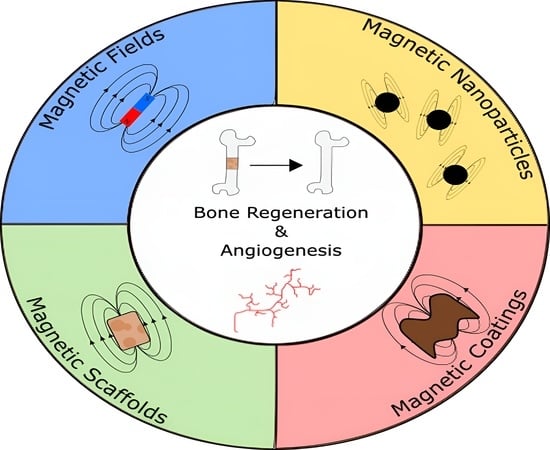
Magnetic Bone Tissue Engineering: Reviewing the Effects of Magnetic Stimulation on Bone Regeneration and Angiogenesis
Abstract
1. Introduction
2. Magnetic Fields Influence on Cell Behaviour
2.1. Influence of Magnetic Stimulation on Bone Regeneration
2.2. Influence of Magnetic Stimulation on Angiogenesis
3. Magnetic Nanoparticles Influence on Cell Behavior
3.1. Magnetic Nanoparticles Production Methods
3.2. Influence of Magnetic Nanoparticles on Bone Regeneration
3.3. Influence of Magnetic Nanoparticles on Angiogenesis
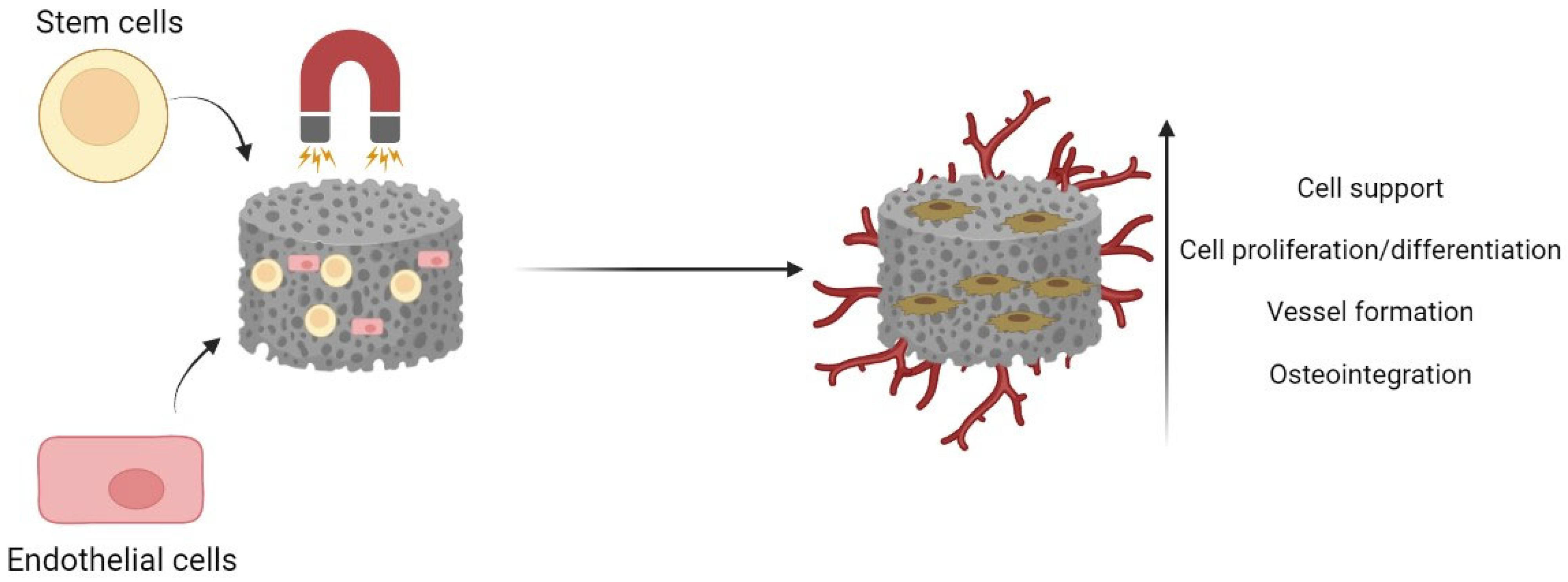
4. Magnetic Scaffolds in Bone Tissue Engineering
4.1. Production Methods of Magnetic Bone Scaffolds
4.2. Influence of Magnetic Scaffolds on Bone Regeneration
4.3. Influence of Magnetic Scaffolds on Angiogenesis
5. Magnetic Coatings in Bone Tissue Engineering
5.1. Production Methods of Magnetic Coatings
5.2. Influence of Magnetic Coatings on Bone Regeneration
6. Further Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASC | Adipose-Derived Mesenchymal Cells |
| ALP | Alkaline Phosphatase |
| BSA | Bovine Serum Albumin |
| CVD | Chemical Vapor Deposition |
| DPSCs | Dental Pulp Stem Cells |
| ECD | Electrochemical Deposition |
| eNOS | Endothelial Nitric Oxide Synthase |
| bFGF | Fibroblast Growth Factor |
| ICA | Icariin |
| MNPs | Magnetic Nanoparticles |
| MAO | Micro Arc Oxidation |
| PEO | Plasma Electrolytic Oxidation |
| PVD | Physical Vapor Deposition |
| PCL | Polycaprolactone |
| PDA | Polydopamine |
| PEG | Polyethylene Glycol |
| PEI | Polyethylenimine |
| PLA | Poly-l-lactide |
| PMMA | Polymethyl Methacrylate |
| ROS | Reactive Oxygen Species |
| SMF | Static Magnetic Field |
| SPIONS | Superparamagnetic Iron Oxide Nanoparticles |
| VEGF | Vascular Endothelial Growth Factor |
References
- Wang, J.; Yao, Q.-Y.; Zhu, H.-Y. Efficacy of bone grafts in jaw cystic lesions: A systematic review. World J. Clin. Cases 2022, 10, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Ferraz, M.P.; Azeredo, J.; Fernandes, M.H.; Gomes, P.S.; Monteiro, F.J. Alginate-nanohydroxyapatite hydrogel system: Optimizing the formulation for enhanced bone regeneration. Mater. Sci. Eng. C. 2019, 105, 109985. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.; Araújo, R.; Quadros, P.A.; Sousa, S.R.; Monteiro, F.J. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Mater. Sci. Eng. C. 2019, 97, 529–538. [Google Scholar] [CrossRef]
- Coelho, C.C.; Sousa, S.R.; Monteiro, F.J. Heparinized nanohydroxyapatite/collagen granules for controlled release of vancomycin. J. Biomed. Mater. Res. Part A. 2015, 103, 3128–3138. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Vielreicher, M.; Bozec, A.; Schett, G.; Friedrich, O. Murine Metatarsus Bone and Joint Collagen-I Fiber Morphologies and Networks Studied With SHG Multiphoton Imaging. Front. Bioeng. Biotechnol. 2021, 9, 455. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone Tissue Engineering: State of the Art and Future Trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Kenkre, J.; Bassett, J. The bone remodelling cycle, Ann. Clin. Biochem. Int. J. Lab. Med. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Nhlapo, N.; Dzogbewu, C.T.; de Smidt, O. Nanofiber Polymers for Coating Titanium-Based Biomedical Implants. Fibers 2022, 10, 36. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Bandyopadhyay, A.; Bose, S. Plasma sprayed fluoride and zinc doped hydroxyapatite coated titanium for load-bearing implants. Surf. Coat. Technol. 2022, 440, 128464. [Google Scholar] [CrossRef]
