Association between Dupilumab and Conjunctivitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
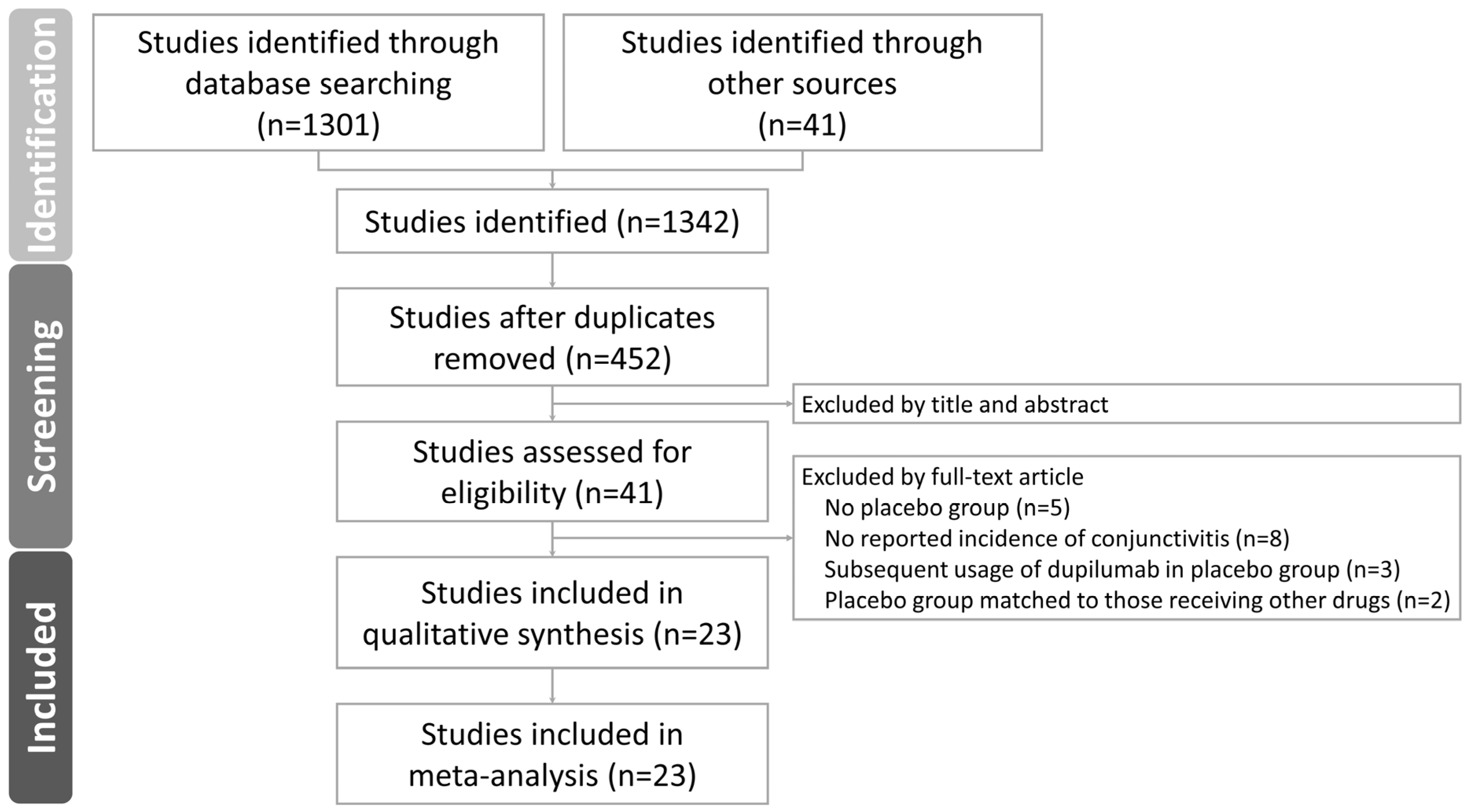
3.1. Outcomes of the Literature Search and Characteristics of Included Studies
3.2. Conjunctivitis in Dupilumab versus Placebo Users
3.3. Conjunctivitis in Dupilumab versus Placebo Users with AD and Non-AD indications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halling, A.S.; Loft, N.; Silverberg, J.I.; Guttman-Yassky, E.; Thyssen, J.P. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 84, 139–147. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kusakabe, M.; Nagai, M.; Yasuda, K.; Yamanishi, K. Dupilumab Effects on Innate Lymphoid Cell and Helper T Cell Populations in Patients with Atopic Dermatitis. JID Innov. Ski. Sci. Mol. Popul. Health 2021, 1, 100003. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Maspero, J.F.; Katelaris, C.H.; Fiocchi, A.G.; Gagnon, R.; de Mir, I.; Jain, N.; Sher, L.D.; Mao, X.; Liu, D.; et al. Dupilumab in Children with Uncontrolled Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 2230–2240. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [Green Version]
- Bachert, C.; Mannent, L.; Naclerio, R.M.; Mullol, J.; Ferguson, B.J.; Gevaert, P.; Hellings, P.; Jiao, L.; Wang, L.; Evans, R.R.; et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA 2016, 315, 469–479. [Google Scholar] [CrossRef]
- Hirano, I.; Dellon, E.S.; Hamilton, J.D.; Collins, M.H.; Peterson, K.; Chehade, M.; Schoepfer, A.M.; Safroneeva, E.; Rothenberg, M.E.; Falk, G.W.; et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis. Gastroenterology 2020, 158, 111–122.e110. [Google Scholar] [CrossRef] [Green Version]
- Dellon, E.S.; Rothenberg, M.E.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Aceves, S.; Sun, X.; et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N. Engl. J. Med. 2022, 387, 2317–2330. [Google Scholar] [CrossRef]
- Neagu, N.; Dianzani, C.; Avallone, G.; Dell’Aquila, C.; Morariu, S.H.; Zalaudek, I.; Conforti, C. Dupilumab ocular side effects in patients with atopic dermatitis: A systematic review. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Popiela, M.Z.; Barbara, R.; Turnbull, A.M.J.; Corden, E.; Martinez-Falero, B.S.; O’Driscoll, D.; Ardern-Jones, M.R.; Hossain, P.N. Dupilumab-associated ocular surface disease: Presentation, management and long-term sequelae. Eye 2021, 35, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Maudinet, A.; Law-Koune, S.; Duretz, C.; Lasek, A.; Modiano, P.; Tran, T.H.C. Ocular Surface Diseases Induced by Dupilumab in Severe Atopic Dermatitis. Ophthalmol. Ther. 2019, 8, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Akinlade, B.; Guttman-Yassky, E.; de Bruin-Weller, M.; Simpson, E.L.; Blauvelt, A.; Cork, M.J.; Prens, E.; Asbell, P.; Akpek, E.; Corren, J.; et al. Conjunctivitis in dupilumab clinical trials. Br. J. Dermatol. 2019, 181, 459–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Thaçi, D.; Simpson, E.L.; Beck, L.A.; Bieber, T.; Blauvelt, A.; Papp, K.; Soong, W.; Worm, M.; Szepietowski, J.C.; Sofen, H.; et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016, 387, 40–52. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Blauvelt, A.; Simpson, E.L.; Tyring, S.K.; Purcell, L.A.; Shumel, B.; Petro, C.D.; Akinlade, B.; Gadkari, A.; Eckert, L.; Graham, N.M.H.; et al. Dupilumab does not affect correlates of vaccine-induced immunity: A randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 158–167.e151. [Google Scholar] [CrossRef] [Green Version]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Worm, M.; Simpson, E.L.; Thaçi, D.; Bissonnette, R.; Lacour, J.P.; Beissert, S.; Kawashima, M.; Ferrándiz, C.; Smith, C.H.; Beck, L.A.; et al. Efficacy and Safety of Multiple Dupilumab Dose Regimens After Initial Successful Treatment in Patients With Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 131–143. [Google Scholar] [CrossRef] [Green Version]
- de Bruin-Weller, M.; Thaçi, D.; Smith, C.H.; Reich, K.; Cork, M.J.; Radin, A.; Zhang, Q.; Akinlade, B.; Gadkari, A.; Eckert, L.; et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: A placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br. J. Dermatol. 2018, 178, 1083–1101. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Paller, A.S.; Siegfried, E.C.; Boguniewicz, M.; Sher, L.; Gooderham, M.J.; Beck, L.A.; Guttman-Yassky, E.; Pariser, D.; Blauvelt, A.; et al. Efficacy and Safety of Dupilumab in Adolescents With Uncontrolled Moderate to Severe Atopic Dermatitis: A Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 44–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paller, A.S.; Siegfried, E.C.; Thaçi, D.; Wollenberg, A.; Cork, M.J.; Arkwright, P.D.; Gooderham, M.; Beck, L.A.; Boguniewicz, M.; Sher, L.; et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J. Am. Acad. Dermatol. 2020, 83, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, L.; Lu, Q.; Gao, X.; Zhu, X.; Yao, X.; Li, L.; Li, W.; Ding, Y.; Song, Z.; et al. The efficacy and safety of dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: A randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2022, 186, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Merola, J.F.; Chiou, A.S.; During, E. 33558 Dupilumab significantly improves sleep disturbance in adults with moderate-to-severe atopic dermatitis: Results of the DUPISTAD study. J. Am. Acad. Dermatol. 2022, 87, AB46. [Google Scholar] [CrossRef]
- Paller, A.S.; Simpson, E.L.; Siegfried, E.C.; Cork, M.J.; Wollenberg, A.; Arkwright, P.D.; Soong, W.; Gonzalez, M.E.; Schneider, L.C.; Sidbury, R.; et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2022, 400, 908–919. [Google Scholar] [CrossRef]
- Patruno, C.; Napolitano, M.; Argenziano, G.; Peris, K.; Ortoncelli, M.; Girolomoni, G.; Offidani, A.; Ferrucci, S.M.; Amoruso, G.F.; Rossi, M.; et al. Dupilumab therapy of atopic dermatitis of the elderly: A multicentre, real-life study. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Fabbrocini, G.; Neri, I.; Stingeni, L.; Boccaletti, V.; Piccolo, V.; Amoruso, G.F.; Malara, G.; De Pasquale, R.; Di Brizzi, E.V.; et al. Dupilumab Treatment in Children Aged 6-11 Years With Atopic Dermatitis: A Multicentre, Real-Life Study. Paediatr. Drugs 2022, 24, 671–678. [Google Scholar] [CrossRef]
