Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review
Abstract
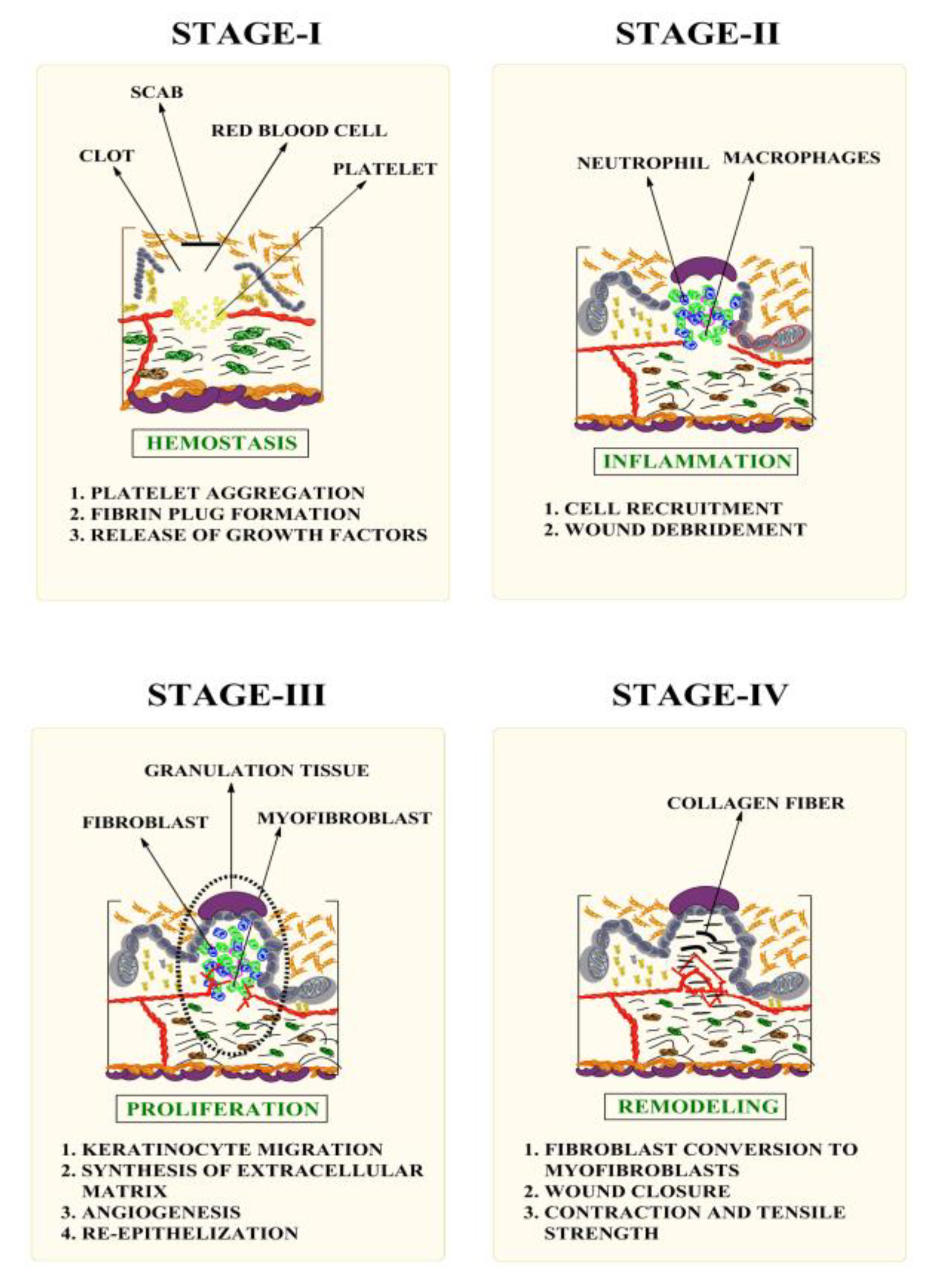
1. Introduction
- a.
- Substantial erosion of the epithelium, including hair, skin, and related glands;
- b.
- Infection of the wound may potentially result in the loss of integument [18];
- c.
- Underlying bacterial growth is present in wounds; hence, if the host is incapable of inhibiting the development of bacteria, the wound would lead to develop an infection [19].
