Applications of Stimuli-Responsive Hydrogels in Bone and Cartilage Regeneration
Abstract
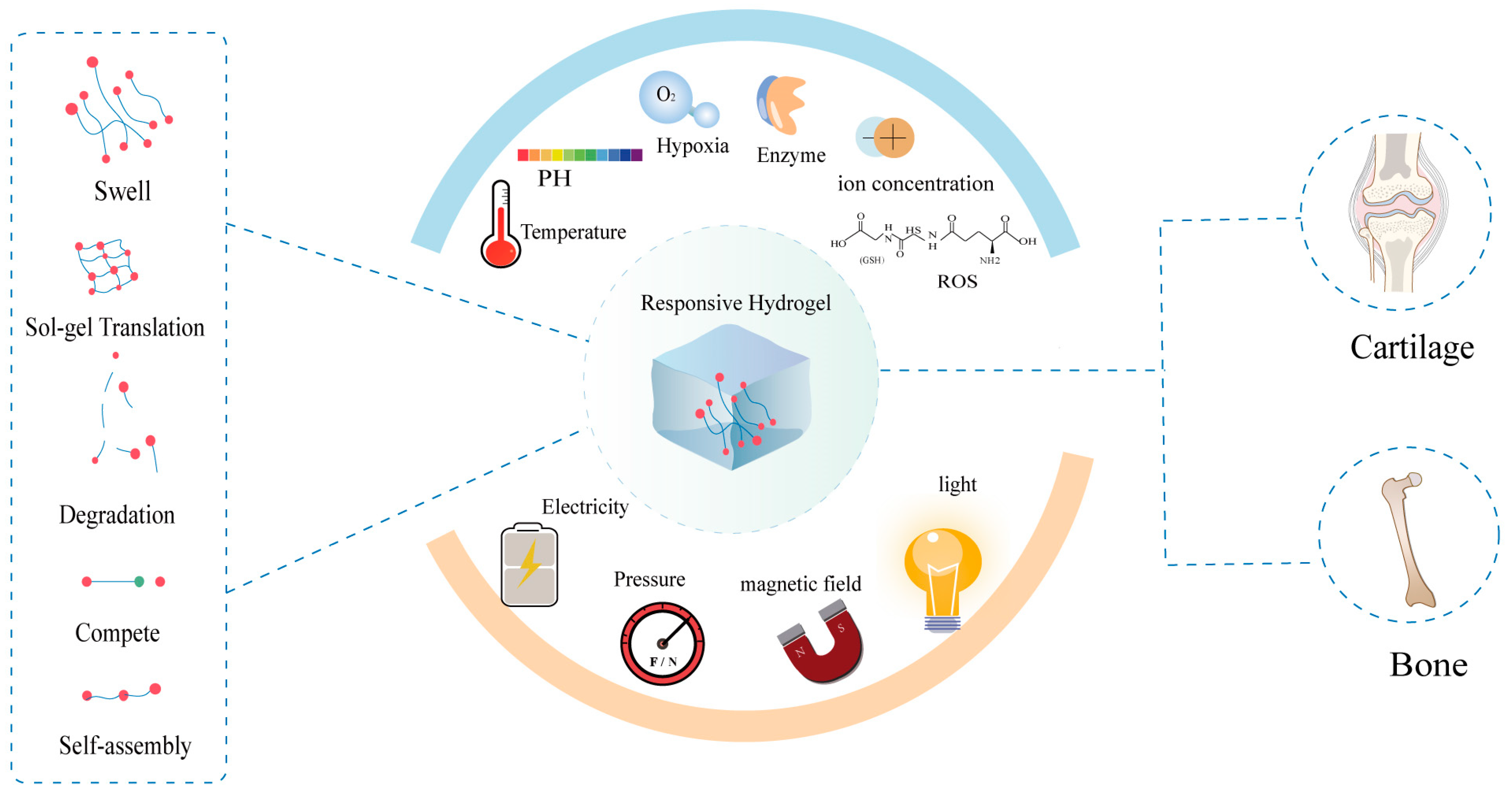
1. Introduction
2. Different Types of Stimuli-Responsive Hydrogel
2.1. Enzyme-Responsive Hydrogels
2.2. pH-Responsive Hydrogels
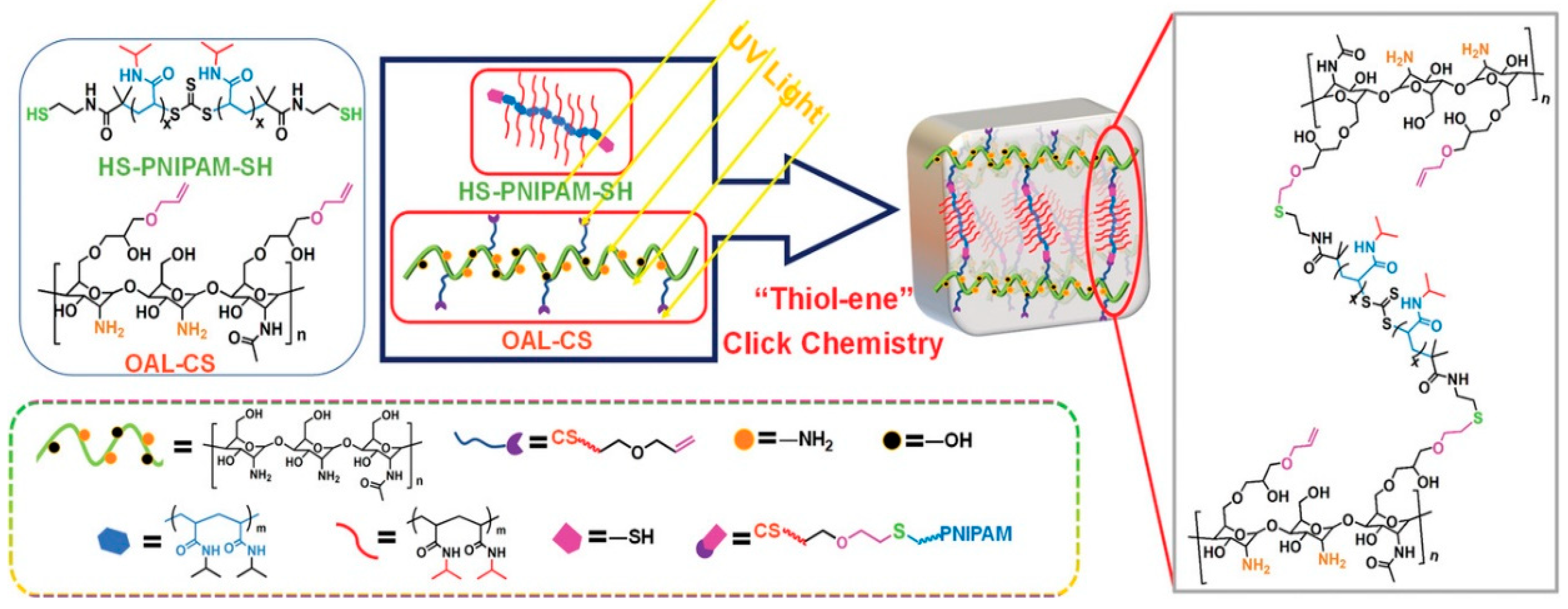
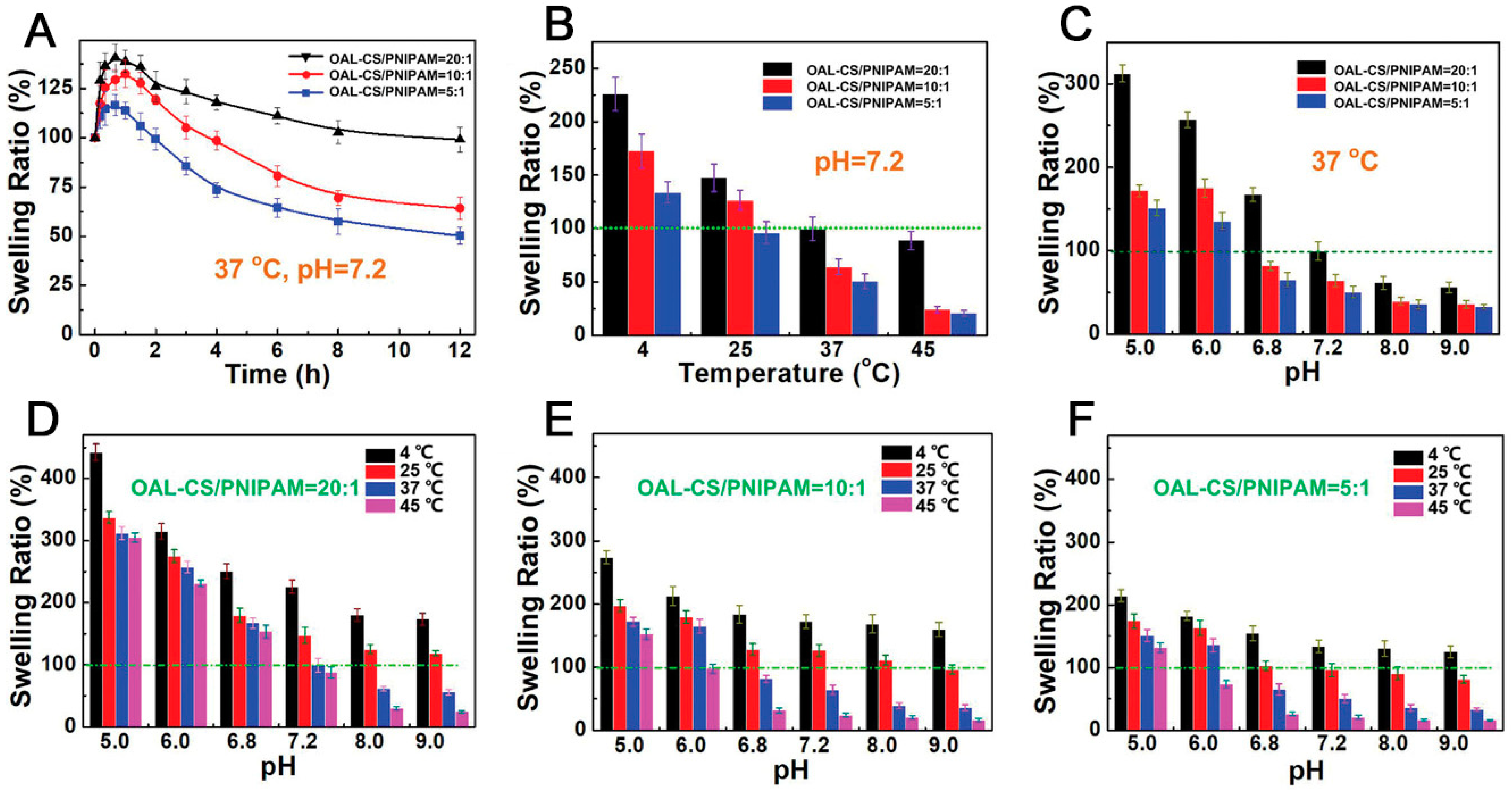
2.3. Temperature-Responsive Hydrogels
2.4. ROS-Responsive Hydrogels
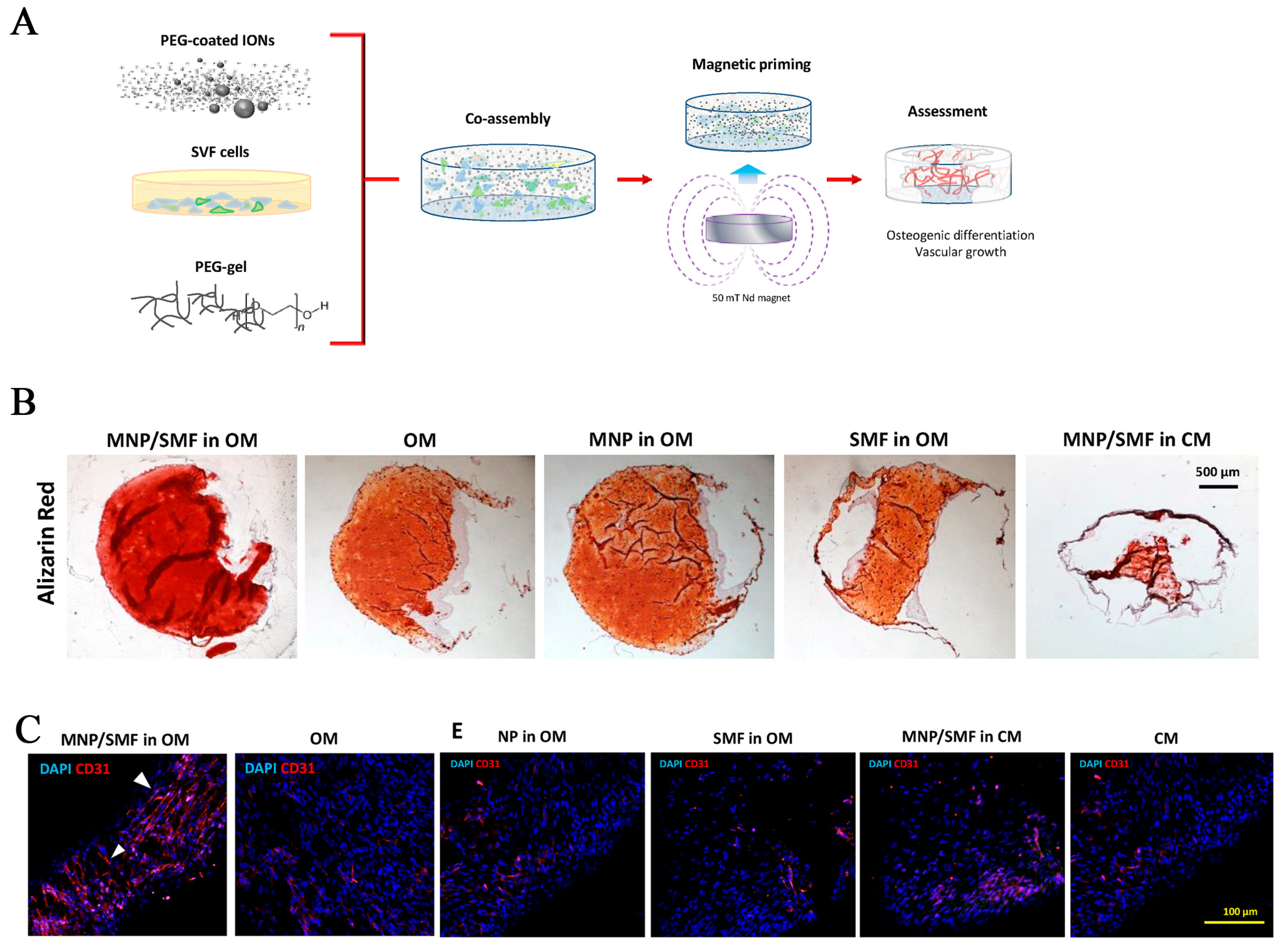
2.5. Magnetic-Responsive Hydrogels
2.6. Photo-Responsive Hydrogels
2.7. Electro-Responsive Hydrogels
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Bonzani, I.C.; George, J.H.; Stevens, M.M. Novel materials for bone and cartilage regeneration. Curr. Opin. Chem. Biol. 2006, 10, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Erggelet, C.; Vavken, P. Microfracture for the treatment of cartilage defects in the knee joint—A golden standard? J. Clin. Orthop. Trauma 2016, 7, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Leite Pereira, C.; Lamghari, M.; Sarmento, B. Advances in nanoenabled 3D matrices for cartilage repair. Acta Biomater. 2022, 150, 1–21. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, R.J.; Mao, J. Bone tissue engineering and regeneration: From discovery to the clinic—An overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite Hydrogels for Bone Regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1329–1343. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Y.; Min, Y.; Sun, J.; Shi, X.; Gao, G.; Sun, L.; Wang, J. Progress and prospect of technical and regulatory challenges on tissue-engineered cartilage as therapeutic combination product. Bioact. Mater. 2023, 20, 501–518. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Luby, A.O.; Ranganathan, K.; Lynn, J.V.; Nelson, N.S.; Donneys, A.; Buchman, S.R. Stem Cells for Bone Regeneration: Current State and Future Directions. J. Craniofac. Surg. 2019, 30, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, G.E.; Carrion, B.; Coleman, R.M.; Ostrowski, A.D. Photoresponsive Polysaccharide-Based Hydrogels with Tunable Mechanical Properties for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 14423–14429. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.A.; Nair, L.S. Injectable hydrogels for bone and cartilage repair. Biomed. Mater. 2012, 7, 024105. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated Hydrogels for Tissue Engineering and Regenerative Medicine. Bioconjug. Chem. 2015, 26, 1984–2001. [Google Scholar] [CrossRef] [PubMed]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Shi, R.; Huang, Y.; Ma, C.; Wu, C.; Tian, W. Current advances for bone regeneration based on tissue engineering strategies. Front. Med. 2019, 13, 160–188. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yu, B.; Pei, X.; Zhou, F. Structural hydrogels. Polymer 2016, 98, 516–535. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Coseri, S. An injectable and self-healing cellulose nanofiber-reinforced alginate hydrogel for bone repair. Carbohydr. Polym. 2023, 300, 120243. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Guan, J.; Mao, Y.; Zhou, P. Hydrogel: A potential therapeutic material for bone tissue engineering. AIP Adv. 2021, 11, 010701. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Phan, V.H.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110862. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.R.; Dolatabadi, R.; Baghani, M. Transient swelling response of pH-sensitive hydrogels: A monophasic constitutive model and numerical implementation. Int. J. Pharm. 2020, 577, 119030. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 758–780. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e1901714. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural Polymer-based Stimuli-responsive Hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef]
- Rizzo, F.; Kehr, N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv. Healthc. Mater. 2021, 10, e2001341. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, H.; Buksh, K.; Noreen, S.; Pervaiz, F.; Maqbool, I. Hydrogels as potential drug-delivery systems: Network design and applications. Ther. Deliv. 2021, 12, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 589–605. [Google Scholar] [CrossRef]
