Blautia coccoides JCM1395T Achieved Intratumoral Growth with Minimal Inflammation: Evidence for Live Bacterial Therapeutic Potential by an Optimized Sample Preparation and Colony PCR Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Experimental Animals
2.3. Bacterial Isolation from Tissues
2.4. Colony PCR
2.5. Histological Analysis
2.6. Blood Biochemical Analyses
2.7. Statistical Analyses
3. Results
3.1. Optimization of Sample Preparation for Colony PCR of 16S rRNA Gene
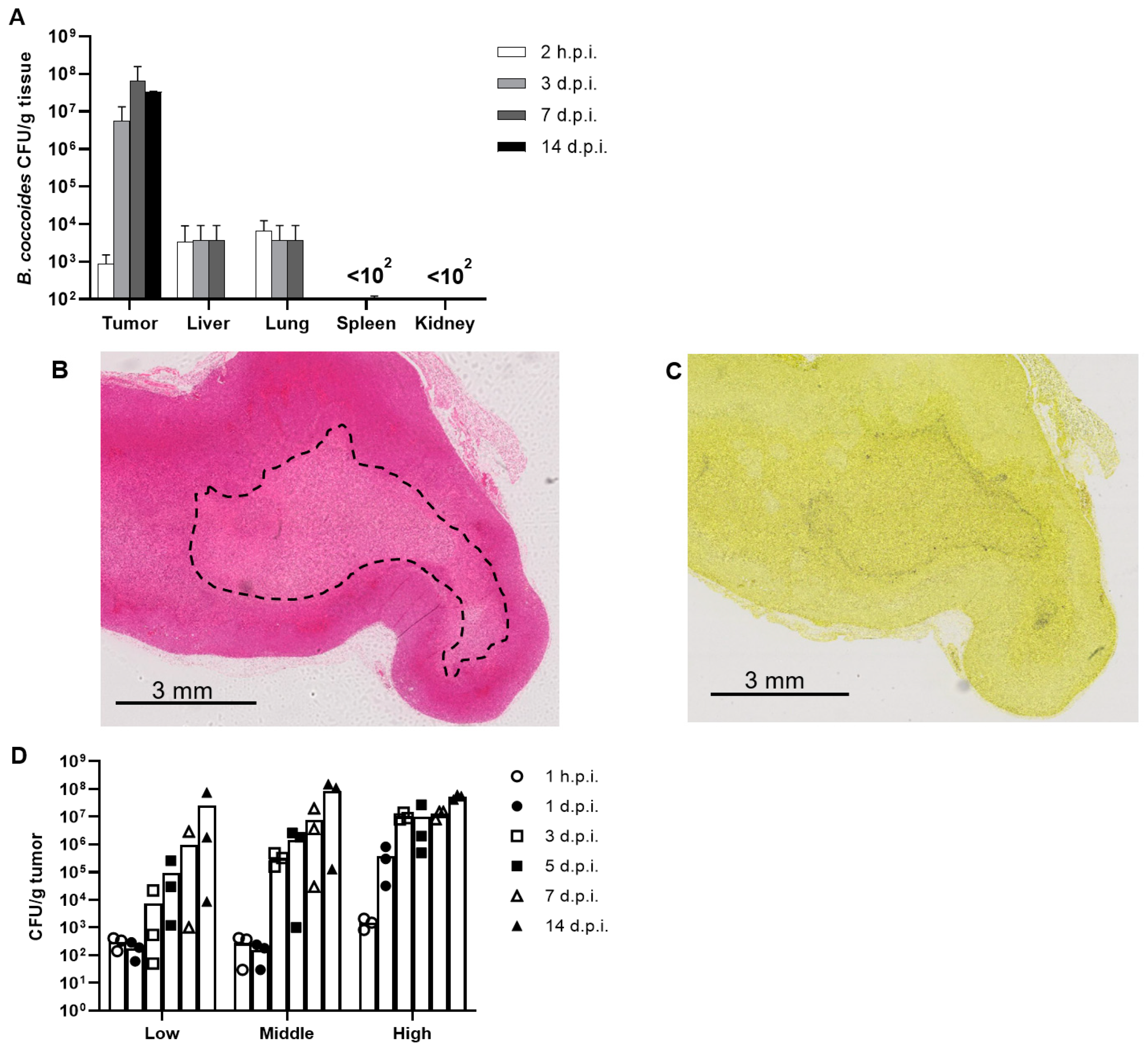
3.2. Identification of Bacteria in Tumors
3.3. Biodistribution of B. coccoides JCM1395T after Intravenous Administration
3.4. Dose Dependency of the Proliferation of B. coccoides JCM1395T in Tumors
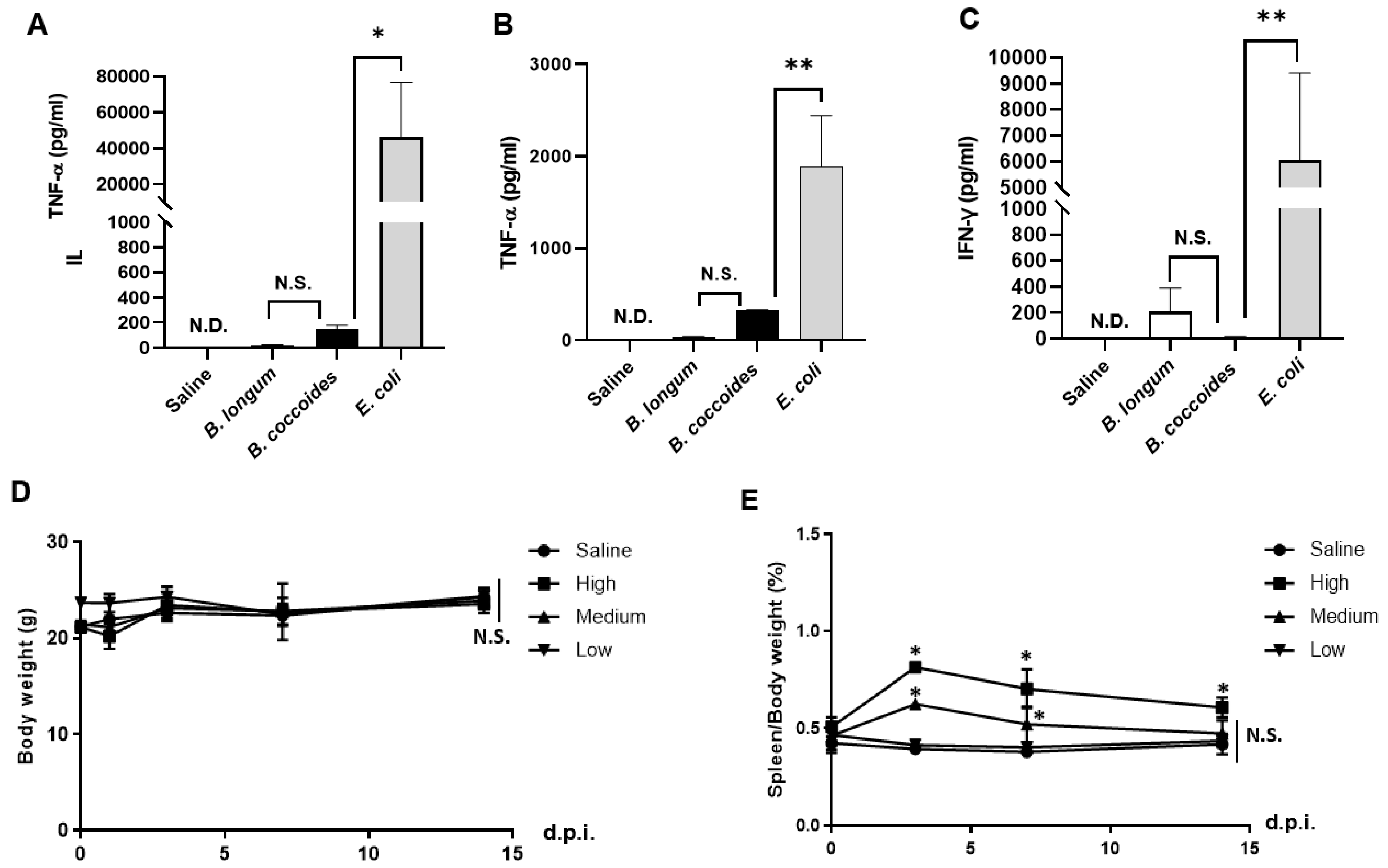
3.5. Adverse Effects after Intravenous Administration of B. coccoides JCM1395T
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riglar, D.T.; Silver, P.S. Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Microbiol. 2018, 16, 214–225. [Google Scholar] [CrossRef]
- Vandenbroucke, K.; Hans, W.; Huysse, J.; Neirynck, S.; Demetter, P.; Remaut, E.; Rottiers, P.; Steidler, L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology 2004, 127, 502–513. [Google Scholar] [CrossRef]
- Forbes, N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 2010, 10, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003, 4, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 2020, 20, 142–152. [Google Scholar] [CrossRef]
- Heimann, D.M.; Rosenberg, S.A. Continuous Intravenous Administration of Live Genetically Modified Salmonella Typhimurium in Patients With Metastatic Melanoma. J. Immunother. 2003, 26, 179–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.-H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.-J.; Hong, Y.; Bom, H.-S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9, eaak9537. [Google Scholar] [CrossRef]
- Ganai, S.; Arenas, R.B.; Forbes, N.S. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br. J. Cancer 2009, 101, 1683–1691. [Google Scholar] [CrossRef]
