles for Enhanced Stability and Anticancer Efficacy. ACS Appl. Mater. Interfaces 2017, 9, 6865–6877. [Google Scholar] [CrossRef]
- Isaacson, K.J.; Jensen, M.M.; Subrahmanyam, N.B.; Ghandehari, H. Matrix-metalloproteinases as targets for controlled delivery in cancer: An analysis of upregulation and expression. J. Control. Release 2017, 259, 62–75. [Google Scholar] [CrossRef]
- Jayatilaka, H.; Umanzor, F.G.; Shah, V.; Meirson, T.; Russo, G.; Starich, B.; Tyle, P.; Lee, J.S.; Khatau, S.; Gil-Henn, H.; et al. Tumor cell density regulates matrix metalloproteinases for enhanced migration. Oncotarget 2018, 9, 32556–32569. [Google Scholar] [CrossRef]
- Ke, W.; Zha, Z.; Mukerabigwi, J.F.; Chen, W.; Wang, Y.; He, C.; Ge, Z. Matrix Metalloproteinase-Responsive Multifunctional Peptide-Linked Amphiphilic Block Copolymers for Intelligent Systemic Anticancer Drug Delivery. Bioconjug. Chem. 2017, 28, 2190–2198. [Google Scholar] [CrossRef]
- Yao, Q.; Dai, Z.; Choi, J.H.; Kim, D.; Zhu, L. Building Stable MMP2-Responsive Multifunctional Polymeric Micelles by an All-in-One Polymer–Lipid Conjugate for Tumor-Targeted Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 32520–32533. [Google Scholar] [CrossRef]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020, 113, 501–511. [Google Scholar] [CrossRef]
- Preethi, S.; Arthiga, K.; Patil, A.B.; Spandana, A.; Jain, V. Review on NAD(P)H dehydrogenase quinone 1 (NQO1) pathway. Mol. Biol. Rep. 2022, 49, 8907–8924. [Google Scholar] [CrossRef]
- Park, J.; Jo, S.; Lee, Y.M.; Saravanakumar, G.; Lee, J.; Park, D.; Kim, W.J. Enzyme-Triggered Disassembly of Polymeric Micelles by Controlled Depolymerization via Cascade Cyclization for Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2021, 13, 8060–8070. [Google Scholar] [CrossRef]
- Della Rocca, Y.; Fonticoli, L.; Rajan, T.S.; Trubiani, O.; Caputi, S.; Diomede, F.; Pizzicannella, J.; Marconi, G.D. Hypoxia: Molecular pathophysiological mechanisms in human diseases. J. Physiol. Biochem. 2022, 78, 739–752. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, W.; Guo, J. Hypoxia-sensitive micelles based on amphiphilic chitosan derivatives for drug-controlled release. Polym. Adv. Technol. 2021, 32, 3113–3122. [Google Scholar] [CrossRef]
- Long, M.; Liu, X.; Huang, X.; Lu, M.; Wu, X.; Weng, L.; Chen, Q.; Wang, X.; Zhu, L.; Chen, Z. Alendronate-functionalized hypoxia-responsive polymeric micelles for targeted therapy of bone metastatic prostate cancer. J. Control. Release 2021, 334, 303–317. [Google Scholar] [CrossRef]
- Sun, X.S.; Jang, M.-S.; Fu, Y.; Lee, J.H.; Lee, D.S.; Li, Y.; Yang, H.Y. Intracellular delivery of cytochrome C using hypoxia-responsive polypeptide micelles for efficient cancer therapy. Mater. Sci. Eng. C 2020, 114, 111069. [Google Scholar] [CrossRef]
- Lu, M.; Huang, X.; Cai, X.; Sun, J.; Liu, X.; Weng, L.; Zhu, L.; Luo, Q.; Chen, Z. Hypoxia-Responsive Stereocomplex Polymeric Micelles with Improved Drug Loading Inhibit Breast Cancer Metastasis in an Orthotopic Murine Model. ACS Appl. Mater. Interfaces 2022, 14, 20551–20565. [Google Scholar] [CrossRef]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent progress and advances in stimuli-responsive polymers for cancer therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Shen, Q.; Xing, D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun. 2019, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef] [PubMed]
- Daund, V.; Chalke, S.; Sherje, A.P.; Kale, P.P. ROS responsive mesoporous silica nanoparticles for smart drug delivery: A review. J. Drug Deliv. Sci. Technol. 2021, 64, 102599. [Google Scholar] [CrossRef]
- Xin, X.; Lin, F.; Wang, Q.; Yin, L.; Mahato, R.I. ROS-Responsive Polymeric Micelles for Triggered Simultaneous Delivery of PLK1 Inhibitor/miR-34a and Effective Synergistic Therapy in Pancreatic Cancer. ACS Appl. Mater. Interfaces 2019, 11, 14647–14659. [Google Scholar] [CrossRef]
- Li, J.; Ke, W.; Wang, L.; Huang, M.; Yin, W.; Zhang, P.; Chen, Q.; Ge, Z. Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2 production for synergistic oxidation-chemotherapy. J. Control. Release 2016, 225, 64–74. [Google Scholar] [CrossRef]
- Pan, Y.; Zholobko, O.; Voronov, A.; Yang, Z. Inversion of Polymeric Micelles Probed by Spin Labeled Peptide Incorporation and Electron Paramagnetic Resonance. J. Phys. Chem. C 2018, 122, 25692–25699. [Google Scholar] [CrossRef]
- Polunin, Y.; Alferiev, I.S.; Brodeur, G.M.; Voronov, A.; Chorny, M. Environment-Sensitive Polymeric Micelles Encapsulating SN-38 Potently Suppress Growth of Neuroblastoma Cells Exhibiting Intrinsic and Acquired Drug Resistance. ACS Pharmacol. Transl. Sci. 2021, 4, 240–247. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Song, X.; Zhu, J.; Sng, J.A.; Li, J.; Wen, Y. Synthesis and Characterization of Palmitoyl-block-poly(methacryloyloxyethyl phosphorylcholine) Polymer Micelles for Anticancer Drug Delivery. Biomacromolecules 2022, 23, 4586–4596. [Google Scholar] [CrossRef]