- He, D.; Zhao, A.S.; Su, H.; Zhang, Y.; Wang, Y.N.; Luo, D.; Gao, Y.; Li, J.A.; Yang, P. An injectable scaffold based on temperature-responsive hydrogel and factor-loaded nanoparticles for application in vascularization in tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, e2100062. [Google Scholar] [CrossRef]
- Ding, C.; Tian, M.; Feng, R.; Dang, Y.; Zhang, M. Novel Self-Healing Hydrogel with Injectable, pH-Responsive, Strain-Sensitive, Promoting Wound-Healing, and Hemostatic Properties Based on Collagen and Chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Le, T.M.D.; Duong, H.T.T.; Thambi, T.; Giang Phan, V.H.; Jeong, J.H.; Lee, D.S. Bioinspired pH- and Temperature-Responsive Injectable Adhesive Hydrogels with Polyplexes Promotes Skin Wound Healing. Biomacromolecules 2018, 19, 3536–3548. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, J.; Hu, Y.; Gao, F.; Pak-Heng Leung, G.; Geng, F.; Fu, C.; Zhang, J. Injectable thermo-responsive nano-hydrogel loading triptolide for the anti-breast cancer enhancement via localized treatment based on “two strikes” effects. Acta Pharm. Sin. B 2020, 10, 2227–2245. [Google Scholar] [CrossRef]
- Liang, J.; Yang, B.; Zhou, X.; Han, Q.; Zou, J.; Cheng, L. Stimuli-responsive drug delivery systems for head and neck cancer therapy. Drug Deliv. 2021, 28, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Koch, A.E. Angiogenesis and its targeting in rheumatoid arthritis. Vascul. Pharmacol. 2009, 51, 1–7. [Google Scholar] [CrossRef]
- Itoh, Y. Metalloproteinases in Rheumatoid Arthritis: Potential Therapeutic Targets to Improve Current Therapies. Prog. Mol. Biol. Transl. Sci. 2017, 148, 327–338. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Raynauld, J.P.; Caron, J.; Mineau, F.; Abram, F.; Dorais, M.; Haraoui, B.; Choquette, D.; Martel-Pelletier, J. Decrease in serum level of matrix metalloproteinases is predictive of the disease-modifying effect of osteoarthritis drugs assessed by quantitative MRI in patients with knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Montes, A.H.; Valle-Garay, E.; Alvarez, V.; Pevida, M.; Garcia Perez, E.; Paz, J.; Meana, A.; Asensi, V. A functional polymorphism in MMP1 could influence osteomyelitis development. J. Bone Miner. Res. 2010, 25, 912–919. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Morse, A.; Mikulec, K.; Peacock, L.; Baldock, P.A.; Kostenuik, P.J.; Little, D.G. Matrix metalloproteinase-driven endochondral fracture union proceeds independently of osteoclast activity. J. Bone Miner. Res. 2013, 28, 1550–1560. [Google Scholar] [CrossRef]
- Allard-Chamard, H.; Haroun, S.; de Brum-Fernandes, A.J. Secreted phospholipase A2 type II is present in Paget’s disease of bone and modulates osteoclastogenesis, apoptosis and bone resorption of human osteoclasts independently of its catalytic activity in vitro. Prostaglandins Leukot. Essent. Fatty Acids 2014, 90, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Knapik, D.M.; Perera, P.; Nam, J.; Blazek, A.D.; Rath, B.; Leblebicioglu, B.; Das, H.; Wu, L.C.; Hewett, T.E.; Agarwal, S.K., Jr.; et al. Mechanosignaling in bone health, trauma and inflammation. Antioxid. Redox Signal 2014, 20, 970–985. [Google Scholar] [CrossRef] [PubMed]
- Staines, K.A.; Madi, K.; Mirczuk, S.M.; Parker, S.; Burleigh, A.; Poulet, B.; Hopkinson, M.; Bodey, A.J.; Fowkes, R.C.; Farquharson, C.; et al. Endochondral Growth Defect and Deployment of Transient Chondrocyte Behaviors Underlie Osteoarthritis Onset in a Natural Murine Model. Arthritis Rheumatol. 2016, 68, 880–891. [Google Scholar] [CrossRef]
- Montonen, M.; Li, T.F.; Lukinmaa, P.L.; Sakai, E.; Hukkanen, M.; Sukura, A.; Konttinen, Y.T. RANKL and cathepsin K in diffuse sclerosing osteomyelitis of the mandible. J. Oral Pathol. Med. 2006, 35, 620–625. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Nakki, A.; Rodriguez-Fontenla, C.; Gonzalez, A.; Harilainen, A.; Leino-Arjas, P.; Heliovaara, M.; Eriksson, J.G.; Tallroth, K.; Videman, T.; Kaprio, J.; et al. Association study of MMP8 gene in osteoarthritis. Connect. Tissue Res. 2016, 57, 44–52. [Google Scholar] [CrossRef]