- Jiang, S.N.; Phan, T.X.; Nam, T.-K.; Nguyen, V.H.; Kim, H.-S.; Bom, H.-S.; E Choy, H.; Hong, Y.; Min, J.-J. Inhibition of Tumor Growth and Metastasis by a Combination of Escherichia coli–mediated Cytolytic Therapy and Radiotherapy. Mol. Ther. 2020, 18, 635–642. [Google Scholar] [CrossRef]
- Anderson, J.C.; Clarke, E.J.; Arkin, A.P.; Voigt, C.A. Environmentally Controlled Invasion of Cancer Cells by Engineered Bacteria. J. Mol. Biol. 2006, 355, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Din, M.O.; Danino, T.; Prindle, A.; Skalak, M.; Selimkhanov, J.; Allen, K.; Julio, E.; Atolia, E.; Tsimring, L.S.; Bhatia, S.N.; et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 2016, 536, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clairmont, C.; Lee, K.C.; Pike, J.; Ittensohn, M.; Low, K.B.; Pawelek, J.; Bermudes, D.; Brecher, S.M.; Margitich, D.; Turnier, J.; et al. Biodistribution and Genetic Stability of the Novel Antitumor Agent VNP20009, a Genetically Modified Strain of Salmonella typhimurium. J. Infect. Dis. 2000, 181, 1996–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazawa, K.; Fujimori, M.; Nakamura, T.; Sasaki, T.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res. Treat. 2003, 66, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Fujimori, M.; Hamaji, Y.; Hama, Y.; Ito, K.-I.; Amano, J.; Taniguchi, S. Genetically engineered Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci. 2006, 97, 649–657. [Google Scholar] [CrossRef]
- Taniguchi, S.; Fujimori, M.; Sasaki, T.; Tsutsui, H.; Shimatani, Y.; Seki, K.; Amano, J. Targeting solid tumors with non-pathogenic obligate anaerobic bacteria. Cancer Sci. 2010, 101, 1925–1932. [Google Scholar] [CrossRef]
- Nuyts, S.; Van Mellaert, L.; Theys, J.; Landuyt, W.; Bosmans, E.; Anné, J.; Lambin, P. Radio-responsive recA promoter significantly increases TNFα production in recombinant clostridia after 2 Gy irradiation. Gene Ther. 2001, 8, 1197–1201. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.C.; Minton, N.P.; Giaccia, A.J.; Brown, J.M. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002, 9, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008, 118, 3132–3142. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2-Associated Clonal T Cell Proliferation in Two Patients after Gene Therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef]