- Son, J.; Yi, G.; Yoo, J.; Park, C.; Koo, H.; Choi, H.S. Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv. Drug Deliv. Rev. 2019, 138, 133–147. [Google Scholar] [CrossRef]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef]
- Sánchez-Moreno, P.; De Vicente, J.; Nardecchia, S.; Marchal, J.A.; Boulaiz, H. Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials 2018, 8, 935. [Google Scholar] [CrossRef]
- Bagheri, A.; Boyer, C.; Lim, M. Synthesis of Light-Responsive Pyrene-Based Polymer Nanoparticles via Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2019, 40, 1800510. [Google Scholar] [CrossRef]
- Xiang, J.; Tong, X.; Shi, F.; Yan, Q.; Yu, B.; Zhao, Y. Near-infrared light-triggered drug release from UV-responsive diblock copolymer-coated upconversion nanoparticles with high monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, G. NIR light-responsive nanocarriers for controlled release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [PubMed]
- Siboro, S.A.P.; Anugrah, D.S.B.; Jeong, Y.T.; Yoo, S.I.; Lim, K.T. Systematic investigation to the effects of near-infrared light exposure on polymeric micelles of poly(ethylene glycol)-block-poly(styrene-alt-maleic anhydride) loaded with indocyanine green. Polym. Degrad. Stab. 2019, 167, 241–249. [Google Scholar] [CrossRef]
- Wei, X.; Liu, L.; Guo, X.; Wang, Y.; Zhao, J.; Zhou, S. Light-Activated ROS-Responsive Nanoplatform Codelivering Apatinib and Doxorubicin for Enhanced Chemo-Photodynamic Therapy of Multidrug-Resistant Tumors. ACS Appl. Mater. Interfaces 2018, 10, 17672–17684. [Google Scholar] [CrossRef]
- Chen, J.; Qian, C.; Ren, P.; Yu, H.; Kong, X.; Huang, C.; Luo, H.; Chen, G. Light-Responsive Micelles Loaded with Doxorubicin for Osteosarcoma Suppression. Front. Pharmacol. 2021, 12, 679610. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2021.679610 (accessed on 30 January 2023). [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. ‘Smart’ materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y.; Ebara, M.; Aoyagi, T.; Narain, R. Recent Advances in Dual Temperature Responsive Block Copolymers and Their Potential as Biomedical Applications. Polymers 2016, 8, 380. [Google Scholar] [CrossRef]
- Di, X.; Kang, Y.; Li, F.; Yao, R.; Chen, Q.; Hang, C.; Xu, Y.; Wang, Y.; Sun, P.; Wu, G. Poly(N-isopropylacrylamide)/polydopamine/clay nanocomposite hydrogels with stretchability, conductivity, and dual light- and thermo- responsive bending and adhesive properties. Colloids Surf. B Biointerfaces 2019, 177, 149–159. [Google Scholar] [CrossRef]
- Yang, J.; Zhai, S.; Qin, H.; Yan, H.; Xing, D.; Hu, X. NIR-controlled morphology transformation and pulsatile drug delivery based on multifunctional phototheranostic nanoparticles for photoacoustic imaging-guided photothermal-chemotherapy. Biomaterials 2018, 176, 1–12. [Google Scholar] [CrossRef]
- Yadav, H.K.S.; Mohammed, A.; Dibi, M. Chapter 12-Advancements in exogeneous techniques for stimuli-sensitive delivery systems. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 447–481. [Google Scholar] [CrossRef]
- An, J.; Hong, H.; Won, M.; Rha, H.; Ding, Q.; Kang, N.; Kang, H.; Kim, J.S. Mechanical stimuli-driven cancer therapeutics. Chem. Soc. Rev. 2023, 52, 30–46. [Google Scholar] [CrossRef]
- Zhao, Z.; Saiding, Q.; Cai, Z.; Cai, M.; Cui, W. Ultrasound technology and biomaterials for precise drug therapy. Mater. Today 2023. [Google Scholar] [CrossRef]
- Wu, P.; Jia, Y.; Qu, F.; Sun, Y.; Wang, P.; Zhang, K.; Xu, C.; Liu, Q.; Wang, X. Ultrasound-Responsive Polymeric Micelles for Sonoporation-Assisted Site-Specific Therapeutic Action. ACS Appl. Mater. Interfaces 2017, 9, 25706–25716. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Cao, J.; Qi, X.; Wu, L.; Shen, S. Ultrasound responsive self-assembled micelles loaded with hypocrellin for cancer sonodynamic therapy. Int. J. Pharm. 2021, 608, 121052. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhai, G.-X. Intelligent polymeric micelles: Development and application as drug delivery for docetaxel. J. Drug Target. 2017, 25, 285–295. [Google Scholar] [CrossRef]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, J.; Su, Y.; Huang, J.; Li, J.; Qiu, L.; Zhan, M.; He, X.; Yuan, W.; Li, Y. Fabrication of thermoresponsive magnetic micelles from amphiphilic poly(phenyl isocyanide) and Fe3O4 nanoparticles for controlled drug release and synergistic thermochemotherapy. Polym. Chem. 2021, 12, 2132–2140. [Google Scholar] [CrossRef]