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioact. Mater. 2022, 9, 221–238. [Google Scholar] [CrossRef]
- Wu, L.; Pei, X.; Zhang, B.; Su, Z.; Gui, X.; Gao, C.; Guo, L.; Fan, H.; Jiang, Q.; Zhao, L.; et al. 3D-printed HAp bone regeneration scaffolds enable nano-scale manipulation of cellular mechanotransduction signals. Chem. Eng. J. 2023, 455, 140699. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Ding, C.; Dong, D.; Shang, P. Regulation of Osteoblast Differentiation and Iron Content in MC3T3-E1 Cells by Static Magnetic Field with Different Intensities. Biol. Trace Elem. Res. 2018, 184, 214–225. [Google Scholar] [CrossRef]
- Sakurai, T.; Terashima, S.; Miyakoshi, J. Enhanced secretion of prostaglandin E2 from osteoblasts by exposure to a strong static magnetic field. Bioelectromagnetics 2008, 29, 277–283. [Google Scholar] [CrossRef]
- Ziegelberger, G.; Vecchia, P.; Hietanen, M.; Ahlbom, A.; Anderson, L.E.; Breitbart, E.; De Gruijl, F.R.; Lin, J.C.; Matthes, R.; Peralta, A.P.; et al. Guidelines on limits of exposure to static magnetic fields. Health Phys. 2009, 96, 504–514. [Google Scholar] [CrossRef]
- Bekhite, M.M.; Figulla, H.R.; Sauer, H.; Wartenberg, M. Static magnetic fields increase cardiomyocyte differentiation of Flk-1+ cells derived from mouse embryonic stem cells via Ca2+ influx and ROS production. Int. J. Cardiol. 2013, 167, 798–808. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, C.; Shang, P. Alterations of Mineral Elements in Osteoblast During Differentiation Under Hypo, Moderate and High Static Magnetic Fields. Biol. Trace Elem. Res. 2014, 162, 153–157. [Google Scholar] [CrossRef]
- Lew, W.Z.; Huang, Y.C.; Huang, K.Y.; Lin, C.T.; Tsai, M.T.; Huang, H.M. Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. J. Tissue Eng. Regen. Med. 2018, 12, 19–29. [Google Scholar] [CrossRef]
- Qian, A.R.; Gao, X.; Zhang, W.; Li, J.B.; Wang, Y.; Di, S.M.; Hu, L.F.; Shang, P. Large Gradient High Magnetic Fields Affect Osteoblast Ultrastructure and Function by Disrupting Collagen I or Fibronectin/αβ1 Integrin. PLoS ONE 2013, 8, e51036. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, L.; Zhu, X.; Wang, J.; Han, L.; Cheng, P.; Jing, D.; Zhang, X.; Shan, Q. Moderate-intensity 4 mT static magnetic fields prevent bone architectural deterioration and strength reduction by stimulating bone formation in streptozotocin-treated diabetic rats. Bone 2018, 107, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.F.; Perea, H.; Hopfner, U.; Ferguson, V.L.; Wintermantel, E. Effects of weak static magnetic fields on endothelial cells. Bioelectromagnetics 2010, 31, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, J.; Wang, X.; Xu, Y.; Liang, Z.; Gu, X.; He, C. Coupling induction of osteogenesis and type H vessels by pulsed electromagnetic fields in ovariectomy-induced osteoporosis in mice. Bone 2022, 154, 116211. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, S.; Genova, T.; Chinigò, G.; Roato, I.; Scarpellino, G.; Kopecka, J.; Altruda, F.; Tolosano, E.; Riganti, C.; Mussano, F.; et al. Endothelial Cells Promote Osteogenesis by Establishing a Functional and Metabolic Coupling With Human Mesenchymal Stem Cells. Front. Physiol. 2022, 12, 813547. [Google Scholar] [CrossRef]