- Fargnoli, M.C.; Esposito, M.; Ferrucci, S.; Girolomoni, G.; Offidani, A.; Patrizi, A.; Peris, K.; Costanzo, A.; Malara, G.; Pellacani, G.; et al. Real-life experience on effectiveness and safety of dupilumab in adult patients with moderate-to-severe atopic dermatitis. J. Dermatol. Treat. 2021, 32, 507–513. [Google Scholar] [CrossRef]
- Ou, Z.; Chen, C.; Chen, A.; Yang, Y.; Zhou, W. Adverse events of Dupilumab in adults with moderate-to-severe atopic dermatitis: A meta-analysis. Int. Immunopharmacol. 2018, 54, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Stingeni, L.; Bianchi, L.; Antonelli, E.; Caroppo, E.S.; Ferrucci, S.M.; Gurioli, C.; Ortoncelli, M.; Fabbrocini, G.; Nettis, E.; Schena, D.; et al. A 52-week update of a multicentre Italian real-world experience on effectiveness and safety of dupilumab in adolescents with moderate-to-severe atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. JEADV 2023, 37, e384–e388. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.S.; Rice, B.A.; Dutt, J.E. Immunopathology of atopic keratoconjunctivitis. Ophthalmology 1991, 98, 1190–1196. [Google Scholar] [CrossRef]
- Wu, K.K.; Borba, A.J.; Deng, P.H.; Armstrong, A.W. Association between atopic dermatitis and conjunctivitis in adults: A population-based study in the United States. J. Dermatol. Treat. 2021, 32, 455–459. [Google Scholar] [CrossRef]
- Hsu, J.I.; Pflugfelder, S.C.; Kim, S.J. Ocular complications of atopic dermatitis. Cutis 2019, 104, 189–193. [Google Scholar] [PubMed]
- Pietruszyńska, M.; Zawadzka-Krajewska, A.; Duda, P.; Rogowska, M.; Grabska-Liberek, I.; Kulus, M. Ophthalmic manifestations of atopic dermatitis. Postep. Dermatol. I Alergol. 2020, 37, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.S.; Ariens, L.F.M.; van Luijk, C.; van der Schaft, J.; Thijs, J.L.; Schuttelaar, M.L.A.; van Wijk, F.; Knol, E.F.; Balak, D.M.W.; van Dijk, M.R.; et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br. J. Dermatol. 2019, 180, 1248–1249. [Google Scholar] [CrossRef] [Green Version]
- Voorberg, A.N.; den Dunnen, W.F.A.; Wijdh, R.H.J.; de Bruin-Weller, M.S.; Schuttelaar, M.L.A. Recurrence of conjunctival goblet cells after discontinuation of dupilumab in a patient with dupilumab-related conjunctivitis. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, e64–e66. [Google Scholar] [CrossRef]
- Barnett, B.P.; Afshari, N.A. Dupilumab-Associated Mucin Deficiency (DAMD). Transl. Vis. Sci. Technol. 2020, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Tauqeer, Z.; Jinno, S.E.; Chung, C.W.; Massaro-Giordano, M.; Bunya, V.Y. Clinical Characteristics and Treatment for Dupilumab-Related Ocular Complications in Atopic Dermatitis Patients. Clin. Ophthalmol. 2022, 16, 947–958. [Google Scholar] [CrossRef]
