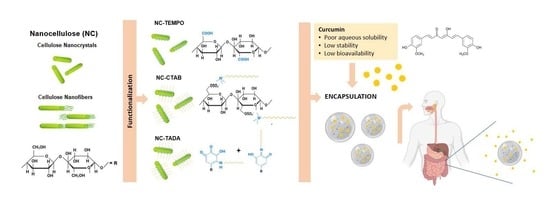
Design of Innovative Biocompatible Cellulose Nanostructures for the Delivery and Sustained Release of Curcumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Production of CNC-TEMPO
2.3. CNC and CNF Modification with CTAB Surfactant
2.4. CNC and CNF Modification with TA and DA
2.5. Characterization
2.5.1. Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy
2.5.2. Powder X-ray Diffraction
2.5.3. Zeta Potential Determination
2.5.4. Microscopy Analysis
2.6. Curcumin Loading
2.7. Curcumin Quantification
2.8. Encapsulation Efficiency, Loading Capacity and Yield
2.9. Curcumin Release Profile
2.10. Cytotoxicity Evaluation
2.11. Statistical Analysis
3. Results
3.1. Characterization of the Carrier Materials
3.1.1. ATR-FT-IR Analysis
3.1.2. PXRD Analysis
3.1.3. Zeta Potential
3.2. Curcumin Binding Analysis
3.2.1. Preliminary Curcumin:Carrier Ratio Screening
3.2.2. ATR-FT-IR Analysis of the Loaded Nanostructures
3.2.3. Curcumin Loading
3.3. Curcumin Release Profile
3.4. Cytotoxicity Evaluation
3.5. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pachuau, L. Application of Nanocellulose for Controlled Drug Delivery. In Nanocellulose and Nanohydrogel Matrices; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 1–19. [Google Scholar]
- Plackett, D.; Letchford, K.; Jackson, J.; Burt, H. A Review of Nanocellulose as a Novel Vehicle for Drug Delivery. Nord. Pulp Pap. Res. J. 2014, 29, 105–118. [Google Scholar] [CrossRef]
- Mohanta, V.; Madras, G.; Patil, S. Layer-by-Layer Assembled Thin Films and Microcapsules of Nanocrystalline Cellulose for Hydrophobic Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 20093–20101. [Google Scholar] [CrossRef]
- Foo, M.L.; Tan, C.R.; Lim, P.D.; Ooi, C.W.; Tan, K.W.; Chew, I.M.L. Surface-Modified Nanocrystalline Cellulose from Oil Palm Empty Fruit Bunch for Effective Binding of Curcumin. Int. J. Biol. Macromol. 2019, 138, 1064–1071. [Google Scholar] [CrossRef]
- Löbmann, K.; Svagan, A.J. Cellulose Nanofibers as Excipient for the Delivery of Poorly Soluble Drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of Hydrophobic and Low-Soluble Food Bioactive Compounds within Different Nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-Loaded Nanocarriers: A Comprehensive Review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of Different Nanocarriers for Encapsulation of Curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 3468–3497. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, J.F.; Bourbon, A.I.; Simões, L.S.; Vicente, A.A.; Coutinho, P.J.G.; Ramos, O.L. Physicochemical Characterisation and Release Behaviour of Curcumin-Loaded Lactoferrin Nanohydrogels into Food Simulants. Food Funct. 2020, 11, 305–317. [Google Scholar] [CrossRef]
- Casanova, F.; Pereira, C.F.; Ribeiro, A.B.; Freixo, R.; Costa, E.; Pintado, M.E.; Fernandes, J.C.; Ramos, Ó.L. Novel Micro- and Nanocellulose-Based Delivery Systems for Liposoluble Compounds. Nanomaterials 2021, 11, 2593. [Google Scholar] [CrossRef]
- George, D.; Begum, K.M.M.S.; Maheswari, P.U. Sugarcane Bagasse (SCB) Based Pristine Cellulose Hydrogel for Delivery of Grape Pomace Polyphenol Drug. Waste Biomass Valorization 2020, 11, 851–860. [Google Scholar] [CrossRef]
- Salem, A.; Ramadan, A.R.; Shoeib, T. Entrapment of β-Carotene and Zinc in Whey Protein Nanoparticles Using the PH Cycle Method: Evidence of Sustained Release Delivery in Intestinal and Gastric Fluids. Food Biosci. 2018, 26, 161–168. [Google Scholar] [CrossRef]