- Nham, T.; Filali, S.; Danne, C.; Derbise, A.; Carniel, E. Imaging of Bubonic Plague Dynamics by In Vivo Tracking of Bioluminescent Yersinia pestis. PLoS ONE 2012, 7, e34714. [Google Scholar] [CrossRef]
- Kocijancic, D.; Felgner, S.; Frahm, M.; Komoll, R.-M.; Iljazovic, A.; Pawar, V.; Rohde, M.; Heise, U.; Zimmermann, K.; Gunzer, F.; et al. Therapy of solid tumors using probiotic Symbioflor-2-restraints and potential. Oncotarget 2016, 7, 22605–22622. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Bi, Y.; Xu, X.; Qiu, Y.; Wang, Q.; Feng, N.; Cui, Y.; Yan, Y.; Zhou, L.; Tan, Y.; et al. Bioluminescent tracking of colonization and clearance dynamics of plasmid-deficient Yersinia pestis strains in a mouse model of septicemic plague. Microbes Infect. 2014, 16, 214–224. [Google Scholar] [CrossRef]
- Kaneuchi, C.; Benno, Y.; Mitsuoka, T. Clostridium coccoides, a new species from the feces of mice. Int. J. Syst. Bacteriol. 1976, 26, 482–486. [Google Scholar] [CrossRef] [Green Version]
- Kurakawa, T.; Ogata, K.; Matsuda, K.; Tsuji, H.; Kubota, H.; Takada, T.; Kado, Y.; Asahara, T.; Takahashi, T.; Nomoto, K. Diversity of Intestinal Clostridium coccoides Group in the Japanese Population, as Demonstrated by Reverse Transcription-Quantitative PCR. PLoS ONE 2015, 10, e0126226. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.F.; Cao, W.W.; Cerniglia, C.E. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microbiol. 1996, 62, 1242–1247. [Google Scholar] [CrossRef] [Green Version]
- Chu, W.-K.; Hung, L.-M.; Hou, C.-W.; Chen, J.-K. Heterogeneous ribonucleoprotein F regulates YAP expression via a G-tract in 3′UTR. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2019, 1862, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, K.R.; Vo, K.T.; Michaelis, S.; Paddon, C. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 1997, 25, 451–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yuan, S.; Xiong, B.; Sun, H.; Ye, L.; Li, J.; Zhang, X.; Bi, C. Development of a fast and easy method for Escherichia coli genome editing with CRISPR /Cas9. Microb. Cell Fact. 2016, 15, 205. [Google Scholar] [CrossRef] [Green Version]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Mocrobiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Sheu, D.-S.; Wang, Y.-T.; Lee, C.-Y. Rapid detection of polyhydroxyalkanoate-accumulating bacteria isolated from the environment by colony PCR. Microbiology 2019, 146, 2019–2025. [Google Scholar] [CrossRef] [Green Version]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Kado, Y.; Takada, T.; Matsumoto, K.; Tanaka, R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal Bifidobacteria. Appl. Environ. Microbiol. 2004, 70, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, C.; Maeda, H.; Kokeguchi, S.; Takashiba, S.; Nishimura, F.; Arai, H.; Fukui, K.; Murayama, Y. Application of denaturing gradient gel electrophoresis (DGGE) to the analysis of microbial communities of subgingival plaque. J. Periodontal Res. 2003, 38, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Traverso, F.R.; Bohr, U.R.; Oyarzabal, O.A.; Rohde, M.; Clarici, A.; Wex, T.; Kuester, D.; Malfertheiner, P.; Fox, J.G.; Backert, S. Morphologic, genetic, and biochemical characterization of Helicobacter Magde-burgensis, a novel species isolated from the intestine of laboratory mice. Helicobacter 2010, 15, 403–415. [Google Scholar] [CrossRef]
- Kimura, N.T.; Taniguchi, S.; Aoki, K.; Baba, T. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res. 1980, 40, 2061–2068. [Google Scholar] [PubMed]
- Zhang, Y.; Cao, W.; Toneri, M.; Zhang, N.; Kiyuna, T.; Murakami, T.; Nelson, S.D.; Dry, S.M.; Li, Y.; Li, S.; et al. Toxicology and efficacy of tumor-targeting Salmonella typhimurium A1-R compared to VNP 20009 in a syngeneic mouse tumor model in immunocompetent mice. Oncotarget 2017, 8, 54616–54628. [Google Scholar] [CrossRef] [Green Version]