2. Fabrication of Nanofibrous Meshes
3. Phytoconstituent-Loaded Nanofibrous Meshes
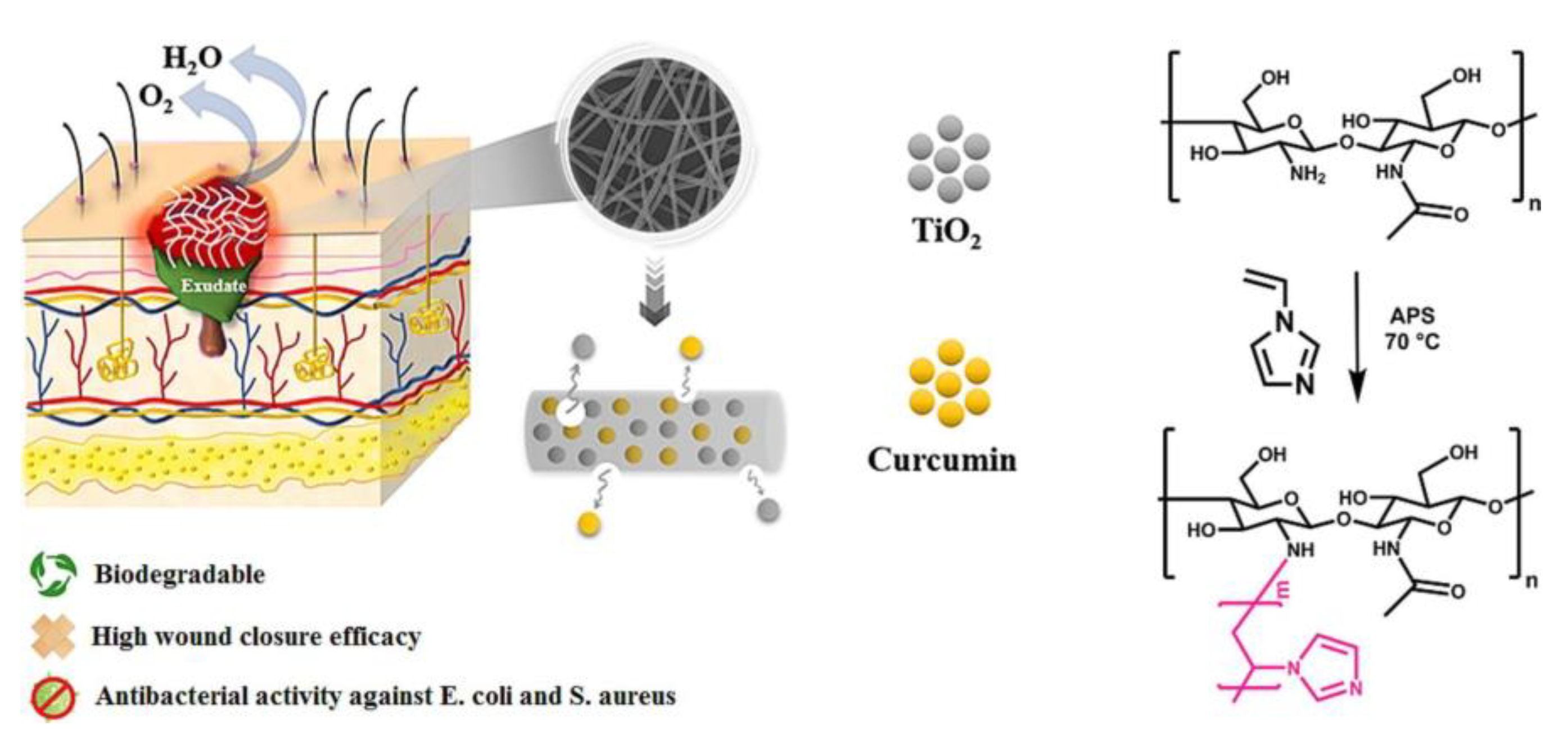
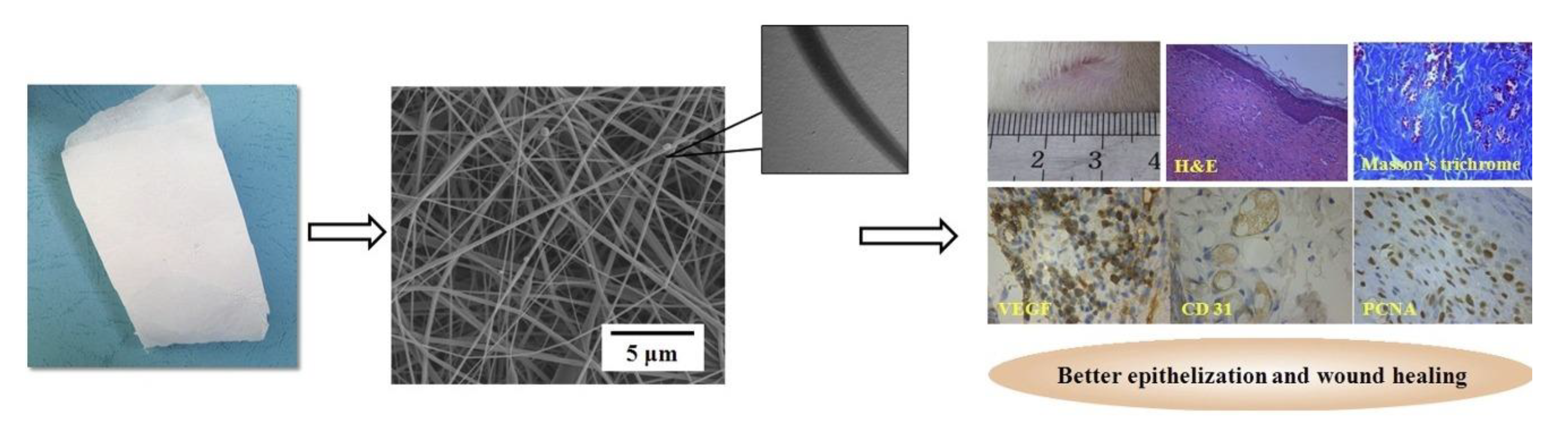
3.1. Curcumin-Loaded Platforms
3.2. Aloe Vera-Incorporated Nanoplatforms
3.3. Centella asiatica Derived Systems
3.4. Azadirachta Indica-Incorporated Wound Dressings
3.5. Tecomella undulata Extract-Based Systems for Enhanced Wound Healing
| Phytoconstituent | Biopolymer | Formulation | Method of Fabrication | Properties of the Prepared Formulation | References |
|---|---|---|---|---|---|
| Curcumin | Gelatin | Indomethacin/gelatin/curcumin nanofibers | Electrospinning | Antioxidant, anti-inflammatory, analgesic properties | [77] |
| Collagen | Curumin-loaded chitosan/poly(ethylene oxide)/collagen (Cho/PEO/Col) nanofibers | Blend–electrospinning process | Anti-inflammatory and anti-infective properties | [78] | |
| Chitosan | Chitosan/curcumin@β-cyclodextrin/silver nanoparticles (CS/Cur@β-CD/AgNPs) | Electrospinning | Antibacterial and antiscarring properties. | [79] | |
| Alginate | Alginate/gelatin sponge combined with curcumin-loaded electrospun fibers (CFAGS) | Electrospinning and interpenetrating polymer network (IPN) strategy | Antibacterial properties | [80] | |
| Cellulose | Graphene oxide/TiO2/curcumin-incorporated cellulose acetate (CA) nanofiber | Electrospinning | Biocompatibility and antimicrobial | [81] | |
| Hyaluronic acid | Bioinspired hyaluronic acid blends immobilized with 3, 4-difluorobenzylidene curcumin (CDF) non-woven nanofiber mats | Electrospinning | Antimicrobial, antibacterial, and anticancer properties | [82] | |
| Aloevera | Gelatin | Hybrid nanofibers fabricated from gelatin/aloevera/poly(ε-caprolactone | Double nozzle electrospinning | Antibacterial activity | [83] |
| Collagen | Zein/Polycaprolactone/Collagen nanofibers | Electrospinning | Anti-infective properties | [84] | |
| Chitosan | Polycaprolactone/chitosan/aloe vera nanofiber membranes | Sloping free surface electrospinning | Antibacterial activity | [85] | |
| Alginate | Aloevera and aqueous leaf extracts of Moringa oleifera calcium alginate-PEG-methyl ether methacrylate (PEGMA) scaffolds | Electrospinning | Anti-inflammatory properties and antimicrobial activity | [86] | |
| Cellulose | Tetracycline hydrochloride (TCH) -loaded poly(caprolactone)/gelatin/aloe vera nanofibers | Electrospinning | Antibacterial activity | [66] | |
| Hyaluronic acid | Ethylcellulose/hydroxypropyl methylcellulosenanofiber mats | Electrospinning | Antibacterial properties | [87] | |
| Centella asiatica | Gelatin | Gelatin/chitosan/nonwoven fabric composite hydrogel wound dressing | Antibacterial activity | [88] | |
| Collagen | Centella asiatica/silver nanoparticle (CA-AgNPs) -loaded Poly caprolactone/ polyethylene oxidePCL/PEO hybrid nanofibers | Mutual electrospinning method | Antimicrobial activity | [69] | |
| Chitosan | Double-layered Polycaprolactone/Poly(vinyl alcohol)_Chitosan-Sodium tripolyphosphate_Centella asiatica (PCL/PVA_CS-TPP_CA) bionanocomposite dressing material | Electrospinning | Anti-inflammatory and antibacterial properties | [89] | |
| Gelatin/collagen/cellulose | Asiatic acid | Electrospinning | Antimicrobial | [90] | |
| Cellulose | Cellulose acetate/centella asiaticananofibers | Electrospinning | Antibacterial activity | [91] |
4. Natural Polymer-Based Nanofibrous Matrices
4.1. Gelatin
4.2. Collagen
4.3. Chitosan
4.4. Alginate
4.5. Cellulose
4.6. Hyaluronic Acid
5. Patent Overview
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.; Marimuthu, T.; Makatini, M.M.; Choonara, Y.E. Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair. Polymers 2022, 14, 5371. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Qiao, L.; Chen, Z.; Liu, B.; Gao, L.; Zhang, L. Wearable Sensor for Continuous Sweat Biomarker Monitoring. Chemosensors 2022, 10, 273. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.T.; Bruschi, M.L. 3D Printing as a Technological Strategy for the Personalized Treatment of Wound Healing. AAPS PharmSciTech 2023, 24, 41. [Google Scholar] [CrossRef]
- Gamble, N. Management of Perioperative Medical Emergencies. In Fundamentals of Operating Department Practice, 2nd ed.; Rodger, D., Henshaw, K., Rawling, P., Miller, S., Eds.; Cambridge University Press: Cambridge, UK, 2022; pp. 143–163. [Google Scholar] [CrossRef]
- Kumar, R.; Keshamma, E.; Kumari, B.; Kumar, A.; Kumar, V.; Janjua, D.; Billah, A.M. Burn Injury Management, Pathophysiology and Its Future Prospectives. J. Res. Appl. Sci. Biotechnol. 2022, 1, 78–89. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Ornitz, D.A.-O.; Itoh, N. New developments in the biology of fibroblast growth factors. WIREs Mech. Dis. 2022, 14, e1549. [Google Scholar] [CrossRef]