- Kou, L.; Jiang, X.; Lin, X.; Huang, H.; Wang, J.; Yao, Q.; Chen, R. Matrix Metalloproteinase Inspired Therapeutic Strategies for Bone Diseases. Curr. Pharm. Biotechnol. 2021, 22, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Song, N.; Zhang, S.X.; Li, H.; Wang, B.; Yang, Y.W. Ca(2+), pH and thermo triple-responsive mechanized Zr-based MOFs for on-command drug release in bone diseases. J. Mater. Chem. B 2016, 4, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.F.; Chia, W.T.; Liu, H.Y.; Hsiao, C.W.; Hsiao, H.C.; Yang, C.M.; Sung, H.W. Inflammation-induced drug release by using a pH-responsive gas-generating hollow-microsphere system for the treatment of osteomyelitis. Adv. Healthc. Mater. 2014, 3, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gao, T.; Wang, Y.; Wang, Z.; Zhang, P.; Liu, J. Environmental pH-controlled loading and release of protein on mesoporous hydroxyapatite nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 158–165. [Google Scholar] [CrossRef]
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012, 8, 358–366. [Google Scholar] [CrossRef]
- Knowles, H.J. Hypoxia-Induced Fibroblast Growth Factor 11 Stimulates Osteoclast-Mediated Resorption of Bone. Calcif. Tissue Int. 2017, 100, 382–391. [Google Scholar] [CrossRef]
- Muz, B.; Khan, M.N.; Kiriakidis, S.; Paleolog, E.M. Hypoxia. The role of hypoxia and HIF-dependent signalling events in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, 201. [Google Scholar] [CrossRef]
- Wegner, A.M.; Haudenschild, D.R. NADPH oxidases in bone and cartilage homeostasis and disease: A promising therapeutic target. J. Orthop. Res. 2020, 38, 2104–2112. [Google Scholar] [CrossRef]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Z.; Song, C.; Kang, H.; Guo, Q.; Dong, Y.; Zhang, Y.; Peng, R.; Guan, H.; Li, F. Schisandrin B Inhibits Osteoclastogenesis and Protects Against Ovariectomy-Induced Bone Loss. Front. Pharmacol. 2020, 11, 1175. [Google Scholar] [CrossRef]
- Chang, S.; Wang, S.; Liu, Z.; Wang, X. Advances of Stimulus-Responsive Hydrogels for Bone Defects Repair in Tissue Engineering. Gels 2022, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Coletta, D.J.; Ibanez-Fonseca, A.; Missana, L.R.; Jammal, M.V.; Vitelli, E.J.; Aimone, M.; Zabalza, F.; Issa, J.P.M.; Alonso, M.; Rodriguez-Cabello, J.C.; et al. Bone Regeneration Mediated by a Bioactive and Biodegradable Extracellular Matrix-Like Hydrogel Based on Elastin-Like Recombinamers. Tissue Eng. Part A 2017, 23, 1361–1371. [Google Scholar] [CrossRef]
- Holloway, J.L.; Ma, H.; Rai, R.; Burdick, J.A. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J. Control. Release 2014, 191, 63–70. [Google Scholar] [CrossRef]
- Hsu, C.W.; Olabisi, R.M.; Olmsted-Davis, E.A.; Davis, A.R.; West, J.L. Cathepsin K-sensitive poly(ethylene glycol) hydrogels for degradation in response to bone resorption. J. Biomed. Mater. Res. 2011, 98, 53–62. [Google Scholar] [CrossRef]
- Aziz, A.H.; Wilmoth, R.L.; Ferguson, V.L.; Bryant, S.J. IDG-SW3 Osteocyte Differentiation and Bone Extracellular Matrix Deposition Are Enhanced in a 3D Matrix Metalloproteinase-Sensitive Hydrogel. ACS Appl. Bio Mater. 2020, 3, 1666–1680. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Bryant, S.J. A MMP7-sensitive photoclickable biomimetic hydrogel for MSC encapsulation towards engineering human cartilage. J. Biomed. Mater. Res. A 2018, 106, 2344–2355. [Google Scholar] [CrossRef]
- Zhao, C.; Qazvini, N.T.; Sadati, M.; Zeng, Z.; Huang, S.; De La Lastra, A.L.; Zhang, L.; Feng, Y.; Liu, W.; Huang, B.; et al. A pH-Triggered, Self-Assembled, and Bioprintable Hybrid Hydrogel Scaffold for Mesenchymal Stem Cell Based Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 8749–8762. [Google Scholar] [CrossRef]
- Saha, S.; Yang, X.B.; Wijayathunga, N.; Harris, S.; Feichtinger, G.A.; Davies, R.P.W.; Kirkham, J. A biomimetic self-assembling peptide promotes bone regeneration in vivo: A rat cranial defect study. Bone 2019, 127, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Rogina, A.; Ressler, A.; Matic, I.; Gallego Ferrer, G.; Marijanovic, I.; Ivankovic, M.; Ivankovic, H. Cellular hydrogels based on pH-responsive chitosan-hydroxyapatite system. Carbohydr. Polym. 2017, 166, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Tsai, M.J.; Brahmayya, M.; Chen, J.P. Bone Regeneration Using Adipose-Derived Stem Cells in Injectable Thermo-Gelling Hydrogel Scaffold Containing Platelet-Rich Plasma and Biphasic Calcium Phosphate. Int. J. Mol. Sci. 2018, 19, 2537. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/beta-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Segredo-Morales, E.; Garcia-Garcia, P.; Reyes, R.; Perez-Herrero, E.; Delgado, A.; Evora, C. Bone regeneration in osteoporosis by delivery BMP-2 and PRGF from tetronic-alginate composite thermogel. Int. J. Pharm. 