- Okano, H.; Onmori, R.; Tomita, N.; Ikada, Y. Effects of a moderate-intensity static magnetic field on VEGF-A stimulated endothelial capillary tubule formation in vitro. Bioelectromagnetics 2006, 27, 628–640. [Google Scholar] [CrossRef]
- Wu, D.; Chang, X.; Tian, J.; Kang, L.; Wu, Y.; Liu, J.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: Release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotech. 2021, 19, 209. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Monteiro, F.J.; Laranjeira, M.S. Duality of iron (III) doped nano hydroxyapatite in triple negative breast cancer monitoring and as a drug-free therapeutic agent. Ceram. Int. 2020, 46, 16590–16597. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Monteiro, F.J.; Laranjeira, M.S. PEGylation of iron doped hydroxyapatite nanoparticles for increased applicability as MRI contrast agents and as drug vehicles: A study on thrombogenicity, cytocompatibility and drug loading. Eur. Polym. J. 2020, 137, 109934. [Google Scholar] [CrossRef]
- Dasari, A.; Xue, J.; Deb, S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials 2022, 12, 757. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Huang, G.; Lu, C.-H.; Yang, H.-H. Magnetic Nanomaterials for Magnetic Bioanalysis. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 89–109. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F. Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J. Environ. Chem. Eng. 2018, 7, 102805. [Google Scholar] [CrossRef]
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single step synthesis, characterization and applications of curcumin functionalized iron oxide magnetic nanoparticles. Mater. Sci. Eng. C 2016, 67, 59–64. [Google Scholar] [CrossRef]
- Scuderi, M.; Esposito, M.; Todisco, F.; Simeone, D.; Tarantini, I.; De Marco, L.; De Giorgi, M.; Nicotra, G.; Carbone, L.; Sanvitto, D.; et al. Nanoscale Study of the Tarnishing Process in Electron Beam Lithography-Fabricated Silver Nanoparticles for Plasmonic Applications. J. Phys. Chem. C 2016, 120, 24314–24323. [Google Scholar] [CrossRef]
- Manescu, V.; Paltanea, G.; Antoniac, I.; Vasilescu, M. Magnetic Nanoparticles Used in Oncology. Materials 2021, 14, 5948. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, B.; Wang, L.; Wang, J.; Li, X.; Yang, G.; Gao, F. Superparamagnetic iron oxide nanoparticles coated with different polymers and their MRI contrast effects in the mouse brains. Appl. Surf. Sci. 2015, 326, 32–38. [Google Scholar] [CrossRef]
- Alromi, D.A.; Madani, S.Y.; Seifalian, A. Emerging Application of Magnetic Nanoparticles for Diagnosis and Treatment of Cancer. Polymers 2021, 13, 4146. [Google Scholar] [CrossRef]
- Liu, X.-D.; Chen, H.; Liu, S.-S.; Ye, L.-Q.; Li, Y.-P. Hydrothermal synthesis of superparamagnetic Fe3O4 nanoparticles with ionic liquids as stabilizer. Mater. Res. Bull. 2015, 62, 217–221. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Moreira, J.A.; Monteiro, F.J.; Laranjeira, M.S. Nanomaterials in cancer: Reviewing the combination of hyperthermia and triggered chemotherapy. J. Control. Release 2022, 347, 89–103. [Google Scholar] [CrossRef]
- Laranjeira, M.S.; Ribeiro, T.P.; Magalhães, A.I.; Silva, P.C.; Santos, J.A.; Monteiro, F.J. Magnetic mesoporous silica nanoparticles as a theranostic approach for breast cancer: Loading and release of the poorly soluble drug exemestane. Int. J. Pharm. 2022, 619, 121711. [Google Scholar] [CrossRef]