- Pognan, F.; Beilmann, M.; Boonen, H.C.M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; et al. The evolving role of investigative toxicology in the pharmaceutical industry. Nat. Rev. Drug Discov. 2023, 1–19. [Google Scholar] [CrossRef]
- Tucker, D. Clinical Evaluation of a Synthetic Hybrid-Scale Matrix in the Treatment of Lower Extremity Surgical Wounds. Cureus 2022, 14, e27452. [Google Scholar] [CrossRef]
- Madaan, R.; Singla, K.R.; Kumar, S.; Dubey, K.A.; Kumar, D.; Sharma, P.; Bala, R.; Singla, S.; Shen, B. Bergenin—A Biologically Active Scaffold: Nanotechnological Perspectives. Curr. Top. Med. Chem. 2022, 22, 132–149. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Chopra, H.; Gandhi, S.; Gautam, K.R.; Kamal, A.M. Bacterial Nanocellulose based Wound Dressings: Current and Future Prospects. Curr. Pharm. Des. 2022, 28, 570–580. [Google Scholar] [CrossRef]
- Dhingra, A.G.; Kaur, M.; Singh, M.; Aggarwal, G.; Nagpal, M. Lock Stock and Barrel of Wound Healing. Curr. Pharm. Des. 2019, 25, 4090–4107. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Liang, Z.-J.; Zhao, B.-Y.; Shi, X.-W.; Zhang, T.; Liu, H.; Yu, X.-H. Delayed diagnosis and management of necrotizing fasciitis of the left lower leg: A case report. Medicine 2022, 101, e31231. [Google Scholar] [CrossRef]
- Qiao, X.; Zhang, W.; Zhao, W. Role of CXCL10 in Spinal Cord Injury. Int. J. Med. Sci. 2022, 19, 2058–2070. [Google Scholar] [CrossRef]
- Walker, P.G.; Niehaus, A.J. Integumentary System. In Medicine and Surgery of Camelids; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 232–302. [Google Scholar] [CrossRef]
- Jari Litany, R.I.; Praseetha, P.K. Tiny tots for a big-league in wound repair: Tools for tissue regeneration by nanotechniques of today. J.Control. Release 2022, 349, 443–459. [Google Scholar] [CrossRef]
- Spinnato, P.; Patel, D.B.; Di Carlo, M.; Bartoloni, A.; Cevolani, L.; Matcuk, G.R.; Crombé, A. Imaging of Musculoskeletal Soft-Tissue Infections in Clinical Practice: A Comprehensive Updated Review. Microorganisms 2022, 10, 2329. [Google Scholar] [CrossRef]
- Durand, B.A.R.N.; Pouget, C.; Magnan, C.; Molle, V.; Lavigne, J.-P.; Dunyach-Remy, C. Bacterial Interactions in the Context of Chronic Wound Biofilm: A Review. Microorganisms 2022, 10, 1500. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of Electrospun Drug-Eluting Nanofibers in Wound Healing: Current and Future Perspectives. Polymers 2022, 14, 2931. [Google Scholar] [CrossRef]
- Haidari, H.; Melguizo-Rodríguez, L.; Cowin, A.J.; Kopecki, Z. Therapeutic potential of antimicrobial peptides for treatment of wound infection. Am. J. Physiol.-Cell Physiol. 2023, 324, C29–C38. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, Y.; Zhang, J.; Zhang, H.; Guo, B. Structural and Functional Design of Electrospun Nanofibers for Hemostasis and Wound Healing. Adv. Fiber Mater. 2022, 4, 1027–1057. [Google Scholar] [CrossRef]
- Shaw, P.; Vanraes, P.; Kumar, N.; Bogaerts, A. Possible Synergies of Nanomaterial-Assisted Tissue Regeneration in Plasma Medicine: Mechanisms and Safety Concerns. Nanomaterials 2022, 12, 3397. [Google Scholar] [CrossRef] [PubMed]
- Kostovich, C.T.; Etingen, B.; Wirth, M.; Patrianakos, J.; Kartje, R.; Baharestani, M.; Weaver, F.M. Outcomes of Telehealth for Wound Care: A Scoping Review. Adv. Skin Wound Care 2022, 35, 394–403. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 74, 103564. [Google Scholar] [CrossRef]
- Shi, W.; Song, N.; Huang, Y.; He, C.; Zhang, M.; Zhao, W.; Zhao, C. Improved Cooling Performance of Hydrogel Wound Dressings via Integrating Thermal Conductivity and Heat Storage Capacity for Burn Therapy. Biomacromolecules 2022, 23, 889–902. [Google Scholar] [CrossRef]
- Sethuram, L.; Thomas, J. Therapeutic applications of electrospun nanofibers impregnated with various biological macromolecules for effective wound healing strategy—A review. Biomed. Pharmacother. 2023, 157, 113996. [Google Scholar] [CrossRef]
- Fan, C.; Xu, Q.; Hao, R.; Wang, C.; Que, Y.; Chen, Y.; Yang, C.; Chang, J. Multi-functional wound dressings based on silicate bioactive materials. Biomaterials 2022, 287, 121652. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2021, 14, 1. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A.M. Emerging treatment strategies in wound care. Int. Wound J. 2022, 19, 1934–1954. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Chidambara Murthy, K.N. Challenges in Healing Wound: Role of Complementary and Alternative Medicine. Front. Nutr. 2022, 8, 791899. [Google Scholar] [CrossRef]