2018, 543, 160–168. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, Q.; Shi, Y.; Li, W.; Fu, Y.; Jin, G. Sintered porous Ti6Al4V scaffolds incorporated with recombinant human bone morphogenetic protein-2 microspheres and thermosensitive hydrogels can enhance bone regeneration. RSC Adv. 2019, 9, 1541–1550. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Sun, J.; Legare, A.; McKee, M.D.; Buschmann, M.D. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage 2005, 13, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Chang, F.; Xu, W.; Ding, J. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 79–87. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.; Chen, J.; Chang, F.; Wang, J.; Ding, J.; Chen, X. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018, 73, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110195. [Google Scholar] [CrossRef] [PubMed]
- Naderi-Meshkin, H.; Andreas, K.; Matin, M.M.; Sittinger, M.; Bidkhori, H.R.; Ahmadiankia, N.; Bahrami, A.R.; Ringe, J. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol. Int. 2014, 38, 72–84. [Google Scholar] [CrossRef]
- Kim, H.K.; Shim, W.S.; Kim, S.E.; Lee, K.H.; Kang, E.; Kim, J.H.; Kim, K.; Kwon, I.C.; Lee, D.S. Injectable in situ-forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng. Part A 2009, 15, 923–933. [Google Scholar] [CrossRef]
- Ding, H.; Li, B.; Liu, Z.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. Decoupled pH- and Thermo-Responsive Injectable Chitosan/PNIPAM Hydrogel via Thiol-Ene Click Chemistry for Potential Applications in Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e2000454. [Google Scholar] [CrossRef]
- Gong, T.; Liu, T.; Zhang, L.; Ye, W.; Guo, X.; Wang, L.; Quan, L.; Pan, C. Design Redox-Sensitive Drug-Loaded Nanofibers for Bone Reconstruction. ACS Biomater. Sci. Eng. 2018, 4, 240–247. [Google Scholar] [CrossRef]
- Gong, T.; Hu, Q.; Nie, X.; Liu, T.; Wang, H. Periodic Dynamic Regulation of MSCs Differentiation on Redox-Sensitive Elastic Switched Substrates. ACS Appl. Bio Mater. 2020, 3, 3612–3620. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Hou, J.; Guo, H.; Liu, C. Bone regeneration using cell-mediated responsive degradable PEG-based scaffolds incorporating with rhBMP-2. Biomaterials 2013, 34, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, Y.; Jia, Z.; Chen, J.; Duan, L.; Liu, W.; Zhu, F.; Liang, Q.; Zhu, W.; You, W.; et al. Development of Magnetic Nanocomposite Hydrogel with Potential Cartilage Tissue Engineering. ACS Omega 2018, 3, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Zhang, G.; Du, G.; Zhan, D.; Cong, Y.; Cheng, Y.; Fu, J. Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf. B Biointerfaces 2013, 103, 318–325. [Google Scholar] [CrossRef]
- Yuan, Z.; Memarzadeh, K.; Stephen, A.S.; Allaker, R.P.; Brown, R.A.; Huang, J. Development of a 3D Collagen Model for the In Vitro Evaluation of Magnetic-assisted Osteogenesis. Sci. Rep. 2018, 8, 16270. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Dasen, B.; Guerrero, J.; Garello, F.; Isu, G.; Born, G.; Ehrbar, M.; Martin, I.; Scherberich, A. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials 2019, 223, 119468. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.A.; Lee, J.; Bharadwaj, N.A.; Ewoldt, R.H.; Kilian, K.A. Temporal Modulation of Stem Cell Activity Using Magnetoactive Hydrogels. Adv. Healthc. Mater. 2016, 5, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Henstock, J.R.; Rotherham, M.; Rashidi, H.; Shakesheff, K.M.; El Haj, A.J. Remotely Activated Mechanotransduction via Magnetic Nanoparticles Promotes Mineralization Synergistically with Bone Morphogenetic Protein 2: Applications for Injectable Cell Therapy. Stem Cells Transl. Med. 2014, 3, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lock, J.; Sallee, A.; Liu, H. Magnetic Nanocomposite Hydrogel for Potential Cartilage Tissue Engineering: Synthesis, Characterization, and Cytocompatibility with Bone Marrow Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 20987–20998. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yan, J.; Tan, H.; Miao, Y.; Hu, X. Magnetic biopolymer nanogels via biological assembly for vectoring delivery of biopharmaceuticals. J. Mater. Chem. B 2014, 2, 8399–8405. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Niu, X.; Lin, Q.; Zhao, B.; Wang, Y.; Zhu, L. An in situ photocrosslinkable platelet rich plasma—Complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017, 62, 179–187. [Google Scholar] [CrossRef]