- Song, Y.; Li, D.; Lu, Y.; Jiang, K.; Yang, Y.; Xu, Y.; Dong, L.; Yan, X.; Ling, D.; Yang, X.; et al. Ferrimagnetic mPEG-b-PHEP copolymer micelles loaded with iron oxide nanocubes and emodin for enhanced magnetic hyperthermia–chemotherapy. Natl. Sci. Rev. 2020, 7, 723–736. [Google Scholar] [CrossRef]
- Bui, Q.N.; Li, Y.; Jang, M.-S.; Huynh, D.P.; Lee, J.H.; Lee, D.S. Redox- and pH-Sensitive Polymeric Micelles Based on Poly(β-amino ester)-Grafted Disulfide Methylene Oxide Poly(ethylene glycol) for Anticancer Drug Delivery. Macromolecules 2015, 48, 4046–4054. [Google Scholar] [CrossRef]
- Su, M.; Xiao, S.; Shu, M.; Lu, Y.; Zeng, Q.; Xie, J.; Jiang, Z.; Liu, J. Enzymatic multifunctional biodegradable polymers for pH- and ROS-responsive anticancer drug delivery. Colloids Surf. B Biointerfaces 2020, 193, 111067. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pang, S.; Li, X.; Li, Y. PH and redox dual-responsive polymeric micelles with charge conversion for paclitaxel delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 2078–2093. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pang, S.; Li, X.; Li, Y. Redox and pH Dual-Responsive Polymeric Micelles with Aggregation-Induced Emission Feature for Cellular Imaging and Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 18489–18498. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Liu, T. Fabrication of Mixed Polymeric Micelles Based on Stimuli-Responsive Amphiphilic Copolymers for Drug Delivery and Controlled Release. NANO 2020, 15, 2050040. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, R.; Zhao, C.; Sun, N.; Luo, H.; Chen, Y.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. Thermo- and pH-dual responsive polymeric micelles with upper critical solution temperature behavior for photoacoustic imaging-guided synergistic chemo-photothermal therapy against subcutaneous and metastatic breast tumors. Theranostics 2018, 8, 4097–4115. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kohestanian, M.; Streb, C. pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C 2020, 108, 110418. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Z.; Liu, B.; Yang, J.; Yang, X.; Yu, Y. A smart drug delivery system responsive to pH/enzyme stimuli based on hydrophobic modified sodium alginate. Eur. Polym. J. 2020, 133, 109779. [Google Scholar] [CrossRef]
- Su, T.; Cheng, F.; Pu, Y.; Cao, J.; Lin, S.; Zhu, G.; He, B. Polymeric micelles amplify tumor oxidative stresses through combining PDT and glutathione depletion for synergistic cancer chemotherapy. Chem. Eng. J. 2021, 411, 128561. [Google Scholar] [CrossRef]
- Wang, N.; Chen, X.-C.; Ding, R.-L.; Yang, X.-L.; Li, J.; Yu, X.-Q.; Li, K.; Wei, X. Synthesis of high drug loading, reactive oxygen species and esterase dual-responsive polymeric micelles for drug delivery. RSC Adv. 2019, 9, 2371–2378. [Google Scholar] [CrossRef]
- Liu, H.; Wang, K.; Yang, C.; Huang, S.; Wang, M. Multifunctional polymeric micelles loaded with doxorubicin and poly(dithienyl-diketopyrrolopyrrole) for near-infrared light-controlled chemo-phototherapy of cancer cells. Colloids Surf. B Biointerfaces 2017, 157, 398–406. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Fang, Y.-H.; Chiu, Y.-J.; Chang, C.-Y.; Lee, C.-H.; Liao, Z.-X.; Wang, L.-F. Light- and Redox-Responsive Block Copolymers of mPEG-SS-ONBMA as a Smart Drug Delivery Carrier for Cancer Therapy. Pharmaceutics 2022, 14, 2594. [Google Scholar] [CrossRef]
- Song, L.; Zhang, B.; Jin, E.; Xiao, C.; Li, G.; Chen, X. A reduction-sensitive thermo-responsive polymer: Synthesis, characterization, and application in controlled drug release. Eur. Polym. J. 2018, 101, 183–189. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, Z.; Peng, S.; Zhang, J.; Wang, W.; Wang, Q.; Lin, W.; Lin, X.; Zu, X.; Luo, H.; et al. pH/redox/UV irradiation multi-stimuli responsive nanogels from star copolymer micelles and Fe3+ complexation for ‘on-demand’ anticancer drug delivery. React. Funct. Polym. 2020, 149, 104532. [Google Scholar] [CrossRef]
- Qu: Reduction/Temperature/pH Multi-Stimuli Responsive...-Google Academic. Available online: https://scholar.google.com/scholar_lookup?title=Reduction%2Ftemperature%2FpH%20multi-stimuli%20responsive%20core%20cross-linked%20polypeptide%20hybrid%20micelles%20for%20triggered%20and%20intracellular%20drug%20release&journal=Colloids%20Surf%20B%20Biointerfaces&doi=10.1016%2Fj.colsurfb.2018.06.015&volume=170&pages=373-381&publication_year=2018&author=Qu%2CJ&author=Wang%2CQY&author=Kl%2CChen&author=Luo%2CJB&author=Zhou%2CQH&author=Lin%2CJ (accessed on 31 January 2023).