- Silva, L.H.; Silva, S.M.; Lima, E.C.; Silva, R.C.; Weiss, D.J.; Morales, M.M.; Cruz, F.F.; Rocco, P.R. Effects of static magnetic fields on natural or magnetized mesenchymal stromal cells: Repercussions for magnetic targeting. Nanomed. Nanotech. Biol. Med. 2018, 14, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, Y.; Zhu, C.; Zhang, W.; Mao, Z.; Gao, C. Fe3O4/BSA particles induce osteogenic differentiation of mesenchymal stem cells under static magnetic field. Acta Biomater. 2016, 46, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Turlej, E.; Kornicka-Garbowska, K.; Zachanowicz, E.; Tomaszewska, A.; Kulpa-Greszta, M.; Pązik, R. Co0.5Mn0.5Fe2O4@PMMA Nanoparticles Promotes Preosteoblast Differentiation through Activation of OPN-BGLAP2-DMP1 Axis and Modulates Osteoclastogenesis under Magnetic Field Conditions. Materials 2021, 14, 5010. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Rojas, J.M.; Sanz-Ortega, L.; Portilla, Y.; Pérez-Yagüe, S.; Barber, D.F. Polyethylenimine-coated superparamagnetic iron oxide nanoparticles impair in vitro and in vivo angiogenesis. Nanomed. Nanotech. Biol. Med. 2019, 21, 102063. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Garot, C.; Bettega, G.; Picart, C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv. Funct. Mater. 2020, 31, 2006967. [Google Scholar] [CrossRef]
- Bigham, A.; Aghajanian, A.H.; Behzadzadeh, S.; Sokhani, Z.; Shojaei, S.; Kaviani, Y.; Hassanzadeh-Tabrizi, S. Nanostructured magnetic Mg2SiO4-CoFe2O4 composite scaffold with multiple capabilities for bone tissue regeneration. Mater. Sci. Eng. C 2019, 99, 83–95. [Google Scholar] [CrossRef]
- Salmani, M.M.; Hashemian, M.; Khandan, A. Therapeutic effect of magnetic nanoparticles on calcium silicate bioceramic in alternating field for biomedical application. Ceram. Int. 2020, 46, 27299–27307. [Google Scholar] [CrossRef]
- Ge, J.; Zhai, M.; Zhang, Y.; Bian, J.; Wu, J. Biocompatible Fe3O4/chitosan scaffolds with high magnetism. Int. J. Biol. Macromol. 2019, 128, 406–413. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Xu, J.; Wang, J.; Gu, X.; Li, P.; Fan, Y. Three-dimensional magnetic fibrous scaffold with icariin expanded by supercritical CO2 for bone tissue engineering under static magnetic field. Compos. Part B Eng. 2021, 226, 109304. [Google Scholar] [CrossRef]
- Mortimer, C.J.; Wright, C. The fabrication of iron oxide nanoparticle-nanofiber composites by electrospinning and their applications in tissue engineering. Biotechnol. J. 2017, 12, 1600693. [Google Scholar] [CrossRef]
- Estévez, M.; Montalbano, G.; Gallo-Cordova, A.; Ovejero, J.G.; Izquierdo-Barba, I.; González, B.; Tomasina, C.; Moroni, L.; Vallet-Regí, M.; Vitale-Brovarone, C.; et al. Incorporation of Superparamagnetic Iron Oxide Nanoparticles into Collagen Formulation for 3D Electrospun Scaffolds. Nanomaterials 2022, 12, 181. [Google Scholar] [CrossRef]
- Makridis, A.; Kazeli, K.; Kyriazopoulos, P.; Maniotis, N.; Samaras, T.; Angelakeris, M. An accurate standardization protocol for heating efficiency determination of 3D printed magnetic bone scaffolds. J. Phys. D Appl. Phys. 2022, 55, 435002. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Khan, R.H.; Suman, R. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma 2019, 11, S118–S124. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Desando, G.; Cavallo, C.; Bartolotti, I.; Shelyakova, T.; Goranov, V.; Brucale, M.; Dediu, V.A.; Fini, M.; et al. Multifunctional 3D-Printed Magnetic Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 3825. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, A.; Gloria, A.; D’Amora, U.; Russo, T.; Panseri, S.; Sandri, M.; Tampieri, A.; Marcacci, M.; Dediu, V.A.; et al. Towards the Design of 3D Fiber-Deposited Poly(-caprolactone)/Iron-Doped Hydroxyapatite Nanocomposite Magnetic Scaffolds for Bone Regeneration. J. Biomed. Nanotechnol. 2015, 11, 1236–1246. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Wang, H.-T.; Wu, T.-L.; Fan, K.-H.; Huang, H.-M.; Chang, W.-J. Fabrication of Fe3 O4/PLLA composites for use in bone tissue engineering. Polym. Compos. 2016, 38, 2881–2888. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Men, Y.; Wang, R.; No, Y.; Zreiqat, H. Personalized 3D printed bone scaffolds: A review. Acta Biomater. 2023, 156, 110–124. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef]
- Rezania, N.; Asadi-Eydivand, M.; Abolfathi, N.; Bonakdar, S.; Mehrjoo, M.; Solati-Hashjin, M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J. Mater. Sci. Mater. Med. 2022, 33, 31. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C 2018, 98, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Paun, I.A.; Calin, B.S.; Mustaciosu, C.C.; Mihailescu, M.; Moldovan, A.; Crisan, O.; Leca, A.; Luculescu, C.R. 3D Superparamagnetic Scaffolds for Bone Mineralization under Static Magnetic Field Stimulation. Materials 2019, 12, 2834. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-M.; Ahn, S.-J.; Park, K.-R.; Kim, M.-J.; Kim, J.-J.; Jin, G.-Z.; Kim, H.-W.; Kim, E.-C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Filippi, M.; Dasen, B.; Guerrero, J.; Garello, F.; Isu, G.; Born, G.; Ehrbar, M.; Martin, I.; Scherberich, A. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials 2019, 223, 119468. [Google Scholar] [CrossRef]
- Hussain, M.; Rizvi, S.A.; Abbas, N.; Sajjad, U.; Shad, M.; Badshah, M.; Malik, A. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Huang, Z.; He, Y.; Chang, X.; Liu, J.; Yu, L.; Wu, Y.; Li, Y.; Tian, J.; Kang, L.; Wu, D.; et al. A Magnetic Iron Oxide/Polydopamine Coating Can Improve Osteogenesis of 3D-Printed Porous Titanium Scaffolds with a Static Magnetic Field by Upregulating the TGF β -Smads Pathway. Adv. Health Mater. 2020, 9, e2000318. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, Z.; Ma, B.; Yu, L.; He, Y.; Xu, D.; Wu, Y.; Wang, H.; Qiu, G. Enhanced in vitro biocompatibility and osteogenesis of titanium substrates immobilized with dopamine-assisted superparamagnetic Fe 3 O 4 nanoparticles for hBMSCs. R. Soc. Open Sci. 2018, 5, 172033. [Google Scholar] [CrossRef]
- Dittler, M.L.; Zelís, P.M.; Beltrán, A.M.; Destch, R.; Grillo, A.C.; Gonzalez, M.C.; Boccaccini, A.R. Magnetic 3D scaffolds for tissue engineering applications: Bioactive glass (45S5) coated with iron-loaded hydroxyapatite nanoparticles. Biomed. Mater. 2021, 16, 055006. [Google Scholar] [CrossRef]
- Paun, I.A.; Popescu, R.C.; Calin, B.S.; Mustaciosu, C.C.; Dinescu, M.; Luculescu, C.R. 3D Biomimetic Magnetic Structures for Static Magnetic Field Stimulation of Osteogenesis. Int. J. Mol. Sci. 2018, 19, 495. [Google Scholar] [CrossRef]
- Tang, B.; Zhuang, J.; Wang, L.; Zhang, B.; Lin, S.; Jia, F.; Dong, L.; Wang, Q.; Cheng, K.; Weng, W.-J. Harnessing Cell Dynamic Responses on Magnetoelectric Nanocomposite Films to Promote Osteogenic Differentiation. ACS Appl. Mater. Interfaces 2018, 10, 7841–7851. [Google Scholar] [CrossRef]