- Li, N.; Xie, L.; Wu, Y.; Wu, Y.; Liu, Y.; Gao, Y.; Yang, J.; Zhang, X.; Jiang, L. Dexamethasone-loaded zeolitic imidazolate frameworks nanocomposite hydrogel with antibacterial and anti-inflammatory effects for periodontitis treatment. Mater. Today Bio 2022, 16, 100360. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, X.; Zhu, Y.; Han, Y.; Shen, J.; Bao, B.; Gao, T.; Lin, J.; Huang, T.; Xu, J.; et al. Tunable and Controlled Release of Cobalt Ions from Metal-Organic Framework Hydrogel Nanocomposites Enhances Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 59051–59066. [Google Scholar] [CrossRef]
- Mac Kenna, N.; Calvert, P.; Morrin, A.; Wallace, G.G.; Moulton, S.E. Electro-stimulated release from a reduced graphene oxide composite hydrogel. J. Mater. Chem. B 2015, 3, 2530–2537. [Google Scholar] [CrossRef]
- Rahimi, N.; Molin, D.G.; Cleij, T.J.; van Zandvoort, M.A.; Post, M.J. Electrosensitive polyacrylic acid/fibrin hydrogel facilitates cell seeding and alignment. Biomacromolecules 2012, 13, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, G.; Liu, S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012, 41, 5933–5949. [Google Scholar] [CrossRef]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Bushetti, S.S.; Appala, R.; Shareef, A.; Imam, S.S.; Singh, M. Stimuli-sensitive hydrogels: A novel ophthalmic drug delivery system. Indian J. Ophthalmol. 2010, 58, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Aggeli, A.; Ingham, E.; McPherson, M.J. Recombinant self-assembling peptides as biomaterials for tissue engineering. Biomaterials 2010, 31, 9395–9405. [Google Scholar] [CrossRef]
- Mu, M.; Li, X.; Tong, A.; Guo, G. Multi-functional chitosan-based smart hydrogels mediated biomedical application. Expert Opin. Drug Deliv. 2019, 16, 239–250. [Google Scholar] [CrossRef]
- De Souza, R.; Zahedi, P.; Allen, C.J.; Piquette-Miller, M. Biocompatibility of injectable chitosan-phospholipid implant systems. Biomaterials 2009, 30, 3818–3824. [Google Scholar] [CrossRef]
- Abrami, M.; Siviello, C.; Grassi, G.; Larobina, D.; Grassi, M. Investigation on the thermal gelation of Chitosan/beta-Glycerophosphate solutions. Carbohydr. Polym. 2019, 214, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; You, M.L.; Ding, Z.Q.; Ye, W.B. A review of emerging bone tissue engineering via PEG conjugated biodegradable amphiphilic copolymers. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 1021–1035. [Google Scholar] [CrossRef]
- Yeon, B.; Park, M.H.; Moon, H.J.; Kim, S.J.; Cheon, Y.W.; Jeong, B. 3D culture of adipose-tissue-derived stem cells mainly leads to chondrogenesis in poly(ethylene glycol)-poly(L-alanine) diblock copolymer thermogel. Biomacromolecules 2013, 14, 3256–3266. [Google Scholar] [CrossRef]
- He, C.; Zhuang, X.; Tang, Z.; Tian, H.; Chen, X. Stimuli-sensitive synthetic polypeptide-based materials for drug and gene delivery. Adv. Healthc. Mater. 2012, 1, 48–78. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, B.; Behl, G. A Comprehensive Outlook of Synthetic Strategies and Applications of Redox-Responsive Nanogels in Drug Delivery. Macromol. Biosci. 2019, 19, e1900071. [Google Scholar] [CrossRef] [PubMed]
- Lakes, A.L.; Jordan, C.T.; Gupta, P.; Puleo, D.A.; Hilt, J.Z.; Dziubla, T.D. Reducible disulfide poly(beta-amino ester) hydrogels for antioxidant delivery. Acta Biomater. 2018, 68, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Badeau, B.A.; DeForest, C.A. Programming Stimuli-Responsive Behavior into Biomaterials. Annu. Rev. Biomed. Eng. 2019, 21, 241–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic Hydrogels and Their Potential Biomedical Applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Shi, W.; Huang, J.; Fang, R.; Liu, M. Imparting Functionality to the Hydrogel by Magnetic-Field-Induced Nano-assembly and Macro-response. ACS Appl. Mater. Interfaces 2020, 12, 5177–5194. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, P.; Shi, X.; Ling, T.; Zeng, W.; Chen, A.; Yang, W.; Zhou, Z. Hierarchically Porous Hydroxyapatite Hybrid Scaffold Incorporated with Reduced Graphene Oxide for Rapid Bone Ingrowth and Repair. ACS Nano 2019, 13, 9595–9606. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.M.; Boyd, N.L. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Steppe, L.; Liedert, A.; Ignatius, A.; Haffner-Luntzer, M. Influence of Low-Magnitude High-Frequency Vibration on Bone Cells and Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 595139. [Google Scholar] [CrossRef]