- Sáiz-Abajo, M.J.; González-Ferrero, C.; Moreno-Ruiz, A.; Romo-Hualde, A.; González-Navarro, C.J. Thermal Protection of β-Carotene in Re-Assembled Casein Micelles during Different Processing Technologies Applied in Food Industry. Food Chem. 2013, 138, 1581–1587. [Google Scholar] [CrossRef]
- Anarjan, N.; Nehdi, I.; Sbihi, H.; Al-Resayes, S.; Malmiri, H.; Tan, C. Preparation of Astaxanthin Nanodispersions Using Gelatin-Based Stabilizer Systems. Molecules 2014, 19, 14257–14265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.-X.; Zhang, N.; Tang, C.-H. Soy Protein Isolate as a Nanocarrier for Enhanced Water Dispersibility, Stability and Bioaccessibility of β -Carotene. J. Sci. Food Agric. 2017, 97, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.T.; Bourbon, A.I.; Pinheiro, A.C.; Fasolin, L.H.; Vicente, A.A. Protein-Based Structures for Food Applications: From Macro to Nanoscale. Front. Sustain. Food Syst. 2018, 2, 77. [Google Scholar] [CrossRef]
- Samadi, N.; Aberoomand Azar, P.; Waqif Husain, S.; Maibach, H.I.; Nafisi, S. Experimental Design in Formulation Optimization of Vitamin K1 Oxide-Loaded Nanoliposomes for Skin Delivery. Int. J. Pharm. 2020, 579, 119136. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, Q.; Diao, C.; Wang, J.; Wu, Z.; Wang, H. Enhanced Physicochemical Stability of Lutein-Enriched Emulsions by Polyphenol-Protein-Polysaccharide Conjugates and Fat-Soluble Antioxidant. Food Hydrocoll. 2020, 101, 105447. [Google Scholar] [CrossRef]
- Pinto, F.; de Barros, D.P.C.; Fonseca, L.P. Design of Multifunctional Nanostructured Lipid Carriers Enriched with α-Tocopherol Using Vegetable Oils. Ind. Crops Prod. 2018, 118, 149–159. [Google Scholar] [CrossRef]
- Guorgui, J.; Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin Formulated in Solid Lipid Nanoparticles Has Enhanced Efficacy in Hodgkin’s Lymphoma in Mice. Arch. Biochem. Biophys. 2018, 648, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Luckanagul, J.A.; Pitakchatwong, C.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-Based Polymer Hybrids for Thermo-Responsive Nanogel Delivery of Curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Inclusion Complexation of Catechin by β-Cyclodextrins: Characterization and Storage Stability. LWT 2017, 86, 555–565. [Google Scholar] [CrossRef]
- Fanta, G.F.; Kenar, J.A.; Felker, F.C. Nanoparticle Formation from Amylose-Fatty Acid Inclusion Complexes Prepared by Steam Jet Cooking. Ind. Crops Prod. 2015, 74, 36–44. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, C.; Dai, L.; Zhan, X.; Gao, Y. Structure, Physicochemical Stability and in Vitro Simulated Gastrointestinal Digestion Properties of β-Carotene Loaded Zein-Propylene Glycol Alginate Composite Nanoparticles Fabricated by Emulsification-Evaporation Method. Food Hydrocoll. 2018, 81, 149–158. [Google Scholar] [CrossRef]
- Sharif, H.R.; Goff, H.D.; Majeed, H.; Shamoon, M.; Liu, F.; Nsor-Atindana, J.; Haider, J.; Liang, R.; Zhong, F. Physicochemical Properties of β-Carotene and Eugenol Co-Encapsulated Flax Seed Oil Powders Using OSA Starches as Wall Material. Food Hydrocoll. 2017, 73, 274–283. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpour, E. Preparation of a Multiple Emulsion Based on Pectin-Whey Protein Complex for Encapsulation of Saffron Extract Nanodroplets. Food Chem. 2017, 221, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic Kenaf Nanocrystalline Cellulose for the Binding of Curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef]