- Tang, B.; Shen, X.; Yang, Y.; Xu, Z.; Yi, J.; Yao, Y.; Cao, M.; Zhang, Y.; Xia, H. Enhanced cellular osteogenic differentiation on CoFe2O4/P(VDF-TrFE) nanocomposite coatings under static magnetic field. Colloids Surfaces B Biointerfaces 2020, 198, 111473. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. A review of techniques for the application of bioactive coatings on metal-based implants to achieve controlled release of active ingredients. Mater. Des. 2022, 217, 110653. [Google Scholar] [CrossRef]
- Zhuang, J.; Lin, S.; Dong, L.; Cheng, K.; Weng, W. Magnetically actuated mechanical stimuli on Fe3O4/mineralized collagen coatings to enhance osteogenic differentiation of the MC3T3-E1 cells. Acta Biomater. 2018, 71, 49–60. [Google Scholar] [CrossRef]
- Lin, S.; Li, J.; Dong, L.; Cheng, K.; Lin, J.; Weng, W. Periodic-Mechanical-Stimulus Enhanced Osteogenic Differentiation of Mesenchymal Stem Cells on Fe3O4/Mineralized Collagen Coatings. ACS Biomater. Sci. Eng. 2019, 5, 6446–6453. [Google Scholar] [CrossRef]
- Lin, S.; Li, J.; Shao, J.; Zhang, J.; He, X.; Huang, D.; Dong, L.; Lin, J.; Weng, W.; Cheng, K. Anisotropic magneto-mechanical stimulation on collagen coatings to accelerate osteogenesis. Colloids Surfaces B Biointerfaces 2021, 210, 112227. [Google Scholar] [CrossRef]
- Zhuang, J.; Lin, J.; Li, J.; Weng, W.; Cheng, K.; Wang, H. Alternating potentials assisted electrochemical deposition of mineralized collagen coatings. Colloids Surfaces B Biointerfaces 2015, 136, 479–487. [Google Scholar] [CrossRef]
- Zhuang, J.; Lin, S.; Dong, L.; Cheng, K.; Weng, W. Magnetically Assisted Electrodeposition of Aligned Collagen Coatings. ACS Biomater. Sci. Eng. 2018, 4, 1528–1535. [Google Scholar] [CrossRef]
- Li, K.; Yan, T.; Xue, Y.; Guo, L.; Zhang, L.; Han, Y. Intrinsically ferromagnetic Fe-doped TiO2 coatings on titanium for accelerating osteoblast response in vitro. J. Mater. Chem. B 2018, 6, 5756–5767. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloy. Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Labusca, L.; Danceanu, C.; Minuti, A.E.; Herea, D.-D.; Ghemes, A.; Rotarescu, C.; Dragos-Pinzaru, O.; Tibu, M.; Marian, G.; Chiriac, H.; et al. Magnetic nanowires substrate increases adipose-derived mesenchymal cells osteogenesis. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Del Bianco, L.; Spizzo, F.; Yang, Y.; Greco, G.; Gatto, M.L.; Barucca, G.; Pugno, N.M.; Motta, A. Silk fibroin films with embedded magnetic nanoparticles: Evaluation of the magneto-mechanical stimulation effect on osteogenic differentiation of stem cells. Nanoscale 2022, 14, 14558–14574. [Google Scholar] [CrossRef] [PubMed]
- Honecker, D.; Bersweiler, M.; Erokhin, S.; Berkov, D.; Chesnel, K.; Venero, D.A.; Qdemat, A.; Disch, S.; Jochum, J.K.; Michels, A.; et al. Using small-angle scattering to guide functional magnetic nanoparticle design. Nanoscale Adv. 2022, 4, 1026–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lv, Y.; Wang, Y.; Kong, D.; Xia, J.; Li, J.; Zhou, Q. Tumor Acidic Microenvironment-Responsive Promodulator Iron Oxide Nanoparticles for Photothermal-Enhanced Chemodynamic Immunotherapy of Cancer. ACS Biomater. Sci. Eng. 2023, 9, 773–783. [Google Scholar] [CrossRef]


| Type of Magnetic Field | Type of Cell | Results | Results In Vivo | Mechanism | Reference | ||
|---|---|---|---|---|---|---|---|
| Static | Pulsed | Intensity | |||||