- Kimura, A.; Takeda, A.; Ikebukuro, T.; Hori, J. Serum IgE reduction and paradoxical eosinophilia associated with allergic conjunctivitis after dupilumab therapy. J. Ophthalmic Inflamm. Infect. 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- de Bruin-Weller, M.; Graham, N.M.H.; Pirozzi, G.; Shumel, B. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin-17 levels?: Reply from the authors. Br. J. Dermatol. 2018, 178, 1220–1221. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Ariens, L.; Thurau, S.; van Luijk, C.; Seegräber, M.; de Bruin-Weller, M. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J. Allergy Clin. Immunol. Pract. 2018, 6, 1778–1780.e1771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Li, J.; Liu, R.; Lao, H.Y.; Fan, Z.; Jin, L.; Liang, L.; Liu, Y. Association of Allergic Conjunctivitis With Health-Related Quality of Life in Children and Their Parents. JAMA Ophthalmol. 2021, 139, 830–837. [Google Scholar] [CrossRef]
- Mikhail, E.; Azizoglu, S.; Gokhale, M.; Suphioglu, C. Questionnaires Assessing the Quality of Life of Ocular Allergy Patients. J. Allergy Clin. Immunol. Pract. 2020, 8, 2945–2952. [Google Scholar] [CrossRef]
- Golden, M.I.; Meyer, J.J.; Patel, B.C. Dry Eye Syndrome. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Suzuki, T. Inflamed Obstructive Meibomian Gland Dysfunction Causes Ocular Surface Inflammation. Investig. Ophthalmol. Vis. Sci. 2018, 59, des94–des101. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Gupta, A.; Tripathy, K. Keratitis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hung, N.; Kang, E.Y.; Lee, T.W.; Chen, T.H.; Shyu, Y.C.; Sun, C.C. The Risks of Corneal Surface Damage in Aqueous-Deficient Dry Eye Disease: A 17-Year Population-Based Study in Taiwan. Am. J. Ophthalmol. 2021, 227, 231–239. [Google Scholar] [CrossRef]
- Hashmi, M.F.; Gurnani, B.; Benson, S. Conjunctivitis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kang, E.Y.; Chen, H.T.; Hsueh, Y.J.; Chen, H.C.; Tan, H.Y.; Hsiao, C.H.; Yeh, L.K.; Wu, W.C. Corneal Sensitivity and Tear Function in Recurrent Corneal Erosion Syndrome. Investig. Ophthalmol. Vis. Sci. 2020, 61, 21. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef]
- Feizi, S.; Javadi, M.A.; Alemzadeh-Ansari, M.; Arabi, A.; Shahraki, T.; Kheirkhah, A. Management of corneal complications in vernal keratoconjunctivitis: A review. Ocul. Surf. 2021, 19, 282–289. [Google Scholar] [CrossRef]
- Achten, R.E.; Bakker, D.S.; van Luijk, C.M.; van der Wal, M.; de Graaf, M.; van Wijk, F.; Zuithoff, N.P.A.; van der Rijst, L.P.; Boesjes, C.M.; Thijs, J.L.; et al. Ocular surface disease is common in moderate-to-severe atopic dermatitis patients. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2022, 52, 801–805. [Google Scholar] [CrossRef]
- Ravn, N.H.; Ahmadzay, Z.F.; Christensen, T.A.; Larsen, H.H.P.; Loft, N.; Rævdal, P.; Heegaard, S.; Kolko, M.; Egeberg, A.; Silverberg, J.I.; et al. Bidirectional association between atopic dermatitis, conjunctivitis, and other ocular surface diseases: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 85, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopal, D.P.; Chetty, U.; O’Donnell, P.; Gajria, C.; Blackadder-Weinstein, J. Implicit bias in healthcare: Clinical practice, research and decision making. Future Healthc. J. 2021, 8, 40–48. [Google Scholar] [CrossRef] [PubMed]