- Lam, N.T.; Saewong, W.; Sukyai, P. Effect of Varying Hydrolysis Time on Extraction of Spherical Bacterial Cellulose Nanocrystals as a Reinforcing Agent for Poly(Vinyl Alcohol) Composites. J. Polym. Res. 2017, 24, 71. [Google Scholar] [CrossRef]
- Xiang, Z.; Gao, W.; Chen, L.; Lan, W.; Zhu, J.Y.; Runge, T. A Comparison of Cellulose Nanofibrils Produced from Cladophora Glomerata Algae and Bleached Eucalyptus Pulp. Cellulose 2016, 23, 493–503. [Google Scholar] [CrossRef]
- Otsuka, I.; Njinang, C.N.; Borsali, R. Simple Fabrication of Cellulose Nanofibers via Electrospinning of Dissolving Pulp and Tunicate. Cellulose 2017, 24, 3281–3288. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent Advances in Surface-Modified Cellulose Nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- De Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Controlled Release of Carvacrol and Curcumin: Bio-Based Food Packaging by Synergism Action of TEMPO-Oxidized Cellulose Nanocrystals and Cyclodextrin. Cellulose 2018, 25, 1249–1263. [Google Scholar] [CrossRef]
- Jackson, J.K.; Letchford, K.; Wasserman, B.Z.; Ye, L.; Hamad, W.Y.; Burt, H.M. The Use of Nanocrystalline Cellulose for the Binding and Controlled Release of Drugs. Int. J. Nanomed. 2011, 6, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Qing, W.; Wang, Y.; Wang, Y.; Zhao, D.; Liu, X.; Zhu, J. The Modified Nanocrystalline Cellulose for Hydrophobic Drug Delivery. Appl. Surf. Sci. 2016, 366, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Saito, T.; Bergström, L.; Isogai, A. Acid-Free Preparation of Cellulose Nanocrystals by TEMPO Oxidation and Subsequent Cavitation. Biomacromolecules 2018, 19, 633–639. [Google Scholar] [CrossRef]
- Shea, F.; Watts, C.E. Dumas Method for Organic Nitrogen. Ind. Eng. Chem. Anal. Ed. 1939, 11, 333–334. [Google Scholar] [CrossRef]
- Hu, Z.; Berry, R.M.; Pelton, R.; Cranston, E.D. One-Pot Water-Based Hydrophobic Surface Modification of Cellulose Nanocrystals Using Plant Polyphenols. ACS Sustain. Chem. Eng. 2017, 5, 5018–5026. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal Crystallinity Index Revisited by the Simulation of X-ray Diffraction Patterns of Cotton Cellulose Iβ and Cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, N.; Bannikova, A.; Evteev, A.; Evdokimov, I.; Kasapis, S. Alginate-Based Encapsulation of Extracts from Beta Vulgaris Cv. Beet Greens: Stability and Controlled Release under Simulated Gastrointestinal Conditions. LWT 2018, 93, 442–449. [Google Scholar] [CrossRef]
- Valo, H.; Kovalainen, M.; Laaksonen, P.; Häkkinen, M.; Auriola, S.; Peltonen, L.; Linder, M.; Järvinen, K.; Hirvonen, J.; Laaksonen, T. Immobilization of Protein-Coated Drug Nanoparticles in Nanofibrillar Cellulose Matrices-Enhanced Stability and Release. J. Control Release 2011, 156, 390–397. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2009; p. 34.
- Shahi, N.; Min, B.; Sapkota, B.; Rangari, V.K. Eco-Friendly Cellulose Nanofiber Extraction from Sugarcane Bagasse and Film Fabrication. Sustainability 2020, 12, 6015. [Google Scholar] [CrossRef]
- Rahimi Kord Sofla, M.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A Comparison of Cellulose Nanocrystals and Cellulose Nanofibres Extracted from Bagasse Using Acid and Ball Milling Methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 35004. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Seixas, M.V.d.S. Obtenção de Nanocellulose a Partir Do Bagaço de Cana-de-Açúcar e Incorporação Em Eva; Escola Politécnica da Universidade de São Paulo: São Paulo, Brazil, 2019. [Google Scholar]