- Merzougui, C.; Miao, F.; Liao, Z.; Wang, L.; Wei, Y.; Huang, D. Electrospun nanofibers with antibacterial properties for wound dressings. J. Biomater. Sci. Polym. Ed. 2022, 33, 2165–2183. [Google Scholar] [CrossRef]
- Priya, S.; Batra, U.; Samshritha, R.N.; Sharma, S.; Chaurasiya, A.; Singhvi, G. Polysaccharide-based nanofibers for pharmaceutical and biomedical applications: A review. Int. J. Biol. Macromol. 2022, 218, 209–224. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Palekar-Shanbhag, P.; Dalal, A.; Navale, T.; Mishra, U. Electrospun Chitosan Nanofibres and its Application. Curr. Drug Ther. 2022, 17, 318–326. [Google Scholar] [CrossRef]
- Niu, Y.-Q.; Liu, J.-H.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C.-H. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef]
- Pattnaik, S.; Swain, K.; Ramakrishna, S. Optimal delivery of poorly soluble drugs using electrospun nanofiber technology: Challenges, state of the art, and future directions. WIREs Nanomed. Nanobiotechnol. 2022, 15, e1859. [Google Scholar] [CrossRef]
- Vasudevan, A.; Tripathi, D.M.; Sundarrajan, S.; Venugopal, J.R.; Ramakrishna, S.; Kaur, S. Evolution of Electrospinning in Liver Tissue Engineering. Biomimetics 2022, 7, 149. [Google Scholar] [CrossRef]
- Acosta, M.; Santiago, M.D.; Irvin, J.A. Electrospun Conducting Polymers: Approaches and Applications. Materials 2022, 15, 8820. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Teoh, X.Y.; Le Hwang, J.; Khong, Z.P.; Sejare, R.; Almashhadani, A.Q.; Assi, R.A.; Chan, S.Y. Electrospinning and its potential in fabricating pharmaceutical dosage form. J. Drug Deliv. Sci. Technol. 2022, 76, 103761. [Google Scholar] [CrossRef]
- Zhi, C.; Shi, S.; Si, Y.; Fei, B.; Huang, H.; Hu, J. Recent Progress of Wearable Piezoelectric Pressure Sensors Based on Nanofibers, Yarns, and Their Fabrics via Electrospinning. Adv. Mater. Technol. 2022, 8, 2201161. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Ghaferi, A.A. Hybrid nanofibers opportunities and frontiers—A review. J. Environ.Chem. Eng. 2022, 10, 108850. [Google Scholar] [CrossRef]
- Vasconcelos, F.; Reis, R.L.; Martins, A.; Neves, N.M. Biomedical Applications of Fibers Produced by Electrospinning, Microfluidic Spinning and Combinations of Both. In Electrospun Nanofibers: Principles, Technology and Novel Applications; Vaseashta, A., Bölgen, N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 251–295. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Dushenkov, S.; Ho, C.-T. Modulation of Inflammatory Genes by Natural Dietary Bioactive Compounds. J. Agric. Food Chem. 2009, 57, 4467–4477. [Google Scholar] [CrossRef]
- Puri, V.; Kanojia, N.; Sharma, A.; Huanbutta, K.; Dheer, D.; Sangnim, T. Natural product-based pharmacological studies for neurological disorders. Front. Pharmacol. 2022, 13, 4675. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, S.; Mathan, S.V.; Tomar, M.S.; Singh, R.K.; Verma, P.K.; Kumar, A.; Kumar, S.; Singh, R.P.; Acharya, A. Therapeutic application of Carica papaya leaf extract in the management of human diseases. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2020, 28, 735–744. [Google Scholar] [CrossRef]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Bahú, J.O.; Melo de Andrade, L.R.; Crivellin, S.; Khouri, N.G.; Sousa, S.O.; Fernandes, L.M.I.; Souza, S.D.A.; Concha, L.S.C.; Schiavon, M.I.R.B.; Benites, C.I.; et al. Rotary Jet Spinning (RJS): A Key Process to Produce Biopolymeric Wound Dressings. Pharmaceutics 2022, 14, 2500. [Google Scholar] [CrossRef]
- Lv, Y.; Yu, Z.; Li, C.; Zhou, J.; Lv, X.; Chen, J.; Wei, M.; Liu, J.; Yu, X.; Wang, C.; et al. Gelatin-based nanofiber membranes loaded with curcumin and borneol as a sustainable wound dressing. Int. J. Biol. Macromol. 2022, 219, 1227–1236. [Google Scholar] [CrossRef]
- Motasadizadeh, H.; Azizi, S.; Shaabani, A.; Sarvestani, M.G.; Sedghi, R.; Dinarvand, R. Development of PVA/Chitosan-g-Poly (N-vinyl imidazole)/TiO2/curcumin nanofibers as high-performance wound dressing. Carbohydr. Polym. 2022, 296, 119956. [Google Scholar] [CrossRef]
- Alabdali, A.Y.M.; Khalid, R.; Kzar, M.; Ezzat, M.O.; Huei, G.M.; Hsia, T.W.; Mogana, R.; Rahman, H.; Razik, B.M.A.; Issac, P.K.; et al. Design, synthesis, in silico and antibacterial evaluation of curcumin derivatives loaded nanofiber as potential wound healing agents. J.King Saud Univ. Sci. 2022, 34, 102205. [Google Scholar] [CrossRef]
- Jose, J.; Pai, A.R.; Gopakumar, D.A.; Dalvi, Y.; Ruby, V.; Bhat, S.G.; Pasquini, D.; Kalarikkal, N.; Thomas, S. Novel 3D porous aerogels engineered at nano scale from cellulose nano fibers and curcumin: An effective treatment for chronic wounds. Carbohydr. Polym. 2022, 287, 119338. [Google Scholar] [CrossRef]