- Dong, Y.; Ma, X.; Huo, H.; Zhang, Q.; Qu, F.; Chen, F. Preparation of quadruple responsive polymeric micelles combining temperature-, pH-, redox-, and UV-responsive behaviors and its application in controlled release system. J. Appl. Polym. Sci. 2018, 135, 46675. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Xie, H.; Zhu, A.; Xu, Y.; Zeng, B.; Luo, W.; Dai, L. Polyion complex micelles formed by azobenzene-based polymer with multi-responsive properties. J. Appl. Polym. Sci. 2021, 138, 50580. [Google Scholar] [CrossRef]
- Bhavadharini, B.; Kavimughil, M.; Malini, B.; Vallath, A.; Prajapati, H.K.; Sunil, C.K. Recent Advances in Biosensors for Detection of Chemical Contaminants in Food—A Review. Food Anal. Methods 2022, 15, 1545–1564. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants-trends and perspective. TrAC Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Rangasamy, G.; Pauline, J.M.N.; Ramaraju, P.; Mohanasundaram, S.; Vo, D.-V.N. Biosensor for heavy metals detection in wastewater: A review. Food Chem. Toxicol. 2022, 168, 113307. [Google Scholar] [CrossRef]
- Xia, N.; Liu, G.; Zhang, S.; Shang, Z.; Yang, Y.; Li, Y.; Liu, L. Oxidase-mimicking peptide-copper complexes and their applications in sandwich affinity biosensors. Anal. Chim. Acta 2022, 1214, 339965. [Google Scholar] [CrossRef]
- Rahmawati, I.; Einaga, Y.; Ivandini, T.A.; Fiorani, A. Enzymatic Biosensors with Electrochemiluminescence Transduction. ChemElectroChem 2022, 9, e202200175. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, H.; Wang, Y.; Zhang, Y.-H.P. Protein engineering for electrochemical biosensors. Curr. Opin. Biotechnol. 2022, 76, 102751. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Jebelli, A.; Vandghanooni, S.; Jahanban-Esfahlan, A.; Baradaran, B.; Amini, M.; Bidar, N.; de la Guardia, M.; Mokhtarzadeh, A.; Eskandani, M. Perspectives and trends in advanced DNA biosensors for the recognition of single nucleotide polymorphisms. Chem. Eng. J. 2022, 441, 135988. [Google Scholar] [CrossRef]
- Huang, Q.-D.; Lv, C.-H.; Yuan, X.-L.; He, M.; Lai, J.-P.; Sun, H. A novel fluorescent optical fiber sensor for highly selective detection of antibiotic ciprofloxacin based on replaceable molecularly imprinted nanoparticles composite hydrogel detector. Sens. Actuators B Chem. 2021, 328, 129000. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Moharramnezhad, M. A promising electrochemiluminescence herbicide sensor based on ternary nanocomposite and boron nitride quantum dots for trace analysis of tribenuron-methyl in environmental samples. Microchem. J. 2021, 168, 106518. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lou, X.-Y.; Liang, F.; Yang, Y.-W. Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coord. Chem. Rev. 2022, 459, 214461. [Google Scholar] [CrossRef]
- Shukla, S.K.; Govender, P.P.; Tiwari, A. Chapter Six-Polymeric Micellar Structures for Biosensor Technology. In Advances in Biomembranes and Lipid Self-Assembly; Iglič, A., Kulkarni, C.V., Rappolt, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 24, pp. 143–161. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Elmasry, M.R.; Lee, Y.-I. Recent advances on amphiphilic polymer-based fluorescence spectroscopic techniques for sensing and imaging. Appl. Spectrosc. Rev. 2019, 54, 204–236. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Kumar, P.S.; Saravanan, R.; Govarthanan, M. Electrochemical sensing system for the analysis of emerging contaminants in aquatic environment: A review. Chemosphere 2022, 294, 133779. [Google Scholar] [CrossRef]
- Brown, K.; McMenemy, M.; Palmer, M.; Baker, M.J.; Robinson, D.W.; Allan, P.; Dennany, L. Utilization of an Electrochemiluminescence Sensor for Atropine Determination in Complex Matrices. Anal. Chem. 2019, 91, 12369–12376. [Google Scholar] [CrossRef]
- Li, S.; Pang, C.; Ma, X.; Wu, Y.; Wang, M.; Xu, Z.; Luo, J. Aggregation-induced electrochemiluminescence and molecularly imprinted polymer based sensor with Fe3O4@Pt nanoparticle amplification for ultrasensitive ciprofloxacin detection. Microchem. J. 2022, 178, 107345. [Google Scholar] [CrossRef]
- Alanazi, K.; Cruz, A.G.; Di Masi, S.; Voorhaar, A.; Ahmad, O.S.; Cowen, T.; Piletska, E.; Langford, N.; Coats, T.J.; Sims, M.R.; et al. Disposable paracetamol sensor based on electroactive molecularly imprinted polymer nanoparticles for plasma monitoring. Sens. Actuators B Chem. 2021, 329, 129128. [Google Scholar] [CrossRef]
- Sulym, I.; Cetinkaya, A.; Yence, M.; Çorman, M.E.; Uzun, L.; Ozkan, S.A. Novel electrochemical sensor based on molecularly imprinted polymer combined with L-His-MWCNTs@PDMS-5 nanocomposite for selective and sensitive assay of tetracycline. Electrochim. Acta 2022, 430, 141102. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Gao, X.; Sun, Z.; Sun, X.; Guo, Y.; Li, F.; Boboriko, N.E. Fluorescent aptasensor based on DNA-AgNCs emitting in the visible red wavelength range for detection of kanamycin in milk. Sens. Actuators B Chem. 2022, 360, 131665. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, Y. Molecularly imprinted micelles for fluorescent sensing of nonsteroidal anti-inflammatory drugs (NSAIDs). React. Funct. Polym. 2021, 158, 104759. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, H.; Zhu, F.; Ge, M.; Liang, G. Sensitive and rapid detection of aliphatic amines in water using self-stabilized micelles of fluorescent block copolymers. J. Hazard. Mater. 2019, 368, 630–637. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Gao, H.; Zhu, F.; Ge, M.; Liang, G. Rapid detection of aromatic pollutants in water using swellable micelles of fluorescent polymers. Sens. Actuators B Chem. 2019, 283, 415–425. [Google Scholar] [CrossRef]
- Han, J.; Cai, Y.; Wang, Y.; Dai, X.; Wang, L.; Li, C.; An, B.; Ni, L. Mixed polymeric micelles as a multifunctional visual thermosensor for the rapid analysis of mixed metal ions with Al3+ and Fe3+. New J. Chem. 2018, 42, 12853–12864. [Google Scholar] [CrossRef]
- Halder, A.; Shikha, D.; Adhikari, A.; Ghosh, R.; Singh, S.; Adhikari, T.; Pal, S.K. Development of A Nano-Sensor (FeNSOR) Based Device for Estimation of Iron Ions in Biological and Environmental Samples. IEEE Sens. J. 2020, 20, 1268–1274. [Google Scholar] [CrossRef]
- Home-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 31 January 2023).