- Cartmell, S.H.; Dobson, J.; Verschueren, S.B.; El Haj, A.J. Development of magnetic particle techniques for long-term culture of bone cells with intermittent mechanical activation. IEEE Trans. Nanobiosci. 2002, 1, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cui, Z.K.; Fan, J.; Fartash, A.; Aghaloo, T.L.; Lee, M. Photocrosslinkable chitosan hydrogels functionalized with the RGD peptide and phosphoserine to enhance osteogenesis. J. Mater. Chem. B 2016, 4, 5289–5298. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; McBain, S.; Dobson, J.; El Haj, A.J. Selective activation of mechanosensitive ion channels using magnetic particles. J. R. Soc. Interface 2007, 5, 855–863. [Google Scholar] [CrossRef]
- Henstock, J.R.; Rotherham, M.; El Haj, A.J. Magnetic ion channel activation of TREK1 in human mesenchymal stem cells using nanoparticles promotes osteogenesis in surrounding cells. J. Tissue Eng. 2018, 9, 2041731418808695. [Google Scholar] [CrossRef]
- Augustyniak, E.; Trzeciak, T.; Richter, M.; Kaczmarczyk, J.; Suchorska, W. The role of growth factors in stem cell-directed chondrogenesis: A real hope for damaged cartilage regeneration. Int. Orthop. 2015, 39, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.; Appel, I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016, 39, 89–96. [Google Scholar] [CrossRef]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; Dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. Stimulus-responsive metal-organic frameworks. Chem. Asian J. 2014, 9, 2358–2376. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Han, I.; Lee, S.; Kim, Y.; Lee, D.N. Novel Metal-Organic Framework-Based Photocrosslinked Hydrogel System for Efficient Antibacterial Applications. ACS Appl. Mater. Interfaces 2020, 12, 20234–20242. [Google Scholar] [CrossRef]
- Ejeian, F.; Razmjou, A.; Nasr-Esfahani, M.H.; Mohammad, M.; Karamali, F.; Ebrahimi Warkiani, M.; Asadnia, M.; Chen, V. ZIF-8 Modified Polypropylene Membrane: A Biomimetic Cell Culture Platform with a View to the Improvement of Guided Bone Regeneration. Int. J. Nanomed. 2020, 15, 10029–10043. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, J.; Ma, Z.; Feng, H.; Chen, S.; Cai, H.; Xue, Y.; Pei, X.; Wang, J.; Wan, Q. 3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 2020, 12, 24437–24449. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, e1807333. [Google Scholar] [CrossRef]
- Agarwala, S. Electrically Conducting Hydrogels for Health care: Concept, Fabrication Methods, and Applications. Int. J. Bioprint. 2020, 6, 273. [Google Scholar] [CrossRef]
- Cirillo, G.; Spizzirri, U.G.; Curcio, M.; Hampel, S.; Vittorio, O.; Restuccia, D.; Picci, N.; Iemma, F. Carbon Nanohybrids as Electro-Responsive Drug Delivery Systems. Mini-Rev. Med. Chem. 2016, 16, 658–667. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Curcio, M.; Cirillo, G.; Spataro, T.; Vittorio, O.; Picci, N.; Hampel, S.; Iemma, F.; Nicoletta, F.P. Recent Advances in the Synthesis and Biomedical Applications of Nanocomposite Hydrogels. Pharmaceutics 2015, 7, 413–437. [Google Scholar] [CrossRef]
- Shang, J.; Shao, Z.; Chen, X. Electrical behavior of a natural polyelectrolyte hydrogel: Chitosan/carboxymethylcellulose hydrogel. Biomacromolecules 2008, 9, 1208–1213. [Google Scholar] [CrossRef]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Jiang, H.; Fan, L.; Yan, S.; Li, F.; Li, H.; Tang, J. Tough and electro-responsive hydrogel actuators with bidirectional bending behavior. Nanoscale 2019, 11, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Jalilinejad, N.; Rabiee, M.; Baheiraei, N.; Ghahremanzadeh, R.; Salarian, R.; Rabiee, N.; Akhavan, O.; Zarrintaj, P.; Hejna, A.; Saeb, M.R.; et al. Electrically conductive carbon-based (bio)-nanomaterials for cardiac tissue engineering. Bioeng. Transl. Med. 2023, 8, e10347. [Google Scholar] [CrossRef]