| Publication (RCT Identifier) | Indication of Dupilumab | Type of RCT | Number of Patients | Age * | Gender # | Drug Survival of Dupilumab | Incidence of Conjunctivitis | Follow-Up Time of AEs |
|---|---|---|---|---|---|---|---|---|
| Beck 2014 (NCT01548404) | Atopic dermatitis | Two-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg QW (n = 55) vs. Placebo QW (n = 54) for 12 weeks | Adult 36.5 (11.69) | 46.8%/53.2% | 74.55% (41/55) | 23.64% (13/55) dupilumab vs. 3.70% (2/54) placebo | 28 weeks |
| Simpson 2016 (NCT02277743) (NCT02277769) | Atopic dermatitis | Three-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg QW (n = 223) vs. Dupilumab 300mg Q2W (n = 224) vs. Placebo QW (n = 224) for 16 weeks | Adult 39.5 (14.31) | 41.9%/58.1% | 90.60% (405/447) | 6.52% (60/920) dupilumab vs. 1.54% (7/456) placebo | 28 weeks |
| Atopic dermatitis | Three-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg QW (n = 239) vs. Dupilumab 300mg Q2W (n = 233) vs. Placebo QW (n = 236) for 15 weeks | Adult 37.1 (14.17) | 42.4%/57.6% | 93.45% (441/473) | 28 weeks | ||
| Thaçi 2016 (NCT01859988) | Atopic dermatitis | Six-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 300 mg QW (n = 63) vs. Dupilumab 300 mg Q2W (n = 64) vs. Dupilumab 200 mg Q2W (n = 62) vs. Dupilumab 300 mg Q4W (n = 65) vs. Dupilumab 100 mg Q4W (n = 65) vs. Placebo QW (n = 61) for 15 weeks | Adult 37.0 (12.06) | 38.3%/61.7% | 73.90% (235/318) | 6.60% (21/318) dupilumab vs. 3.28% (2/61) placebo | 32 weeks |
| Blauvelt 2017 (NCT02260986) | Atopic dermatitis | Three-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg QW (n = 319) vs. Dupilumab 300mg Q2W (n = 106) vs. Placebo QW (n = 315) for 51 weeks | Adult 37.1 (13.46) | 39.7%/60.3% | 91.75% (371/425) | 17.88% (76/425) dupilumab vs. 7.94% (25/315) placebo | 52 weeks |
| Bruin-Weller 2018 (NCT02755649) | Atopic dermatitis | Three-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 300mg QW +TCS (n = 110) vs. Dupilumab 300mg Q2W+TCS (n = 107) vs. Placebo QW +TCS (n = 108) for 16 weeks | Adult 38.4 (13.13) | 38.8%/61.2% | 99.08% (215/217) | 22.12% (48/217) dupilumab vs. 11.11% (12/108) placebo | 28 weeks |
| Blauvelt 2019 (NCT02210780) | Atopic dermatitis | Two-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg QW (n = 97) vs. Placebo QW (n = 97) for 15 weeks | Adult 39.6 (13.77) | 51.0%/49.0% | 91.75% (89/97) | 8.25% (8/97) dupilumab vs. 0.00% (0/97) placebo | 32 weeks |
| Guttman-Yassky 2019 (NCT01979016) | Atopic dermatitis | Two-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 200mg QW (n = 27) vs. Placebo QW (n = 27) for 15 weeks | Adult 41.3 (14.81) | 44.4%/55.6% | 96.30% (26/27) | 11.11% (3/27) dupilumab vs. 3.70% (1/27) placebo | 32 weeks |
| Paller 2020 (NCT03345914} | Atopic dermatitis | Three-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 300mg Q4W +TCS (n = 120) vs. Dupilumab 300/100mg Q2W+TCS (n = 122) vs. Placebo QW +TCS (n = 120) for 16 weeks | Child 8.5 (1.72) | 50.1%/49.9% | 97.13% (237/244) | 10.74% (26/242) dupilumab vs. 4.17% (5/120) placebo | 28 weeks |