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef]
- Keam, B.; Lee, K.-W.; Lee, S.-H.; Kim, J.-S.; Wu, H.-G.; Eom, K.-Y.; Kim, S.; Ahn, S.-H.; Chung, E.-J.; Kwon, S.K.; et al. A Phase II Study of Genexol-PM and Cisplatin as Induction Chemotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma. Oncologist 2019, 24, 751-e231. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, M.D.R.; Soto-Figueroa, C.; Galicia-Garcia, T.; Ruiz-Santos, R.; Vicente, L. Micellar phase control of poly(acrylic-acid-co-acrylonitrile) polymeric micelles via upper critical solution temperature: Removal process of organic molecules. Chem. Phys. Lett. 2022, 787, 139224. [Google Scholar] [CrossRef]
- Ray, S. Design and Applications of Theranostic Nanomedicines; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Dahiya, S.; Dahiya, R. Chapter 13-Smart drug delivery systems and their clinical potential. In Smart Polymeric Nano-Constructs in Drug Delivery; Vyas, S.P., Agrawal, U., Sharma, R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 401–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, T.; Gou, J.; Zhang, L.; Tao, X.; Tian, B.; Tian, P.; Yu, D.; Song, J.; Liu, X.; et al. Strategies for improving the payload of small molecular drugs in polymeric micelles. J. Control. Release 2017, 261, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, F.; Qi, H.; Li, F.; Xin, T.; Xu, J.; Ye, T.; Sheng, N.; Yang, X.; Pan, W. Multifunctional Tumor-Targeting Nanocarriers Based on Hyaluronic Acid-Mediated and pH-Sensitive Properties for Efficient Delivery of Docetaxel. Pharm. Res. 2014, 31, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Arafa, K.K.; El-Sherbiny, I.M. Chapter 20-Polymeric micelles with cleavable links for drug delivery. In Polymeric Micelles for Drug Delivery; Kesharwani, P., Greish, K., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 515–530. [Google Scholar] [CrossRef]
- Melim, C.; Jarak, I.; Veiga, F.; Figueiras, A. The potential of micelleplexes as a therapeutic strategy for osteosarcoma disease. 3 Biotech 2020, 10, 147. [Google Scholar] [CrossRef]
- Li, J.; Liu, P. Facile Synthesis of a Redox-Responsive Hyperbranched Polymer Prodrug as a Unimolecular Micelle for the Tumor-Selective Drug Delivery. Bioconjugate Chem. 2022, 33, 411–417. [Google Scholar] [CrossRef]
- Ray, M.; Lee, Y.-W.; Scaletti, F.; Yu, R.; Rotello, V.M. Intracellular delivery of proteins by nanocarriers. Nanomedicine 2017, 12, 941–952. [Google Scholar] [CrossRef]
- Teo, S.L.Y.; Rennick, J.J.; Yuen, D.; Al-Wassiti, H.; Johnston, A.P.R.; Pouton, C.W. Unravelling cytosolic delivery of cell penetrating peptides with a quantitative endosomal escape assay. Nat. Commun. 2021, 12, 3721. [Google Scholar] [CrossRef]
- Dong, X.; Guo, X.; Liu, G.; Fan, A.; Wang, Z.; Zhao, Y. When self-assembly meets topology: An enhanced micelle stability. Chem. Commun. 2017, 53, 3822–3825. [Google Scholar] [CrossRef]
- Mousavizadeh, A.; Jabbari, A.; Akrami, M.; Bardania, H. Cell targeting peptides as smart ligands for targeting of therapeutic or diagnostic agents: A systematic review. Colloids Surf. B Biointerfaces 2017, 158, 507–517. [Google Scholar] [CrossRef]
- Yan, L.; Li, X. Biodegradable Stimuli-Responsive Polymeric Micelles for Treatment of Malignancy. Curr. Pharm. Biotechnol. 2016, 17, 227–236. [Google Scholar] [CrossRef]






| Polymer | Chemical Structure | Properties | Ref. | |
|---|---|---|---|---|
| Hydrophilic polymers | PEG | 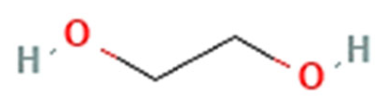 | Has been used in clinically approved nanoformulations including polymeric micelles (Genexol® PM). | [12,14,15,16] |
| Dextran |  | Included into block and graft copolymers as a component. Has been utilized as an excipient in clinically approved injectable pharmaceuticals (Feraheme®). Variable molecular weight. Biodegradable. | ||
| Poly(N-(2-hydroxypropyl)methacrylamide) (PHPMA) | 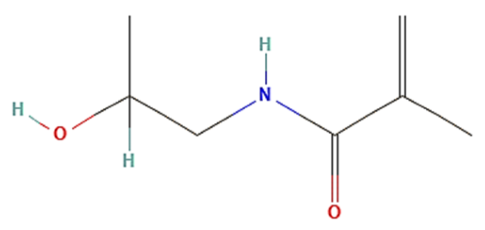 | Biocompatible, non-toxic, non-charged, and non-immunogenic. | ||
| Hydrophobic polymers | PLA |  | Clinically tested PLA-based polymeric micellar systems (Genexol® and Nanoxel®) are available. | |
| PLGA |  | Clinicians utilize PLGA (Vicryl®) as a biodegradable surgical suture. Biodegradable. | ||
| Poly(β-benzyl-l-aspartate) |  | The benzyl group’s presence increases hydrophobicity. Biodegradable. | ||
| Poly(γ-benzyl-α, l-glutamate) | 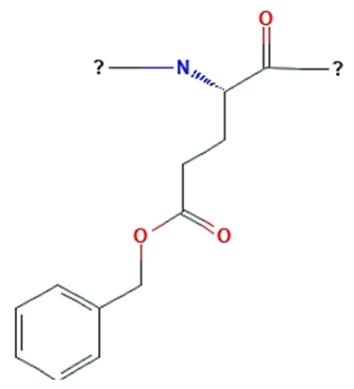 | The hydrophobicity can be adjusted by benzyl group’s presence. Extremely high loading capacity for a variety of poorly soluble medications (such as paclitaxel and etoposide). Broad library of polymer architectures. | ||