- Agnes Mary, S.; Giri Dev, V.R. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J. Text. Inst. 2015, 106, 886–895. [Google Scholar] [CrossRef]
- Agnes Mary, S.; Giri Dev, V.R. In vivo bioactivity of herbal-drug-incorporated nanofibrous matrixes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.; Marques, A.P.; Caridade, S.G.; Teixeira, P.; Sousa, C.; Mano, J.F.; Reis, R.L. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M. Characteristics of aloe vera incorporated poly(ε-caprolactone)/gum tragacanth nanofibers as dressings for wound care. J. Ind. Text. 2017, 47, 1464–1477. [Google Scholar] [CrossRef]
- Suganya, S.; Venugopal, J.; Agnes Mary, S.; Ramakrishna, S.; Lakshmi, B.S.; Giri Dev, V.R. Aloe vera incorporated biomimetic nanofibrous scaffold: A regenerative approach for skin tissue engineering. Iran. Polym. J. 2014, 23, 237–248. [Google Scholar] [CrossRef]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe Vera Hydrogel Films for Biomedical Applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Pandimadevi, M.; Menda, J.P.; Reddy, T.; Deepika, R.; Sastry, T.P. Preparation and Characterization of Wound Healing Composites of Chitosan, Aloe Vera and Calendula officinalis—A Comparative Study. Am.J.Phytomed. Clin. Ther. 2014, 2, 61–76. [Google Scholar]
- Thompson, Z.; Rahman, S.; Yarmolenko, S.; Sankar, J.; Kumar, D.; Bhattarai, N. Fabrication and Characterization of Magnesium Ferrite-Based PCL/Aloe Vera Nanofibers. Materials 2017, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Baghersad, S.; Hivechi, A.; Bahrami, S.H.; Brouki Milan, P.; Siegel, R.A.; Amoupour, M. Optimal Aloe vera encapsulated PCL/Gel nanofiber design for skin substitute application and the evaluation of its in vivo implantation. J. Drug Deliv. Sci. Technol. 2022, 74, 103536. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Niu, Z.; Zhong, K.; Liu, T.; Yang, M.; Ji, L.; Hu, W. A review of pharmacokinetic and pharmacological properties of asiaticoside, a major active constituent of Centella asiatica (L.) Urb. J. Ethnopharmacol. 2023, 302, 115865. [Google Scholar] [CrossRef] [PubMed]
- Sofi, H.S.; Rashid, R.; Amna, T.; Hamid, R.; Sheikh, F.A. Recent advances in formulating electrospun nanofiber membranes: Delivering active phytoconstituents. J. Drug Deliv. Sci. Technol. 2020, 60, 102038. [Google Scholar] [CrossRef]
- Bozkaya, O.; Arat, E.; Gün Gök, Z.; Yiğitoğlu, M.; Vargel, I. Production and characterization of hybrid nanofiber wound dressing containing Centella asiatica coated silver nanoparticles by mutual electrospinning method. Eur. Polym. J. 2022, 166, 111023. [Google Scholar] [CrossRef]
- Guo, J.; Wang, T.; Yan, Z.; Ji, D.; Li, J.; Pan, H. Preparation and evaluation of dual drug-loaded nanofiber membranes based on coaxial electrostatic spinning technology. Int. J. Pharm. 2022, 629, 122410. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Du, L.; Jin, Y. Preparation of asiaticoside-loaded coaxially electrospinning nanofibers and their effect on deep partial-thickness burn injury. Biomed. Pharmacother. 2016, 83, 33–40. [Google Scholar] [CrossRef]
- Shrivastava, P.; Lakshmi, D.G.; Sri, S.; Chauhan, D.; Solanki, P. Potential of electrospun cellulose acetate nanofiber mat integrated with silver nanoparticles from Azadirachta indica as antimicrobial agent. J. Polym. Res. 2020, 27, 1–11. [Google Scholar]
- Rajora, A.D.; Bal, T. Evaluating neem gum-polyvinyl alcohol (NGP-PVA) blend nanofiber mat as a novel platform for wound healing in murine model. Int. J. Biol. Macromol. 2023, 226, 760–771. [Google Scholar] [CrossRef]
- Suganya, S.; Senthil Ram, T.; Lakshmi, B.S.; Giridev, V.R. Herbal drug incorporated antibacterial nanofibrous mat fabricated by electrospinning: An excellent matrix for wound dressings. J. Appl. Polym. Sci. 2011, 121, 2893–2899. [Google Scholar] [CrossRef]
- Chellamani, K.; Veerasubramanian, D.; Sudharsan, J. Wound Healing Ability of Herbal Drug Incorporated PCL (Poly(ε-caprolactone)) Wound Dressing. JAIR 2014, 2, 622–626. [Google Scholar]
- Assar, D.H.; Elhabashi, N.; Mokhbatly, A.-A.A.; Ragab, A.E.; Elbialy, Z.I.; Rizk, S.A.; Albalawi, A.E.; Althobaiti, N.A.; Al Jaouni, S.; Atiba, A. Wound healing potential of licorice extract in rat model: Antioxidants, histopathological, immunohistochemical and gene expression evidences. Biomed. Pharmacother. 2021, 143, 112151. [Google Scholar] [CrossRef]
- Gulsun, T.; Inal, M.; Akdag, Y.; Izat, N.; Oner, L.; Sahin, S. The development and characterization of electrospun gelatin nanofibers containing indomethacin and curcumin for accelerated wound healing. J. Drug Deliv. Sci. Technol. 2022, 67, 103000. [Google Scholar] [CrossRef]