| X | __ | 500 nT 0.2 T 16 T | MC3T3-E1 | Concentration of minerals increase alongside the strength of SMF; higher degree of differentiation | _____ | Osteoblasts in differentiation accumulated more mineral elements than non-differentiated cell cultures | [18] |
| X | __ | 16T | MC3T3-E1 | Iron accumulation inside the cells | _____ | Expression of transferrin receptor one and ferroportin 1; associated with increased ALP activity and mineralization | [14] |
| X | __ | 4 mT | ___ | _____ | Prevent bone deterioration in diabetic mice | Treatment led to high levels of osteocalcin; increased number of osteoblasts; upregulation of BMP2 and Runx2 genes | [21] |
| X | __ | 60 uT 120 uT | HUVECs | 40% increase in proliferation | _____ | Endothelial cells’ functionality increased with the application of SMF, which upregulated eNOS expression | [22] |
| __ | X | ___ | ___ | Increase in endothelial cells in the metaphysis of long bones | Prevent bone loss in a mouse model of postmenopausal osteoporosis | PEMF-induced osteogenesis and expansion of types H vessels may be mediated by HIF-1α signaling in these endothelial cells | [23] |
| Material | Methods | Type of Magnetic Field | Type of Cell | Results | Results In Vivo | Mechanism | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Static | Other | Intensity | |||||||
| Iron oxide nanoparticles | Coprecipitation | X | ___ | 0.3–0.45 T | Stem cells | Temporary decrease in cell proliferation and viability | _____ | Iron in magnetized MSCs aggravated the loss of viability | [42] |
| Fe3O4/BSA nanoparticles (200 nm) | Desolvation | X | ___ | 1 T | Stem cells | Increase in the differentiation of stem cells into osteogenic cells | _____ | Higher level of nanoparticle internalization; proliferation decreased, probably due to cell differentiation | [43] |
| Co0.5Mn0.5Fe2O4@PMMA | Microwave-driven non-hydrolytic approach | X | ___ | 0.2 T | Pre-existing bone cells | Restore the balance of osteoblasts and osteoclasts activity in the condition of osteoporosis | _____ | Stimulated integrins, improving preosteoblast activity and inhibiting osteoclasts | [44] |
| Gelatinous sponges with SPIONs | Coprecipitation | __ | X | 1.5 T 7 T | ___ | _____ | Increased bone density and trabecular volume; new bone formation and blood vessel formation in the sockets of rats | SPIONs are taken up by osteoblasts and vascular endothelial cells, leading to improved bone formation and blood vessel formation | [45] |
| PEI-coated SPIONs | Coprecipitation | __ | __ | ___ | HUVECs | Negatively affected the functionality of primary HUVECs; decreased blood vessel number at tumor sites | _____ | SPIONs lead the cells to produce more reactive oxygen species, disrupting the cells’ actin cytoskeleton activity | [46] |
| Material | Methods | Type of Magnetic Field | Type of Cell | Results | Results In Vivo | Mechanism | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Static | Other | Intensity | |||||||
| Calcium phosphate scaffold with IONPs | Compression | X | __ | 35 mT | hDPSCs | Formation of bone enhanced by 22.2% | _____ | Probably due to physical forces generated by the magnetic field and the presence of magnetic nanoparticles within the scaffold | [63] |
| PCL fiber scaffold with IONPs | Depressurization of subcritical CO2 fluid | X | ___ | 15 mT | MC3T3-E1 | Cell growth | _____ | Great cell penetration, collagen deposition and angiogenesis | [52] |