| Amphiphilic block copolymers | Poly(ethylene oxide)–PEO |  | PEOn-PPOm-PEOn copolymers are frequently employed in pharmaceutical formulations as non-active pharmaceutical components. Clinical trials for SP1049C, Pluronic®-based PMs entrapping (DOX). Marketed as poloxamers (Pluronic®). Biocompatible. | |
| PEOn-PPOm-PEOn |
| Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Direct dissolution | - Suitable for extremely hydrophilic molecular low weight polymers - Simple method - Organic approach (without solvents) - Possibility of scaling up | - A minimal medication load - Not appropriate for the majority of hydrophobic copolymers or drugs | [25,26] |
| Thin-film hydration/solvent evaporation | - High capacity for loading drugs - Possibility of scaling up | - Only usable for copolymers with high hydrophilic–lipophilic balance - Difficulty in removing the free drug and organic solvents from the formulation - Requires expensive equipment - Time-consuming | [25] |
| Oil in water emulsion | - High capacity for loading drugs - Restricted size range of micelles | - Using chlorinated solvents in the formulation makes it difficult to remove the free drug and organic solvents, which is not optimal for the environment. - Unrealistic for scaling up | [25] |
| Dialysis | - High capacity for loading drugs - Commonly employed for formulations at the laboratory scale | - Difficulty in eliminating organic solvents and free drugs from the formulation - Unrealistic for scaling up - Time-consuming - Significant amounts of water are necessary | [27] |
| Polymers | Anticancer Agent | Cancer Cell Lines | Experimental Condition & Therapeutic Outcomes | Ref. |
|---|---|---|---|---|
| Poly(L-lactic acid)-b-polylysine/poly(D-lactic acid)-b-methoxy poly(ethylene glycol) (PLLA-b-PLys/PDLA-b-mPEG) | DOX | 4T1 breast cancer cells | Exp. Cond.: In vitro Outcomes: - The DOX-loaded micelles exhibited a slower drug release behavior and a weaker efficacy of intracellular proliferation inhibition than PLLA-b-PLys micelles - DOX-loaded stereocomplexed micelles exhibited lower growth inhibition efficiency of both HepG2 and 4T1 cells | [113] |
| Poly(ethylene glycol)-imino-poly(benzyl-l-aspartate) (PIPAH) | Hydroxycamptothecin | MCF-7 cells | Exp. Cond.: In vitro and in vivo Outcomes: - The particle size of the PIPAH micelles did not change at pH 7.4 but increased at pH 6.0 and 5.0, respectively. - Only 25.5% of the encapsulated drug was released under normal physiological conditions (pH 7.4) within 12 h; drug release from the PIPAH micelles in pH 6.0 and 5.0 buffer solutions was noticeably accelerated - Delivery using the PPAH micelles resulted in substantially lower tumor accumulation | [114] |
| Poly{α-[4-(diethylamino)methyl-1,2,3-triazol]-caprolactone-co-caprolactone}-b-poly(2-methacryloyloxyethylphosphorylcholine) (PDCL-PMPC) | DOX | 4T1 breast cancercells | Exp. Cond.: In vitro, in vivo, ex vivo Outcomes: - The accumulation release account of DOX from PDCL-PMPC micelles was 20% at pH 7.4, but up to 75% at pH 5.0 - At low pH such as a subcellular organelle environment, PDCL-PMPC micelles release DOX quickly - Reasonable in vivo antitumor effect | [115] |
| Heparin-alpha-tocophero- cis-aconitic anhydride (HEP-CA-TOC) | Docetaxel | MCF-7 and 4TI breast cancercells | Exp. Cond.: In vitro Outcomes: - Docetaxel release rate increased as the pH of the medium decreased - In the acidic environment of the tumor cells, CA bond starts to hydrolyze and accelerate the release and endosome escape of docetaxel leading to more cytotoxicity and treatment efficiency | [116] |
| Poly (acrylic acid)-b-polycaprolactone (PAA-b-PCL) | Gambogenic acid | HepG2 cells | Exp. Cond.: In vitro, in vivo Outcomes: - Only 16.64 % of gambogenic acid was released within the first 12 h - The encapsulation by micelles could enhance both the cytotoxicity and the circulation of gambogenic acid in the body | [117] |
| Eudragit® S100 | Quercetin | CT26 murine colon carcinoma cells | Exp. Cond.: In vitro Outcomes: - In vitro release testing showed a delay in drug release in acidic pH, but complete release within 24 h at pH 7.2. - Dose-dependent decrease in cell viability | [118] |
| Cyclodextrins- Acrylic/maleic copolymer | Capecitabine | Exp. Cond.: In vitro Outcomes: - The release of Capecitabine at pH 1.2 within 2 h is only 4%; at pH 7.4, 70.4% was released within 12 h and 97% was released within 36 h | [119] | |
| N-deacetyl hyaluronic acid dodecylamine | DOX | MCF-7 cells | Exp. Cond.: In vitro and in vivo Outcomes: - Micelles possessed excellent serum stability at pH 7.4 and very low cytotoxicity - Micelles exhibited antitumor therapeutic efficacy with remarkably low systemic toxicity in vivo | [120] |