- Jirofti, N.; Golandi, M.; Movaffagh, J.; Ahmadi, F.S.; Kalalinia, F. Improvement of the Wound-Healing Process by Curcumin-Loaded Chitosan/Collagen Blend Electrospun Nanofibers: In Vitro and In Vivo Studies. ACS Biomater. Sci. Eng. 2021, 7, 3886–3897. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Lun, X.; Sheng, H.; Yan, A. Effects of wound dressing based on the combination of silver@curcumin nanoparticles and electrospun chitosan nanofibers on wound healing. Bioengineered 2022, 13, 4328–4339. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Yan, Z.; Li, Y.; Ji, D.; Yun, K.; Su, Y.; Liu, D.; Pan, W. A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int. J. Biol. Macromol. 2021, 182, 1339–1350. [Google Scholar] [CrossRef]
- Prakash, J.; Venkataprasanna, K.S.; Bharath, G.; Banat, F.; Niranjan, R.; Venkatasubbu, G.D. In-vitro evaluation of electrospun cellulose acetate nanofiber containing Graphene oxide/TiO2/Curcumin for wound healing application. Coll. Surf. A Physicochem. Eng. Asp. 2021, 627, 127166. [Google Scholar] [CrossRef]
- Patel, P.R.; Singam, A.; Iyer, A.K.; Gundloori, R.V.N. Bioinspired hyaluronic acid based nanofibers immobilized with 3, 4- difluorobenzylidene curcumin for treating bacterial infections. J. Drug Deliv. Sci. Technol. 2022, 74, 103480. [Google Scholar] [CrossRef]
- Baghersad, S.; Hajir Bahrami, S.; Mohammadi, M.R.; Mojtahedi, M.R.M.; Milan, P.B. Development of biodegradable electrospun gelatin/aloe-vera/poly(ε-caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Mater. Sci. Eng. C 2018, 93, 367–379. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xu, L. Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int. J. Biol. Macromol. 2020, 160, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Elizalde, I.; Bernáldez-Sarabia, J.; Moreno-Ulloa, A.; Vilanova, C.; Juárez, P.; Licea-Navarro, A.; Castro-Ceseña, A.B. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr. Polym. 2019, 206, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Mohebian, Z.; Tajmohammadi, I.; Yavari Maroufi, L.; Ramezani, S.; Ghorbani, M. A Novel Aloe Vera-Loaded Ethylcellulose/Hydroxypropyl Methylcellulose Nanofibrous Mat Designed for Wound Healing Application. J. Polym. Environ. 2022, 30, 867–877. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Shen, Y.; Liu, F.; Zhou, Y.; Wu, H.; Liu, Q.; Deng, B. Preparation of Centella asiatica loaded gelatin/chitosan/nonwoven fabric composite hydrogel wound dressing with antibacterial property. Int. J. Biol. Macromol. 2021, 192, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Mouro, C.; Fangueiro, R.; Gouveia, I.C. Preparation and Characterization of Electrospun Double-layered Nanocomposites Membranes as a Carrier for Centella asiatica (L.). Polymers 2020, 12, 2653. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Barnthip, N.; Muakngam, A. Preparation of Cellulose Acetate Nanofibers Containing Centella Asiatica Extract by Electrospinning Process as the Prototype of Wound-Healing Materials. J. Bionano. Sci. 2014, 8, 313–318. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, A.; Puri, V.; Singh, I. Preparation and characterization of biocomposite films of carrageenan/locust bean gum/montmorrillonite for transdermal delivery of curcumin. BioImpacts 2019, 9, 37–43. [Google Scholar] [CrossRef]
- Sharma, A.; Puri, V.; Kakkar, V.; Singh, I. Formulation and Evaluation of Silymarin-Loaded Chitosan-Montmorilloite Microbeads for the Potential Treatment of Gastric Ulcers. J. Funct. Biomater. 2018, 9, 52. [Google Scholar] [CrossRef]
- Sharma, A.; Puri, V.; Kumar, P.; Singh, I.; Huanbutta, K. Development and Evaluation of Rifampicin Loaded Alginate–Gelatin Biocomposite Microfibers. Polymers 2021, 13, 1514. [Google Scholar] [CrossRef]
- Sharma, A.; Puri, V.; Kumar, P.; Singh, I. Rifampicin-Loaded Alginate-Gelatin Fibers Incorporated within Transdermal Films as a Fiber-in-Film System for Wound Healing Applications. Membranes 2021, 11, 7. [Google Scholar] [CrossRef]
- Sharma, A.; Puri, V.; Kumar, P.; Singh, I. Biopolymeric, Nanopatterned, Fibrous Carriers for Wound Healing Applications. Curr. Pharm. Des. 2020, 26, 4894–4908. [Google Scholar] [CrossRef]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y.; Gao, J.; Wang, D.; Xia, D.; Liang, C.; Li, N.; Xu, R. Synergistic Effect of Co-Delivering Ciprofloxacin and Tetracycline Hydrochloride for Promoted Wound Healing by Utilizing Coaxial PCL/Gelatin Nanofiber Membrane. Int. J. Mol. Sci. 2022, 23, 1895. [Google Scholar] [CrossRef]