- Bakkari, M.E.; Bindiganavile, V.; Goncalves, J.; Boluk, Y. Preparation of Cellulose Nanofibers by TEMPO-Oxidation of Bleached Chemi-Thermomechanical Pulp for Cement Applications. Carbohydr. Polym. 2019, 203, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, I.P.; Wirjosentono, B.; Tamrin; Ismail, H.; Mendez, J.A. Thermal and Morphology Properties of Cellulose Nanofiber from TEMPO-Oxidized Lower Part of Empty Fruit Bunches (LEFB). Open Chem. 2019, 17, 526–536. [Google Scholar] [CrossRef]
- Yang, J.; Cohen Stuart, M.A.; Kamperman, M. Jack of All Trades: Versatile Catechol Crosslinking Mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Isogai, A.; Zhou, Y. Diverse Nanocelluloses Prepared from TEMPO-Oxidized Wood Cellulose Fibers: Nanonetworks, Nanofibers, and Nanocrystals. Curr. Opin. Solid State Mater. Sci. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Kim, H.J.; Roy, S.; Rhim, J.W. Effects of Various Types of Cellulose Nanofibers on the Physical Properties of the CNF-Based Films. J. Environ. Chem. Eng. 2021, 9, 106043. [Google Scholar] [CrossRef]
- Wicaksono, R.; Syamsu, K.; Yuliasih, I.; Nasir, M. Cellulose Nanofibers from Cassava Bagasse: Characterization and Application on Tapioca-Film. Chem. Mater. Res. 2013, 3, 79–87. [Google Scholar]
- Simões, L.S.; Araújo, J.F.; Vicente, A.A.; Ramos, O.L. Design of β-Lactoglobulin Micro- and Nanostructures by Controlling Gelation through Physical Variables. Food Hydrocoll. 2020, 100, 105357. [Google Scholar] [CrossRef] [Green Version]
- Ghalandari, B.; Divsalar, A.; Saboury, A.A.; Parivar, K. β-Lactoglobulin Nanoparticle as a Chemotherapy Agent Carrier for Oral Drug Delivery System. J. Iran. Chem. Soc. 2015, 12, 613–619. [Google Scholar] [CrossRef]
- Ntoutoume, G.M.A.N.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V.; et al. Development of Curcumin-Cyclodextrin/Cellulose Nanocrystals Complexes: New Anticancer Drug Delivery Systems. Bioorganic Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef]
- Gamelas, J.A.F.; Pedrosa, J.; Lourenço, A.F.; Mutjé, P.; González, I.; Chinga-Carrasco, G.; Singh, G.; Ferreira, P.J.T. On the Morphology of Cellulose Nanofibrils Obtained by TEMPO-Mediated Oxidation and Mechanical Treatment. Micron 2015, 72, 28–33. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath Udeni Gunathilake, T.M.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a Nonionic Surfactant on Curcumin Delivery of Nanocellulose Reinforced Chitosan Hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Dobberpuhl, M.R.; Maher, D.M.; Jaggi, M.; Chauhan, S.C. Design of Curcumin Loaded Cellulose Nanoparticles for Prostate Cancer. Curr. Drug Metab. 2012, 13, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-Loaded Polysaccharides-Based Complex Particles Obtained by Polyelectrolyte Complexation and Ionic Gelation. I-Particles Obtaining and Characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Kolakovic, R.; Laaksonen, T.; Peltonen, L.; Laukkanen, A.; Hirvonen, J. Spray-Dried Nanofibrillar Cellulose Microparticles for Sustained Drug Release. Int. J. Pharm. 2012, 430, 47–55. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, T.; Liu, L.; Wang, X.; Wu, P.; Chen, Z.; Ni, C.; Zhang, J.; Hu, F.; Huang, J. Novel Micelle Formulation of Curcumin for Enhancing Antitumor Activity and Inhibiting Colorectal Cancer Stem Cells. Int. J. Nanomed. 2012, 7, 4487–4497. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Shen, Z.L.; Zhai, S.; Xu, J.L.; Liang, H.; Shen, Q.; Li, Q.Y. Transport of Curcumin Derivatives in Caco-2 Cell Monolayers. Eur. J. Pharm. Biopharm. 2017, 117, 123–131. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity Tests of Cellulose Nanofibril-Based Structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Armendáriz-Barragán, B.; Zafar, N.; Badri, W.; Galindo-Rodríguez, S.A.; Kabbaj, D.; Fessi, H.; Elaissari, A. Plant Extracts: From Encapsulation to Application. Expert Opin. Drug Deliv. 2016, 13, 1165–1175. [Google Scholar] [CrossRef]