- Volpi, M.; Paradiso, A.; Costantini, M.; Swieszkowski, W. Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Chen, C.; Xu, X.; Annabi, N.; Zadeh, H.H.; Wu, B.M.; Khademhosseini, A.; Shi, S.; Moshaverinia, A. Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors. Ann. Biomed. Eng. 2016, 44, 1908–1920. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A.; Rahighi, R. Rolled graphene oxide foams as three-dimensional scaffolds for growth of neural fibers using electrical stimulation of stem cells. Carbon 2016, 97, 71–77. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Mogadam, E.; Yu, C.H.; Kimball, W.; Annabi, N. Rational Design of Microfabricated Electroconductive Hydrogels for Biomedical Applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shahsavar, M. Graphene nanogrids for selective and fast osteogenic differentiation of human mesenchymal stem cells. Carbon 2013, 59, 200–211. [Google Scholar] [CrossRef]
- Rueda-Gensini, L.; Serna, J.A.; Cifuentes, J.; Cruz, J.C.; Munoz-Camargo, C. Graphene Oxide-Embedded Extracellular Matrix-Derived Hydrogel as a Multiresponsive Platform for 3D Bioprinting Applications. Int. J. Bioprint. 2021, 7, 353. [Google Scholar] [CrossRef]
- Raslan, A.; Saenz Del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zou, L.; Jiang, L.; Zhao, Z.; Liu, J. Osteoinductive and antimicrobial mechanisms of graphene-based materials for enhancing bone tissue engineering. J. Tissue Eng. Regen. Med. 2021, 15, 915–935. [Google Scholar] [CrossRef]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Z. Biomedical applications of chitosan-graphene oxide nanocomposites. iScience 2022, 25, 103629. [Google Scholar] [CrossRef]
- Zhihui, K.; Min, D. Application of Graphene Oxide-Based Hydrogels in Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Jain, K.G.; Gupta, D.; Raghav, P.K.; Chaudhuri, R.; Pinky; Shakeel, A.; Arora, V.; Sharma, H.; Debnath, D.; et al. Graphene nanofiber composites for enhanced neuronal differentiation of human mesenchymal stem cells. Nanomedicine 2021, 16, 1963–1982. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-W.; Hu, S.-H.; Chen, Y.-W.; Chen, S.-Y. Characterization and drug release behavior of highly responsive chip-like electrically modulated reduced graphene oxide–poly(vinyl alcohol) membranes. J. Mater. Chem. 2012, 22, 17311–17320. [Google Scholar] [CrossRef]
- Wang, Y. Programmable hydrogels. Biomaterials 2018, 178, 663–680. [Google Scholar] [CrossRef]
- Short, A.R.; Koralla, D.; Deshmukh, A.; Wissel, B.; Stocker, B.; Calhoun, M.; Dean, D.; Winter, J.O. Hydrogels That Allow and Facilitate Bone Repair, Remodeling, and Regeneration. J. Mater. Chem. B 2015, 3, 7818–7830. [Google Scholar] [CrossRef] [PubMed]



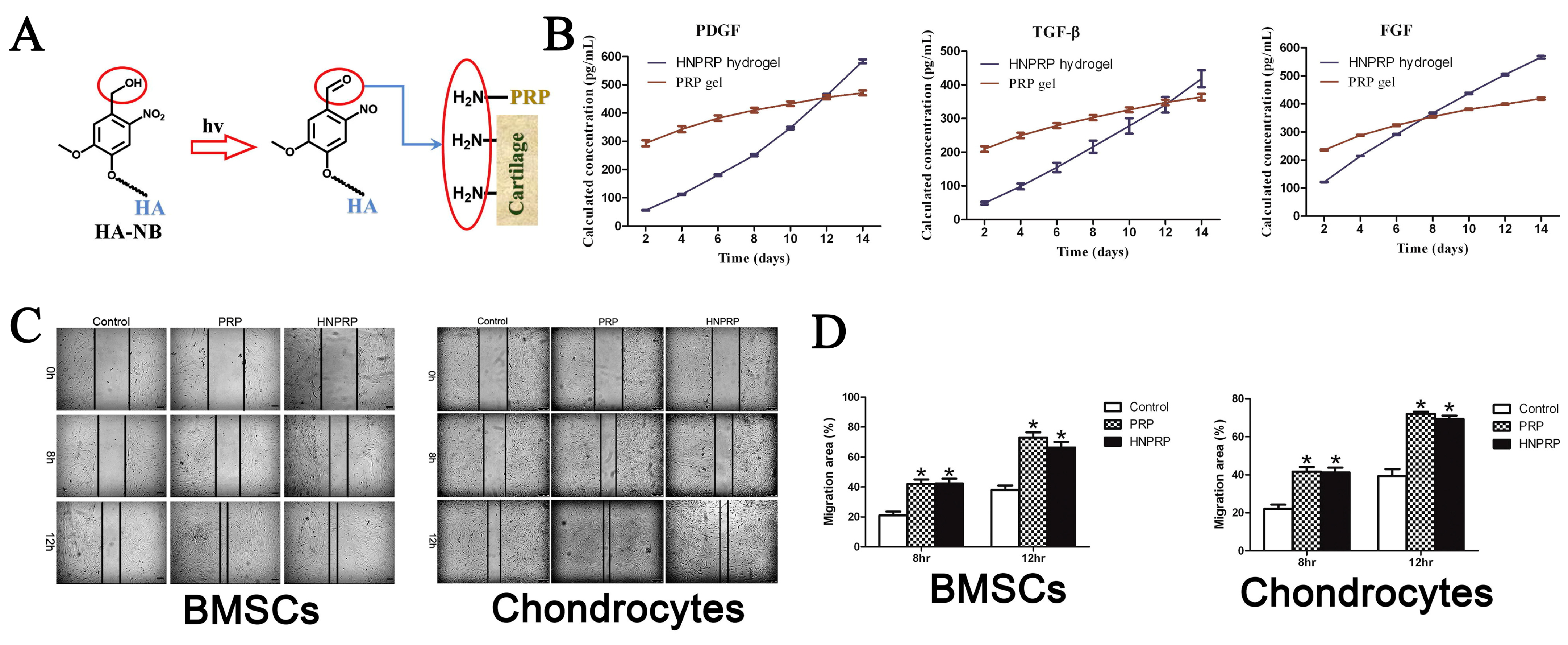

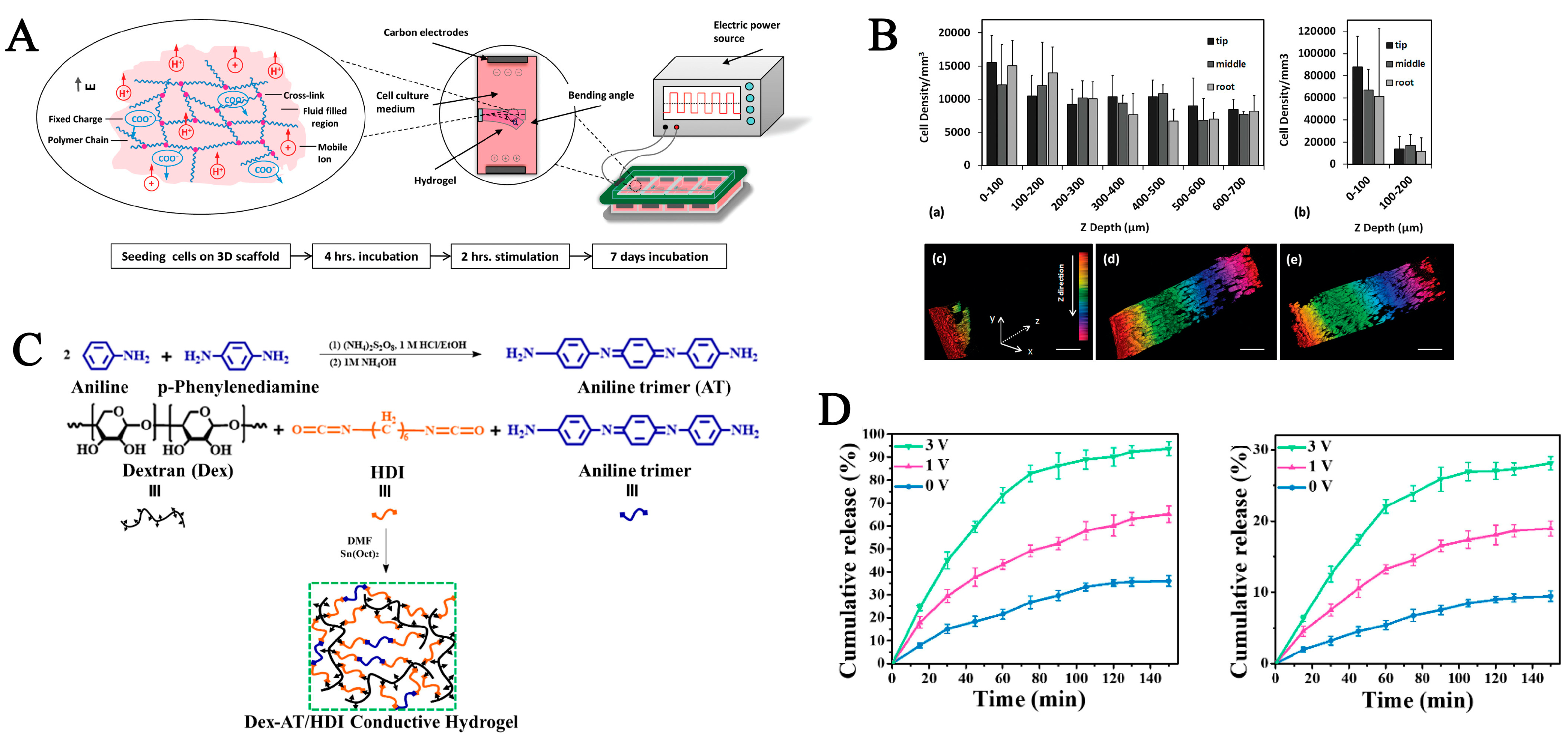
| Stimuli | Components | Growth Factor/ Cell/Drug | Synthesis Methods | Gelation Time | Application | Ref. |
|---|---|---|---|---|---|---|
| Enzyme | Elastin-like recombinamers elastase-sensitive domain | BMP-2, RGD | Crosslinking | / | Bone regeneration | [65] |
| MMP7-sensitive peptide, maleimide-modified hyaluronic acid, RGD | SDF-1α and BMP-2 | Crosslinking | 5 min | Bone regeneration | [66] | |
| Poly(ethyleneglycol) diacrylate (PEGDA), cathepsin-K-sensitive peptide GGGMGPSGWGGK (GPSG) | / | Crosslinking | 1 h | Selective degradation | [67] | |
| Polyethylene glycol | ID-SW3 | Crosslinking | 10 min | Cell differentiation | [68] | |
| PEG norbornene, thiolated chondroitin sulfates, GRGDS, MMP7-sensitive peptide | hMSCs | Crosslinking | 8 min | Cartilage regeneration | [69] | |
| pH | Carboxymethyl chitosan, amorphous calcium phosphate | BMP-9 | Self-assembly | 30 min | Bone regeneration | [70] |
| (SAP)P11-4(CH3COQQRF EWEFEQQQNH2) | HDPSCs | Self-assembly | / | Bone regeneration | [71] | |
| Chitosan, hydroxyapatite | Fibroblasts | Crosslinking | 4 min | Cell growth | [72] | |
| Temperature | Hyaluronic acid-g-chitosan-g-poly (N-isopropylacrylamide-g-poly) | rASCs | Crosslinking | / | Osteoblastic differentiation, ECM mineralization | [73] |
| Zn, chitosan, β-glycerophosphate | MSCs | Crosslinking | 5 min | Osteoblast differentiation of MSCs | [74] | |
| PoloxamineT-1307, alginate, calcium chloride | 17β-estradiol, BMP-2, PRGF | Crosslinking | 15 min | Bone regeneration | [75] | |
| Ti6AI4V, chitosan thioglycolic acid | BMP-2 | Crosslinking | 2.62 ± 0.87 min | Bone regeneration | [76] | |
| Poly(ethylene glycol) -b-poly(L-alanine) | / | Crosslinking | 10 min | Chondrogenic differentiation of ADSCs | [77] | |