| Worm 2020 (NCT02395133) | Atopic dermatitis | Four-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg QW/Q2W (n = 169) vs. Dupilumab 300mg Q4W (n = 86) vs. Dupilumab 300mg Q8W (n = 84) vs. Placebo QW (n=83) for 36 weeks | Adult 38.2 (14.46) | 46.2%/53.8% | 90.27% (306/339) | 4.73% (16/338) dupilumab vs. 4.88% (4/82) placebo | 36 weeks |
| Simpson 2020 (NCT03054428) | Atopic dermatitis | Three-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 300/200mg Q2W (n = 82) vs. Dupilumab 300mg Q4W (n = 84) vs. Placebo Q2W (n = 85) for 16 weeks | Adolescent 14.5 (1.70) | 41.0%/59.0% | 96.39% (160/166) | 10.30% (17/165) dupilumab vs. 4.71% (4/85) placebo | 28 weeks |
| Merola 2022 (NCT04033367) | Atopic dermatitis | Two-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 300mg Q2W (n = 127) vs. Placebo Q2W (n = 61) for 12 weeks | Adult 35.7 (14.89) | 51.6%/48.4% | 96.06% (122/127) | 9.45% (12/127) dupilumab vs. 4.92% (3/61) placebo | 12 weeks |
| Paller 2022 (NCT03346434) | Atopic dermatitis | Two-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 400/200mg Q4W + TCS (n = 83) vs. Placebo Q2W + TCS (n = 79) for 12 weeks | Child 4.0 (0.8) | 61.1%/38.9% | 100.00% (83/83) | 4.82% (4/83) dupilumab vs. 0.00% (0/78) placebo | 28 weeks |
| Zhao 2022 (NCT03912259) | Atopic dermatitis | Two-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 300mg Q2W (n = 82) vs. Placebo Q2W (n=83) for 16 weeks | Adult 30.6 (11.49) | 28.5%/71.5% | 92.68% (76/82) | 18.29% (15/82) dupilumab vs. 6.02% (5/83) placebo | 28 weeks |
| Wenzel 2016 (NCT01854047) | Asthma | Five-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg Q2W (n = 154) vs. Dupilumab 200mg Q2W (n = 157) vs. Dupilumab 300mg Q4W (n = 150) vs. Dupilumab 100mg Q4W (n = 157) vs. Placebo Q2W (n = 158) for 22 weeks | Adult 48.6 (13.00) | 63.1%/36.9% | 92.14% (563/611) | 1.15% (7/611) dupilumab vs. 1.27% (2/158) placebo | 40 weeks |
| Castro 2018 (NCT02414854) | Asthma | Four-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg Q2W (n = 633) vs. Dupilumab 200mg Q2W (n = 631) vs. Placebo 300mg Q2W (n = 321) vs. Placebo 200mg Q2W (n = 317) for 50 weeks | Adult 47.9 (15.30) | 62.9%/37.1% | 92.41% (1168/1264) | 1.74% (22/1263) dupilumab vs. 2.37% (15/634) placebo | 64 weeks |
| Rabe 2018 (NCT02528214) | Asthma | Two-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg Q2W (n = 103) vs. Placebo Q2W (n = 107) for 24 weeks | Adult 51.3 (12.60) | 60.5%/39.5% | 97.09% (100/103) | 0.97% (1/103) dupilumab vs. 0.93% (1/107) placebo | 36 weeks |
| Bacharier 2021 (NCT02948959) | Asthma | Two-arm, triple-blind, placebo-controlled RCT | Dupilumab 200/100mg Q2W (n = 273) vs. Placebo Q2W (n=135) for 52 weeks | Child 8.9 (1.6) | 35.8%/64.2% | 90.84% (248/273) | 2.58% (7/271) dupilumab vs. 6.72% (9/134) placebo | 64 weeks |
| Bachert 2016 (NCT01920893) | CRSwNP | Two-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg QW (n = 30) vs. Placebo QW (n = 30) for 15 weeks | Adult 48.4 (9.40) | 43.3%/56.7% | 93.33% (28/30) | 0.00% (0/30) dupilumab vs. 3.33% (1/30) placebo | 32 weeks |