- Khandaker, M.; Alkadhem, N.; Progri, H.; Nikfarjam, S.; Jeon, J.; Kotturi, H.; Vaughan, M.B. Glutathione Immobilized Polycaprolactone Nanofiber Mesh as a Dermal Drug Delivery Mechanism for Wound Healing in a Diabetic Patient. Process 2022, 10, 512. [Google Scholar] [CrossRef]
- Xiang, J.; Zhou, L.; Xie, Y.; Zhu, Y.; Xiao, L.; Chen, Y.; Zhou, W.; Chen, D.; Wang, M.; Cai, L.; et al. Mesh-like electrospun membrane loaded with atorvastatin facilitates cutaneous wound healing by promoting the paracrine function of mesenchymal stem cells. Stem Cell Res. Ther. 2022, 13, 190. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, I.; Dheer, D.; Nagpal, M.; Kumar, P.; Venkatesh, D.N.; Puri, V.; Singh, I. A propitious role of marine sourced polysaccharides: Drug delivery and biomedical applications. Carbohydr. Polym. 2023, 308, 120448. [Google Scholar] [CrossRef]
- Dheer, D.; Arora, D.; Jaglan, S.; Rawal, R.K.; Shankar, R. Polysaccharides based nanomaterials for targeted anti-cancer drug delivery. J. Drug Target. 2017, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Sun, H.; Jiang, Z.; Wang, S.; Liu, C.; Zou, M.; Ju, R.; Feng, Z.; Liu, W.; Wang, T.; et al. Study on repair of abdominal wall defect rats with hernia mesh coated with chitosan-based photosensitive hydrogel. Carbohydr. Polym. 2022, 291, 119577. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Z.; Liu, J.; Dong, P.; Tian, F.; Li, F.; Meng, X. Electrospun kaolin-loaded chitosan/PEO nanofibers for rapid hemostasis and accelerated wound healing. Int. J. Biol. Macromol. 2022, 217, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mubarak, N.M.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A Critical Review on the Synthesis of Natural Sodium Alginate Based Composite Materials: An Innovative Biological Polymer for Biomedical Delivery Applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential Food and Nutraceutical Applications of Alginate: A Review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef]
- Lu, H.; Butler, J.A.; Britten, N.S.; Venkatraman, P.D.; Rahatekar, S.S. Natural Antimicrobial Nano Composite Fibres Manufactured from a Combination of Alginate and Oregano Essential Oil. Nanomaterials 2021, 11, 2062. [Google Scholar] [CrossRef]
- Olmos-Juste, R.; Olza, S.; Gabilondo, N.; Eceiza, A. Tailor-Made 3D Printed Meshes of Alginate-Waterborne Polyurethane as Suitable Implants for Hernia Repair. Macromol. Biosci. 2022, 22, 2200124. [Google Scholar] [CrossRef]
- Rostamitabar, M.; Ghahramani, A.; Seide, G.; Jockenhoevel, S.; Ghazanfari, S. Drug loaded cellulose–chitosan aerogel microfibers for wound dressing applications. Cellulose 2022, 29, 6261–6281. [Google Scholar] [CrossRef]
- Panthi, K.P.; Gyawali, A.; Pandeya, S.; Sharma Bhusal, M.L.; Neupane, B.B.; Tiwari, A.P.; Joshi, M.K. The Encapsulation of Bioactive Plant Extracts into the Cellulose Microfiber Isolated from G. optiva Species for Biomedical Applications. Membranes 2022, 12, 1089. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Raei, M.; Hushmandi, K.; Zarrabi, A.; Voelcker, N.H.; Aref, A.R.; et al. Hyaluronic acid-based nanoplatforms for Doxorubicin: A review of stimuli-responsive carriers, co-delivery and resistance suppression. Carbohydr. Polym. 2021, 272, 118491. [Google Scholar] [CrossRef]
- Dheer, D.; Gupta, R.; Singh, D.; Magotra, A.; Singh, G.; Gupta, P.N.; Shankar, R. Hyaluronic Acid-Tacrolimus Bioconjugate: Synthesis, Characterization, and Pharmacokinetic Investigation of an Acid-Responsive Macromolecular Prodrug. ACS Appl. Bio. Mater. 2019, 2, 4728–4736. [Google Scholar] [CrossRef]
- Gruppuso, M.; Iorio, F.; Turco, G.; Marsich, E.; Porrelli, D. Hyaluronic acid/lactose-modified chitosan electrospun wound dressings—Crosslinking and stability criticalities. Carbohydr. Polym. 2022, 288, 119375. [Google Scholar] [CrossRef]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Ghilan, A.; Rusu, D.; Simionescu, N.; Tartau, L.M. Nanostructured hyaluronic acid-based hydrogels encapsulating synthetic/ natural hybrid nanogels as promising wound dressings. Biochem. Eng. J. 2022, 179, 108341. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, B.; Tian, D.; Liu, J.; Yu, A.; Wan, Y. Thiolated hyaluronic acid/silk fibroin dual-network hydrogel incorporated with bioglass nanoparticles for wound healing. Carbohydr. Polym. 2022, 288, 119334. [Google Scholar] [CrossRef]
- Jianfeng, L.; Mengya, D.; Jia, M.; Jianyong, L.; Zesheng, H.; Sixian, P.; Yongqi, Z. Medical Dressing Based on Humifuse Euphorbia Herb Extract and Preparation Method Thereof. China Patent CN114904041A, 16 August 2022. [Google Scholar]