- Leschner, S.; Westphal, K.; Dietrich, N.; Viegas, N.; Jablonska, J.; Lyszkiewicz, M.; Lienenklaus, S.; Falk, W.; Gekara, N.O.; Loessner, H.; et al. Tumor Invasion of Salmonella enterica Serovar Typhimurium Is Accompanied by Strong Hemorrhage Promoted by TNF-α. PLoS ONE 2009, 4, e6692. [Google Scholar] [CrossRef] [Green Version]
- Felgner, S.; Kocijancic, D.; Frahm, M.; Heise, U.; Rohde, M.; Zimmermann, K.; Falk, C.; Erhardt, M.; Weiss, S. Engineered Salmonella enterica serovar typhimurium overcomes limitations of an-ti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology 2017, 7, e1382791. [Google Scholar] [CrossRef] [Green Version]
- Westphal, K.; Leschner, S.; Jablonska, J.; Loessner, H.; Weiss, S. Containment of Tumor-Colonizing Bacteria by Host Neutrophils. Cancer Res. 2008, 68, 2952–2960. [Google Scholar] [CrossRef] [Green Version]
- Coutermarsh-Ott, S.L.; Broadway, K.M.; Scharf, B.E.; Allen, I.C. Effect of Salmonella enterica serovar Typhimurium VNP20009 and VNP20009 with restored chemotaxis on 4T1 mouse mammary carcinoma progression. Oncotarget 2017, 8, 33601–33613. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Sasaki, T.; Fujimori, M.; Yazawa, K.; Kano, Y.; Amano, J.; Taniguchi, S.I. Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci. Biotechnol. Biochem. 2014, 66, 2362–2366. [Google Scholar] [CrossRef] [Green Version]
- Cronin, M.; Morrissey, D.; Rajendran, S.; El Mashad, S.M.; van Sinderen, D.; O’Sullivan, G.C.; Tangney, M. Orally Administered Bifidobacteria as Vehicles for Delivery of Agents to Systemic Tumors. Mol. Ther. 2010, 18, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Z.; Mao, S.; Ma, B.; Zhou, S.; Deng, L.; Liu, T.; Cui, D.; Zhao, Y.; He, J.; et al. Antitumor effect of sFlt-1 gene therapy system mediated by Bifidobacterium Infantis on Lewis lung cancer in mice. Cancer Gene Ther. 2001, 18, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Takahashi, M.; Watanabe, Y. Potential usefulness of Brevibacillus for bacteria cancer therapy: Intratumoral provision of tumor necrosis factor-α and anticancer effects. Cancer Gene Ther. 2018, 25, 47–57. [Google Scholar] [CrossRef] [PubMed]