| Bachert 2019 (NCT02898454) (NCT02912468) | CRSwNP | Three-arm, quadruple-blind, placebo-controlled RCT | Dupilumab 300mg Q2W (n = 150) vs. Dupilumab 300mg Q2W then Q4W (n = 145) vs. Placebo Q2W (n = 153) for 52 weeks | Adult 51.95 (12.45) | 37.7%/62.3% | 96.27% (284/295) | 1.59% (7/440) dupilumab vs. 0.35% (1/282) placebo | 24 weeks |
| CRSwNP | Two-arm, double-blind, placebo-controlled RCT | Dupilumab 300mg Q2W (n = 143) vs. Placebo Q2W (n = 133) for 24 weeks | Adult 50.49 (13.39) | 42.8%/57.2% | 96.50% (138/143) | 24 weeks | ||
| Hirano 2020 (NCT02379052) | EE | Two-arm, triple-blind, placebo-controlled RCT | Dupilumab 300mg QW (n = 23) vs. Placebo QW (n = 24) for 12 weeks | Adult 34.7 (10.94) | 51.1%/48.9% | 78.26% (18/23) | 0.00% (0/23) dupilumab vs. 0.00% (0/24) placebo | 28 weeks |
| Dellon 2020 (NCT03633617) | EE | Five-arm, quadruple-blind, placebo-controlled RCT | Part A Dupilumab 300mg QW (n = 42) vs. Placebo QW (n = 39) for 24 weeks Part B Dupilumab 300mg QW (n = 80) vs. Dupilumab 300mg Q2W (n = 81) vs. Placebo QW (n = 79) for 24 weeks | Adult 28.1 (13.12) | 63.7%/36.3% | 94.09% (191/203) | 1.48% (3/203) dupilumab vs. 1.71% (2/117) placebo | 24 weeks |
| Methods | Models | Odds Ratio (95%Cl) | Relative Risk (95%Cl) |
|---|---|---|---|
| Conjunctivitis in dupilumab versus placebo users | |||
| Mantel–Haenszel | Random effects | 2.01 (1.38–2.93) | 1.89 (1.34–2.67) |
| Inverse variance | Random effects | 2.01 (1.39–2.92) | 1.89 (1.34–2.66) |
| Conjunctivitis in dupilumab versus placebo users with AD | |||
| Mantel–Haenszel | Random effects | 2.71 (2.06–3.55) | 2.43 (1.89–3.12) |
| Inverse variance | Random effects | 2.71 (2.06–3.55) | 2.43(1.85–3.61) |
| Conjunctivitis in dupilumab versus placebo users with non-AD indications | |||
| Mantel–Haenszel | Random effects | 0.71 (0.44–1.14) | 0.71 (0.44–1.13) |
| Inverse variance | Random effects | 0.70 (0.42–1.14) | 0.71 (0.44–1.13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-Y.; Wang, C.-Y.; Wang, F.-Y.; Kang, E.Y.-C.; Hwang, Y.-S. Association between Dupilumab and Conjunctivitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceutics 2023, 15, 1031. https://doi.org/10.3390/pharmaceutics15041031
Lin T-Y, Wang C-Y, Wang F-Y, Kang EY-C, Hwang Y-S. Association between Dupilumab and Conjunctivitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceutics. 2023; 15(4):1031. https://doi.org/10.3390/pharmaceutics15041031
Chicago/Turabian StyleLin, Tzu-Yi, Ching-Ya Wang, Fang-Ying Wang, Eugene Yu-Chuan Kang, and Yih-Shiou Hwang. 2023. "Association between Dupilumab and Conjunctivitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Pharmaceutics 15, no. 4: 1031. https://doi.org/10.3390/pharmaceutics15041031
APA StyleLin, T.-Y., Wang, C.-Y., Wang, F.-Y., Kang, E. Y.-C., & Hwang, Y.-S. (2023). Association between Dupilumab and Conjunctivitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceutics, 15(4), 1031. https://doi.org/10.3390/pharmaceutics15041031