| Poly(lactide-co-glycolide)-block-poly(ethylene glycol) -block-poly(lactide-co-glycolide) | BMSCs | Polymerization | / | Chondrogenic differentiation of BMSCs and cartilage repair | [78] | |
| L-Phenylalanine,poly(L-alanine-co-L-phenylalanine)-block-poly(ethylene glycol) -block-poly(L-alanine-co-L-phenylalanine) | BMSCs | Crosslinking | / | Cartilage repair | [79] | |
| β-tricalcium phosphate, hyaluronic acid corn silk extract-nanosilver | MSCs | Osteogenic differentiation of MSCs | [80] | |||
| β-glycerophosphate, chitosan, hydroxyethyl cellulose | Primary articular chondrocytes | Crosslinking | Few minutes | Cartilage regeneration | [77] | |
| β-glycerophosphate, chitosan, Hydroxyethyl cellulose | TGF-β3, hMSCs | Crosslinking | 20 min | Chondrogenic differentiation of hMSCs | [81] | |
| Sulfamethazine oligomer, Poly(e-caprolactone-co-lactide)-Poly(e-caprolactone-co-lactide)-poly | hMSCs, BMP-2 | Crosslinking | / | Bone regeneration | [82] | |
| C6-OH allyl-modified chitosan, Poly(N-isopropyl acrylamide) | / | Crosslinking | 60 s | Drug delivery | [83] | |
| ROS | Poly-LRB-ethylene oxiPeo, Poly (ethyl lactone), redox-responsive c-6A PEG-PCL | BMP-2 | Crosslinking, electrospinning | / | Controlled release, bone regeneration | [84] |
| A mixture of six-arm poly (ethylene glycol)-poly (ε-caprolactone)-3,3′-dithiodipropionic acid gels, six-arm poly(ethylene glycol)-poly(ε-caprolactone)-acryloyl | / | Crosslinking | / | Bone regeneration | [85] | |
| Polyethylene glycol | rhBMP-2 | Crosslinking | / | Controlled release, bone regeneration | [86] | |
| Magnetic field | Polyvinyl alcohol, nano-hydroxyapatite, magnetic nanoparticles (Fe2O3) | BMSCs | Crosslinking, ultrasonic dispersion. | / | Cell growth, chondrogenic differentiation | [87] |
| Nano-hydroxyapatite, poly(vinyl alcohol) | Osteoblasts | Freeze–thawing | / | Cell adhesion and proliferation | [88] | |
| Collagen, iron oxide nanoparticles | MG-63 | Crosslinking, co-assembly | / | Cell proliferation, bone formation | [89] | |
| Polyethylene glycol | SVF cell | Crosslinking | / | Osteogenesis, vascularization | [90] | |
| Polyacrylamide, carbonyl iron | MSCs | Crosslinking, co-assembly | / | Osteogenesis, vascularization | [91] | |
| Collagen, RGD or TREK1K+ | BMP-2, nanoparticle-labeled hMSCs | / | / | Bone formation | [92] | |
| Collagen type II, hyaluronic acid, polyethylene glycol | BMSCs | Crosslinking | / | Cell adhesion, magnetic guidance | [93] | |
| Chitosan, Heparin | BMP-2 | Watson–Crick pairing, co-assembly | / | Cell viability, delivery of growth factors | [94] | |
| Light | Alginate-acrylamide hybrid gels (AlgAam), ferric iron | ATDCs, BMSCs | Crosslinking | / | Cartilage formation | [13] |
| Hyaluronic acid | PRP | Crosslinking | / | Proliferation and migration of BMSCs and chondrocytes | [95] | |
| Zeolitic imidazolate frameworks-8, methacrylic, polyphosphoester (PPEMA), methacrylic gelatin (GelMA) | Dexamethasone | Crosslinking | 20 s | Drug delivery | [96] | |
| 2-ethylimidazole (eIm), zeolitic imidazolate framework-67 (ZIF-67), gelatin methacrylate (GelMA) | Co-icons | Crosslinking | / | Drug delivery, vascularization, bone formation | [97] | |
| Electrictiy | Jeffamine polyetheramine, polyethylene glycol diglycidyl ether (PEGDGE), rGO | Methyl orange | Crosslinking, co-assembly | / | Drug delivery | [98] |
| Fibrin, acrylic acid | pSMC | Free-radical polymerization and crosslinking | / | Cell migration | [99] | |
| Dextran, aniline trimer, hexamethylene diisocyanate | / | Crosslinking | Drug delivery | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Xing, X.; Deng, Y.; Li, Z. Applications of Stimuli-Responsive Hydrogels in Bone and Cartilage Regeneration. Pharmaceutics 2023, 15, 982. https://doi.org/10.3390/pharmaceutics15030982
Ni X, Xing X, Deng Y, Li Z. Applications of Stimuli-Responsive Hydrogels in Bone and Cartilage Regeneration. Pharmaceutics. 2023; 15(3):982. https://doi.org/10.3390/pharmaceutics15030982
Chicago/Turabian StyleNi, Xiaoqi, Xin Xing, Yunfan Deng, and Zhi Li. 2023. "Applications of Stimuli-Responsive Hydrogels in Bone and Cartilage Regeneration" Pharmaceutics 15, no. 3: 982. https://doi.org/10.3390/pharmaceutics15030982
APA StyleNi, X., Xing, X., Deng, Y., & Li, Z. (2023). Applications of Stimuli-Responsive Hydrogels in Bone and Cartilage Regeneration. Pharmaceutics, 15(3), 982. https://doi.org/10.3390/pharmaceutics15030982