- Sopeña, F.; Maqueda, C.; Morillo, E. Controlled Release Formulations of Herbicides Based on Micro-Encapsulation. Cienc. e Investig. Agrar. 2009, 36, 27–42. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Zhao, Y. Chitosan-Cellulose Nanocrystal Microencapsulation to Improve Encapsulation Efficiency and Stability of Entrapped Fruit Anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.; Laaksonen, T. Nanofibrillar Cellulose Films for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef] [PubMed]









| Sample | Zeta Potential (mV) |
|---|---|
| CNC | −45.03 ± 0.79 a |
| CNF | −34.20 ± 1.03 b |
| CNC-TEMPO | −34.37 ± 1.04 b |
| CNF-TEMPO | −30.90 ± 1.68 c |
| CNC-CTAB | −27.97 ± 2.01 c |
| CNF-CTAB | −17.47 ± 2.25 d |
| CNC-TADA | −22.03 ± 0.84 d,e |
| CNF-TADA | −24.90 ± 1.37 e |
| Ratio Curcumin:CNC | EE (%) | LC (%) | Theoric LC (%) |
|---|---|---|---|
| 1:2 | 56.64 ± 0.76 a | 26.90 ± 0.83 a | 33.33 |
| 1:3 | 81.26 ± 0.21 b | 24.57 ± 0.30 b | 25.00 |
| 1:5 | 79.46 ± 0.25 c | 16.04 ± 0.42 c | 16.67 |
| 1:10 | 78.31 ± 0.19 d | 8.82 ± 0.23 d | 9.09 |
| 1:15 | 74.97 ± 0.83 e | 3.13 ± 0.86 e | 6.25 |
| Delivery System | Yield (%) | EE (%) | LC (%) | ZP (mV) |
|---|---|---|---|---|
| CNC | 68.01 ± 3.22 a | 84.29 ± 3.06 a | 31.00 ± 3.41 a | −49.83 ± 2.75 |
| CNF | 91.22 ± 3.64 b | 84.81 ± 4.79 a | 24.72 ± 2.12 b | −19.67 ± 5.52 |
| CNC-TEMPO | 43.33 ± 4.01 c | 54.52 ± 3.29 b | 31.50 ± 3.45 a | −26.13 ± 0.58 |
| CNF-TEMPO | 68.90 ± 2.73 a | 85.39 ± 0.28 a | 25.41 ± 2.70 b | −25.13 ± 0.90 |
| CNC-CTAB | 90.36 ± 3.24 b,d | 90.23 ± 1.58 c | 24.78 ± 3.47 b | −21.97 ± 1.86 |
| CNF-CTAB | 68.95 ± 3.40 a | 81.27 ± 2.97 a | 26.98 ± 3.29 b | −15.37 ± 1.18 |
| CNC-TADA | 83.06 ± 2.89 d | 99.85 ± 0.23 d | 30.10 ± 1.06 a | −20.90 ± 2.43 |
| CNF-TADA | 93.25 ± 2.16 b | 99.84 ± 0.31 d | 26.80 ± 2.29 b | −21.91 ± 1.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, F.; Pereira, C.F.; Ribeiro, A.B.; Costa, E.M.; Freixo, R.; Castro, P.M.; Fernandes, J.C.; Pintado, M.; Ramos, Ó.L. Design of Innovative Biocompatible Cellulose Nanostructures for the Delivery and Sustained Release of Curcumin. Pharmaceutics 2023, 15, 981. https://doi.org/10.3390/pharmaceutics15030981
Casanova F, Pereira CF, Ribeiro AB, Costa EM, Freixo R, Castro PM, Fernandes JC, Pintado M, Ramos ÓL. Design of Innovative Biocompatible Cellulose Nanostructures for the Delivery and Sustained Release of Curcumin. Pharmaceutics. 2023; 15(3):981. https://doi.org/10.3390/pharmaceutics15030981
Chicago/Turabian StyleCasanova, Francisca, Carla F. Pereira, Alessandra B. Ribeiro, Eduardo M. Costa, Ricardo Freixo, Pedro M. Castro, João C. Fernandes, Manuela Pintado, and Óscar L. Ramos. 2023. "Design of Innovative Biocompatible Cellulose Nanostructures for the Delivery and Sustained Release of Curcumin" Pharmaceutics 15, no. 3: 981. https://doi.org/10.3390/pharmaceutics15030981
APA StyleCasanova, F., Pereira, C. F., Ribeiro, A. B., Costa, E. M., Freixo, R., Castro, P. M., Fernandes, J. C., Pintado, M., & Ramos, Ó. L. (2023). Design of Innovative Biocompatible Cellulose Nanostructures for the Delivery and Sustained Release of Curcumin. Pharmaceutics, 15(3), 981. https://doi.org/10.3390/pharmaceutics15030981