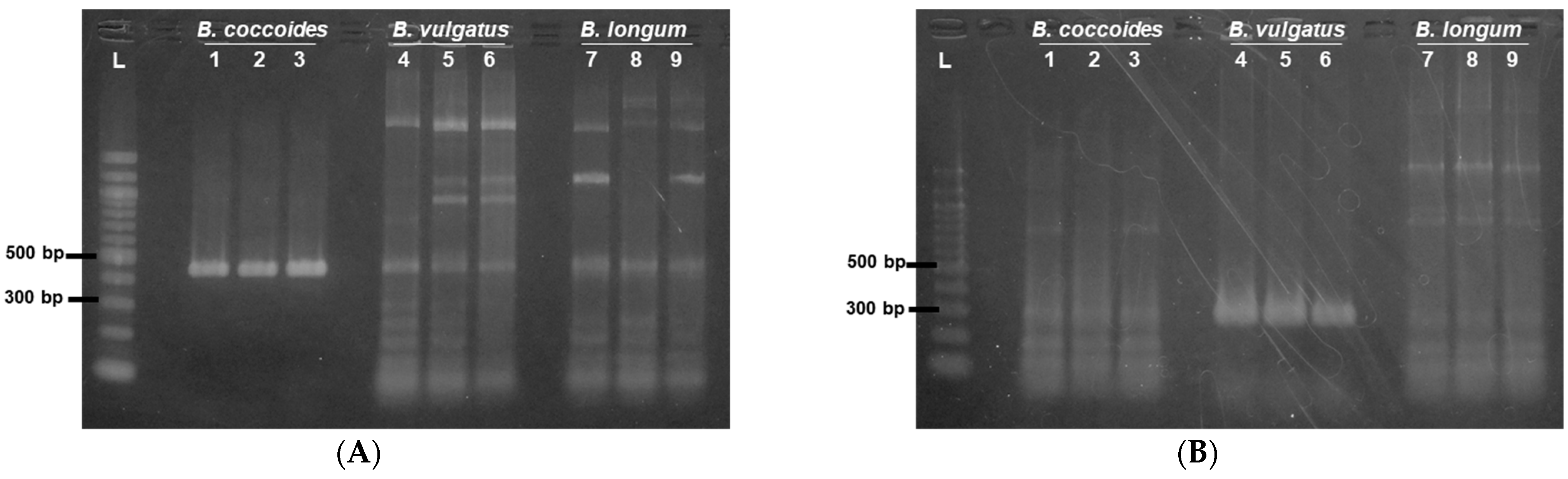

| Species | Oligonucleotide Sequence (5′→3′) | Amplicon Size | Reference |
|---|---|---|---|
| Blautia coccoides JCM1395T | F: 5′-AAATGACGGTACCTGACTAA-3′ R: 5′-CTTTGAGTTTCATTCTTGCGAA-3′ | 438 bp | Kurakawa et al. (2015) [24] |
| Bacteroides vulgatus JCM5826T | F: 5′-GCATCATGAGTCCGCATGTTC-3′ R: 5′-TCCATACCCGACTTTATTCCTT-3′ | 267 bp | Wang et al. (1996) [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, S.; Sukowati, E.W.; Shigeno, Y.; Takahashi, M.; Kato, A.; Benno, Y.; Yamashita, F.; Mukai, H. Blautia coccoides JCM1395T Achieved Intratumoral Growth with Minimal Inflammation: Evidence for Live Bacterial Therapeutic Potential by an Optimized Sample Preparation and Colony PCR Method. Pharmaceutics 2023, 15, 989. https://doi.org/10.3390/pharmaceutics15030989
Nomura S, Sukowati EW, Shigeno Y, Takahashi M, Kato A, Benno Y, Yamashita F, Mukai H. Blautia coccoides JCM1395T Achieved Intratumoral Growth with Minimal Inflammation: Evidence for Live Bacterial Therapeutic Potential by an Optimized Sample Preparation and Colony PCR Method. Pharmaceutics. 2023; 15(3):989. https://doi.org/10.3390/pharmaceutics15030989
Chicago/Turabian StyleNomura, Shoko, Erike W. Sukowati, Yuko Shigeno, Maiko Takahashi, Akari Kato, Yoshimi Benno, Fumiyoshi Yamashita, and Hidefumi Mukai. 2023. "Blautia coccoides JCM1395T Achieved Intratumoral Growth with Minimal Inflammation: Evidence for Live Bacterial Therapeutic Potential by an Optimized Sample Preparation and Colony PCR Method" Pharmaceutics 15, no. 3: 989. https://doi.org/10.3390/pharmaceutics15030989
APA StyleNomura, S., Sukowati, E. W., Shigeno, Y., Takahashi, M., Kato, A., Benno, Y., Yamashita, F., & Mukai, H. (2023). Blautia coccoides JCM1395T Achieved Intratumoral Growth with Minimal Inflammation: Evidence for Live Bacterial Therapeutic Potential by an Optimized Sample Preparation and Colony PCR Method. Pharmaceutics, 15(3), 989. https://doi.org/10.3390/pharmaceutics15030989