| Polymeric scaffold with IONPs | Two-photon polymerization | X | ___ | 1.3 T | MG-63 | Increased mineralization up to 50% | Faster bone regeneration in initial animal tests | SMFs promote cell attachment and the early-stage mineralization of nanoparticle-free osteoblast-like cells | [64] |
| PCL-MNP scaffold | Space holder | X | ___ | 15 mT | HUVECs | Tubular cell formation | _____ | Increase expression of key angiogenic genes | [65] |
| Material | Methods | Type of Magnetic Field | Type of Cell | Results | Results In Vivo | Mechanism | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Static | Other | Intensity | |||||||
| Chit/Col matrix with Hap and IONPs | Spin coating | X | __ | 110 mT 180 mT 250 mT | MG-63 | Enhanced cell differentiation and tissue mineralization Reduced cell proliferation | _____ | Probably due to deformations of the structure caused by the SMF, providing strain stimulation to the cells | [71] |
| Collagen with IONPs | Electron capture dissociation (AP-ECD) | X | ___ | 100 mT | MC3T3-E1 | Enhanced osteogenic differentiation | _____ | Deformation of the coating under SMF provides mechanical stimulation to the cells | [75] |
| IONPs and Polydopamine (PDA) | Electron capture dissociation (AP-ECD) | X | ___ | 15 mT | hBMSCs | Enhanced proliferation and osteogenic differentiation | Increased mineralization and new bone formation | Upregulation of osteogenic factors and TGFβ-Smads signaling pathway | [76] |
| Collagen with IONPs | Electrodeposition | ___ | X | 1500 and 2800 Oe | MSCs | Enhanced cell adhesion, proliferation, and differentiation | _____ | Upregulation of integrin β1 and of YAP/TAZ transcription factors | [79] |
| Nickel nanowires | Electrodeposition | ___ | X | 4 mT | ASCs | Osteogenic conversion of the ASC | _____ | Topography of the coating and MF caused tensile and shear forces on the cell membrane | [82] |
| Collagen with IONPs | Eletrochemical deposition | X | ___ | 280 and 300 mT (in vitro and in vivo, respectively) | BMSCs | Upregulation of differentiation markers | Increased bone formation Enhanced angiogenesis | Direction of the SMF parallel to the collagen alignment in the coating promoted directional mechanical stimulation. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.P.; Flores, M.; Madureira, S.; Zanotto, F.; Monteiro, F.J.; Laranjeira, M.S. Magnetic Bone Tissue Engineering: Reviewing the Effects of Magnetic Stimulation on Bone Regeneration and Angiogenesis. Pharmaceutics 2023, 15, 1045. https://doi.org/10.3390/pharmaceutics15041045
Ribeiro TP, Flores M, Madureira S, Zanotto F, Monteiro FJ, Laranjeira MS. Magnetic Bone Tissue Engineering: Reviewing the Effects of Magnetic Stimulation on Bone Regeneration and Angiogenesis. Pharmaceutics. 2023; 15(4):1045. https://doi.org/10.3390/pharmaceutics15041045
Chicago/Turabian StyleRibeiro, Tiago P., Miguel Flores, Sara Madureira, Francesca Zanotto, Fernando J. Monteiro, and Marta S. Laranjeira. 2023. "Magnetic Bone Tissue Engineering: Reviewing the Effects of Magnetic Stimulation on Bone Regeneration and Angiogenesis" Pharmaceutics 15, no. 4: 1045. https://doi.org/10.3390/pharmaceutics15041045
APA StyleRibeiro, T. P., Flores, M., Madureira, S., Zanotto, F., Monteiro, F. J., & Laranjeira, M. S. (2023). Magnetic Bone Tissue Engineering: Reviewing the Effects of Magnetic Stimulation on Bone Regeneration and Angiogenesis. Pharmaceutics, 15(4), 1045. https://doi.org/10.3390/pharmaceutics15041045