- Rongmei, Z.; Junmin, W.; Hui, W.; Feng, L.; Bin, W.; Zhiqin, P. Preparation Method of Wound Dressing Based on Shaddock Peel Pectin-Oxidized Chitosan Composite Hydrogel. China Patent CN114712553A, 8 July 2022. [Google Scholar]
- Yuping, W.; Hongwei, Z.; Xinbiao, W.; Guoliang, X.; Guohua, Q.; Meng, L. Wood Pulp/Polyester Composite Wiping Material Having Sandwich Structure. WO Patent WO2022078081A1, 21 April 2022. [Google Scholar]
- Guoliang, Z.; Zhenrui, H. Collagen Freeze-Dried Fiber and Preparation Method and Application Thereof. China Patent CN114632022A, 17 June 2022. [Google Scholar]
- Lin, L. Non-Woven Hemostatic Gauze and Preparation Method and Application Thereof. China Patent CN114748670A, 15 July 2022. [Google Scholar]
- Zhongli, L.; Xinyi, L.; Zhaoxu, L.; Yuan, W.; Ruyue, L.; Yu, Z. Nano Short Peptide R-LIFE-1 and Application Thereof in Medicines, Medical Cosmetology and Biomedicine. China Patent CN114989249A, 2 September 2022. [Google Scholar]
- Abudula, T.; Gauthaman, K.; Alshahrie, A.; Salah, N.; Memic, A. Biodegradable Core-Shell Fibrous Scaffolds for Controlled Oxygen and Drug Release. U.S. Patent 11124897B1, 21 September 2021. [Google Scholar]
- Datt, R.; Kumar, R.; Pandey, S.; Shrivastava, P. Multifunctional Formulation Composed of Natural Ingredients and Its Preparation/Manufacturing Method. European Patent ES2885052T3, 16 June 2021. [Google Scholar]
- Mirzaei, E.; Majidi, R.F.; Sarkar, S.; Rezayat, S.M. Electro Spun Nanofibrous Wound Dressing and a Method of Synthesizing the Same. U.S. Patent 9101508B2, 11 August 2015. [Google Scholar]
- Pillay, V.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Mayet, N. Wound Dressing. U.S. Patent 10080816, 25 September 2018. [Google Scholar]



| Sr. No. | Patent Details | Biopolymer Used | Goal | Reference |
|---|---|---|---|---|
| 1. | CN114904041A (2022) | Sodium alginate | Biodegradable fiber-based medical dressing formulated, incorporating sodium alginate with Humifuse euphoria extract | [120] |
| 2. | CN114712553A (2022) | Pectin and chitosan | Biopolymer-based composite hydrogel has resulted in excellent wound-healing rate, especially in terms of it surface area | [121] |
| 3. | WO2022078081A1 (2022) | Chitosan and alginic acid | Treatment of wood pulp fiber with biopolymers led to the development of composite wear-resistant material | [122] |
| 4. | CN114632022A (2022) | Collagen | Recombinant human type III collagen-related freeze-dried fiber showed increased water retention and improved tissue construct | [123] |
| 5. | CN114748670A China (2022) | Sodium alginate | Polyester fibers (non-woven gauze) and sodium alginate (hemostatic material) are adhered on the substrate exhibiting stable action and better wound-healing effect | [124] |
| 6. | CN114989249A (2022) | Hyaluronic acid | The short peptide was self-assembled into nanofibers for wound repair dressing in ultraviolet rays’ skin injury | [125] |
| 7. | US11124897B1 (2021) | Chitin and lignin | Polycaprolactone-coated biopolymer-based nanofiber scaffolds loaded with antimicrobial agent promoted chronic wound-healing property | [126] |
| 8. | ES2885052T3 (2021) | Curcuma longa, Emblicaofficinalis, and Camellia sinensis extracts | Physicochemical treatment of natural compounds in the form of fibers/gel without using polymer, observing multiple-use bandage for wound-healing application | [127] |
| 9. | US9101508B2 (2015) | Chitosan | Sandwich nanolayers have been constructed into fibrous sheets using biopolymer with Melilotusofficinalis for wound dressing | [128] |
| 10. | US10080816B2 (2018) | Lyophilized hyaluronic acid (HA) hydrogel | Stimuli-responsive wound dressing containing a lyophilized polymeric (hyaluronic acid) hydrogel and several devices inserted in it, each gadget can create biofilms or electrospun fiber mats from chitosan and hypromellose | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Dheer, D.; Singh, I.; Puri, V.; Kumar, P. Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review. Pharmaceutics 2023, 15, 1058. https://doi.org/10.3390/pharmaceutics15041058
Sharma A, Dheer D, Singh I, Puri V, Kumar P. Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review. Pharmaceutics. 2023; 15(4):1058. https://doi.org/10.3390/pharmaceutics15041058
Chicago/Turabian StyleSharma, Ameya, Divya Dheer, Inderbir Singh, Vivek Puri, and Pradeep Kumar. 2023. "Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review" Pharmaceutics 15, no. 4: 1058. https://doi.org/10.3390/pharmaceutics15041058
APA StyleSharma, A., Dheer, D., Singh, I., Puri, V., & Kumar, P. (2023). Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review. Pharmaceutics, 15(4), 1058. https://doi.org/10.3390/pharmaceutics15041058