| Dual/Multiple Responsive | Micellar System | Anti-Cancer Medication | Ref. |
|---|---|---|---|
| Dual-responsive | |||
| pH/Thermo | Poly (β-amino ester)-grafted disulfide methylene oxide poly (ethylene glycol) (PAE-g-DSMPEG) | DOX | [182] |
| PEG-poly (ω- pentadecalactone-co-N-methyldiethyleneamine sebacate-co-2, 2′-thiodiethylene sebacate) (mPEG-b-PAE-ss-DOX, mPEG-b-PAE-cis-DOX) | [183] | ||
| Methoxypoly(ethylene glycol)-cystamine-poly(L-glutamic acid)-imidazole (mPEG-SS-PGA-IM) | PTX | [184] | |
| PEG-poly(tetrapheny-lethene-co-2-azepane ethyl methacrylate) (mPEG-P(TPE-co-AEMA) | DOX | [185] | |
| Polyphosphazene (PPZ) | DOX | [186] | |
| PEG-b-poly(acrylamide-co-acrylonitrile-co-vinylimidazole) copolymer (mPEG-PAAV) | DOX and IR780 (NIR absorber) | [187] | |
| Poly(methacrylic acid)-b-poly(N-isopropylacrylamid (PMAA-b-PNIPAM) | DOX | [188] | |
| pH/enzyme | Hydrophobic modified alginate | DOX | [189] |
| Redox/ROS | PEG(-b-PCL-Ce6)-b-PBEMA | [190] | |
| ROS/enzyme | N-isopropylacrylamide (NIPAM)–boronic esters | [191] | |
| Light/thermo | Poly(dithienyl-diketopyrrolopyrrole) (PDPP–F127) | [192] | |
| Light/redox | PEG- hydrophobic o-nitrobenzyl methacrylate (mPEG-SS-pONBMA) | [193] | |
| Redox/thermo | mPEG2k-b-400DTPA-b-mPEG2 (PEG-DTPA) | Nile Red (NR) | [194] |
| Multiple-responsive | |||
| pH/redox/UV | PCL -b-poly (acrylic acid) -b-poly (poly (ethylene glycol) methyl ether methacrylate) (6AS-PCL-PAA-PPEGMA) | DOX | [195] |
| Redox/temperature/pH | Poly(γ-benzyl-L-glutamate) (PBLG) and N-isopropylacrylamide (NIPPAM) | [196] | |
| Temperature/pH/redox/UV | Poly(ethylene glycol)-ss-[poly(dimethylaminoethyl methacrylate)-copoly(2-nitrobenzyl methacrylate)] [PEG-ss-(PDMAEMA-co-PNBM)] | NR | [197] |
| Light/pH/temperature/redox | Azobenzene-based amphiphilic copolymers P (MMA-co-PEGMA-co-NIPAM-co-HAZOMA) with ionized carboxyl and P (MMA-co-PEGMA-co-NIPAM-co-NNAZOMA) | [198] | |
| Trade Name | Drug/ Ingredient | Copolymer | Development Stage | Properties | Identifier | Ref. |
|---|---|---|---|---|---|---|
| Genexol®—PM | PTX | mPEG-b-PDLLA | Approved in South Korea, Philippines, India, Vietnam, and Indonesia; Phase I-IV | Improved or equivalent therapeutic efficacy for metastatic breast cancer, non-small cell lung cancer, advanced gastric cancer, and irresectable thymic epithelial tumors; increased drug dosage without premedication and added toxicity | NCT00111904 NCT01023347 NCT00886717 NCT01276548 NCT00882973 NCT00877253 | [222] |
| Nanoxel® | PTX | PVP-b-PNIPAM | Approved in India; Phase I-III | Similar therapeutic efficacy, and fewer side effects when compared to Taxol®; treating advanced breast cancer, but potential clinical trial deficiency | NCT03614364 NCT04066335 NCT00915369 NCT02639858 NCT03585673 NCT02982395 | [222] |
| Nanoxel® M | Docetaxel | mPEG-b-PDLLA | Approved in South Korea; Phase I-III | Similar PK profiles and antitumor efficacy compared to Taxotere®; reduced side effects | NCT04066335 NCT02639858 NCT03585673 NCT02982395 NCT03614364 NCT00915369 | [222] |
| Zisheng® | PTX | mPEG-PDLLA | Approved in China; Phase I, III | Enhanced therapeutic efficacy and good patient acceptance for the treatment of non-small cell lung cancer | CTR20150217 CTR20130637 ChiCTR-ONC-14005123 ChiCTR-IPR-15006252 | [222] |
| NK105 | PTX | mPEG-b-modified P(Asp) | Phase III | Increased plasma AUC, a decreased occurrence of peripheral sensory neuropathy, and similar overall effectiveness to Taxol ® for metastatic or recurring breast cancer were all observed | NCT01644890 | [222] |
| NK911 | DOX | PEG-b-P(Asp-DOX) | Phase I | A better set of PK characteristics when compared to Doxil and the free medication; no fusion-related events; early indications of a therapeutic response | - | [222] |
| NK012 | SN-38 | PEG-b-P(Glu-sN-38) | Phase I, II | Patients with unresectable metastatic colorectal cancer experienced similar treatment effectiveness and decreased incidents of serious diarrhea compared to those receiving standard care; patients suffering with refractory lung cancer experienced two complete responses and a 22% overall response rate | NCT00951054 NCT00951613 NCT00542958 NCT01238939 NCT01238952 | [222] |
| NC-6004 | Cisplatin | PEG-b-P(Glu) | Phase I-III | Increased tolerability in patients with advanced solid tumors and decreased cisplatin-related toxicity | NCT02043288 NCT02817113 | [222] |
| NC-4016 | DACHPt | PEG-b-P(Glu) | Phase I | In a gastric cancer xenograft model, combination treatment with NC-6300 and NC-4016 had superior anticancer efficacy and reduced toxicity | NCT03168035 | [222] |
| K-912/NC-6300 | Epirubicin | PEG-b-P(Asp-epirubicin) | Phase I-II | Less toxic than traditional epirubicin, patient tolerance with a variety of solid tumors; early angiosarcoma activity | NCT03168061 | [222] |
| CPC634 (CriPec®) | Docetaxel | mPEG-b-P(HPMAm-Lacn -docetaxel) | Phase I-II | Early indications of anti-tumor activity and increased intratumoral docetaxel exposure were noted, as was high dosage skin toxicity | NCT02442531 NCT03742713 NCT03712423 | [222] |
| BIND-014 | Docetaxel | PLA-PEG-GL | Phase I-II | Antitumor activity in metastatic castration-resistant prostate cancer; good tolerance and tolerable toxicity; clinically active and well-tolerated in stage III/IV non-small cell lung cancer | NCT01812746 NCT01792479 NCT01300533 NCT02283320 NCT02479178 | [222] |
| SP1049C | DOX | Pluronic® L61/F127 | Phase I-II | Patients suffering from gastroesophageal junction and esophageal cancer showed therapeutic effectiveness as a single drug and a tolerable safety profile | - | [222] |
| Resveratrol from Vineatrol 30® extract incorporated into micelles | Resveratrol | Phase I | Estimation of pharmacokinetics and safety | NCT02944097 | [222] | |
| PTX micelles | PTX | Phase I | Investigated for efficacy in advanced solid tumors | NCT04778839 | [222] | |
| PTX micelles for injection | PTX | Phase I | Investigated for efficvacy in gynecological cancer | NCT02739529 | [222] | |
| ONM-100 | Indocyanine green | Phase II | Investigated for the detection of cancer in patients with solid tumors (breast cancer, head and neck squamous cell carcinoma, colorectal cancer, prostate cancer, ovarian cancer, urothelial carcinoma, non-small cell lung cancer) undergoing routine surgery | NCT03735680 | [222] |
| Limitations | Options | Ref. |
|---|---|---|
| Low drug loading | - increasing the drug-polymer compatibility - cross-linking of the core and shell of self-assembled polymeric micelles - electrostatic interactions - the micelles can be coated one layer at a time - particles that resemble micelles - lipids should incorporate drug-attached polymers - polymeric prodrugs | [228,229] |
| High CMC | - lengthening the hydrophobic block’s chain - micelle cores can be decorated with different fatty acids, and a benzyl group can be added. | [230] |
| Rapid clearance | - PEGylation approach - Cross-linking with stimuli-sensitive linkers | [231] |
| Low efficiency in drug delivery | - the use of high-affinity targeting ligands - cross-linking with diverse stimuli-sensitive linkers - intracellular redox-responsive drug release | [232,233] |
| Low selectivity | - PEGylation approach - the use of high-affinity targeting ligands | [228,229,231] |
| Low capacity to disrupt membranes | - the use of hydrophobic moieties and cationic groups - the use of polymers with buffering capacity at endosomal pH - the use of high-affinity targeting ligands | [234,235] |
| Low stability | - PEGylation approach - Covalent cross-linking methods that result in shell cross-linked micelles, or core cross-linked ones - covalent cross-linking strategies: photo/UV dimerization, di-functional cross-linkers, click cross-linking method, silicon chemistry method, and reversible boronate ester bond - non-covalent cross-linking by means of micelle cores complexation - modifying the hydrophilic/hydrophobic block ratios of the micelles - increasing the crystallinity of hydrophobic groups - inorganic materials introduction into the core or shell in order to function as structural stabilizers | [230,236] |
| Toxicity and immunogenicity | - PEGylation approach - using pH-sensitive micelles - using high affinity targeting ligands - using biodegradable and biocompatible micellar systems | [142,237] |
| Non-biodegradability and non-biocompatibility | - the use of biodegradable micellar systems such as: PEG, PLA, PCL, mPEG-PDLLA, poly(L-histidine) | [238] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negut, I.; Bita, B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976. https://doi.org/10.3390/pharmaceutics15030976
Negut I, Bita B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics. 2023; 15(3):976. https://doi.org/10.3390/pharmaceutics15030976
Chicago/Turabian StyleNegut, Irina, and Bogdan Bita. 2023. "Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery" Pharmaceutics 15, no. 3: 976. https://doi.org/10.3390/pharmaceutics15030976
APA StyleNegut, I., & Bita, B. (2023). Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics, 15(3), 976. https://doi.org/10.3390/pharmaceutics15030976








