Confocal Raman Spectroscopy for Assessing Bioequivalence of Topical Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. HPLC Analysis
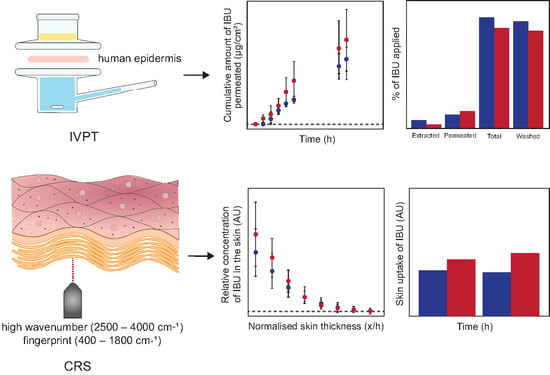
2.2.2. IVPT and Mass Balance Studies
2.2.3. Confocal Raman Spectroscopy In Vivo
2.2.4. Data Analysis
3. Results and Discussion
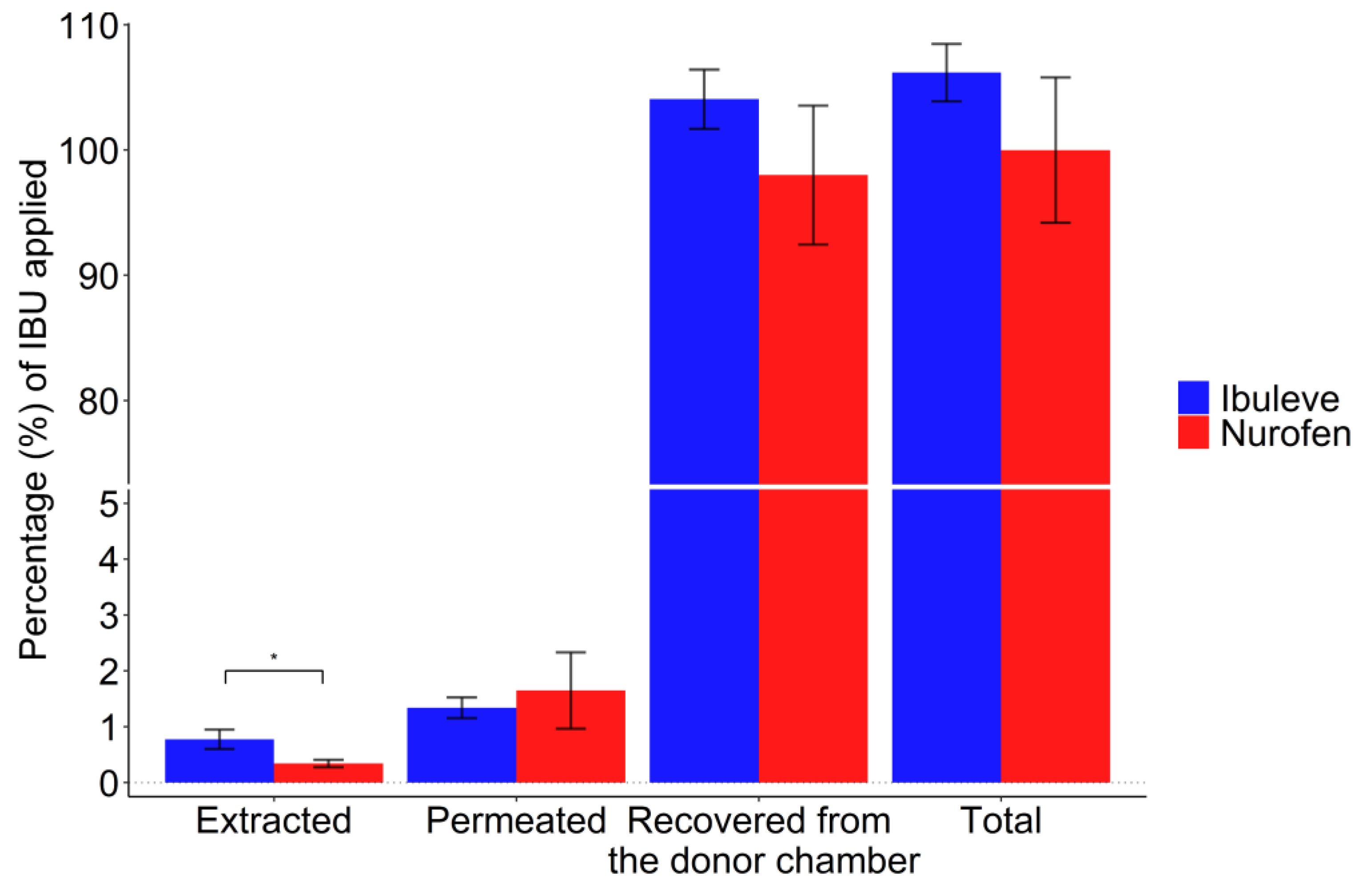
3.1. IVPT and Mass Balance Studies
3.2. Confocal Raman Spectroscopy In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raney, S.G.; Luke, M.C. A new paradigm for topical generic drug products: Impact on therapeutic access. J. Am. Acad. Dermatol. 2020, 82, 1570–1571. [Google Scholar] [CrossRef]
- U.S. Department of Health & Human Services. CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=320&showFR=1 (accessed on 14 February 2023).
- Grosser, S.; Park, M.; Raney, S.G.; Rantou, E. Determining Equivalence for Generic Locally Acting Drug Products. Stat. Biopharm. Res. 2015, 7, 337–345. [Google Scholar] [CrossRef]
- Narkar, Y. Bioequivalence for Topical Products—An Update. Pharm. Res. 2010, 27, 2590–2601. [Google Scholar] [CrossRef]
- Shah, V.P.; Flynn, G.L.; Yacobi, A.; Maibach, H.I.; Bon, C.; Fleischer, N.M.; Franz, T.J.; Kaplan, S.A.; Kawamoto, J.; Lesko, L.J.; et al. Bioequivalence of Topical Dermatological Dosage Forms–Methods of Evaluation of Bioequivalence. Ski. Pharmacol. Physiol. 1998, 11, 117–124. [Google Scholar] [CrossRef]
- Franz, T.J.; Lehman, P.A.; Raney, S.G. Use of Excised Human Skin to Assess the Bioequivalence of Topical Products. Ski. Pharmacol. Physiol. 2009, 22, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Krishnaiah, Y.S.R.; Xu, X.; Rahman, Z.; Yang, Y.; Katragadda, U.; Lionberger, R.; Peters, J.R.; Uhl, K.; Khan, M.A. Development of performance matrix for generic product equivalence of acyclovir topical creams. Int. J. Pharm. 2014, 475, 110–122. [Google Scholar] [CrossRef]
- Lehman, P.A.; Raney, S.G.; Franz, T.J. Percutaneous Absorption in Man: In vitro–in vivo Correlation. Ski. Pharmacol. Physiol. 2011, 24, 224–230. [Google Scholar] [CrossRef]
- Skelly, J.P.; Shah, V.P.; Maibach, H.I. FDA and AAPS report of the workshop on principles and practices of in vitro percutaneous penetration studies: Relevance to bioavailability and bioequivalence. Pharm. Res. 1987, 4, 265–267. [Google Scholar] [CrossRef]
- Shin, S.H.; Rantou, E.; Raney, S.G.; Ghosh, P.; Hassan, H.; Stinchcomb, A. Cutaneous Pharmacokinetics of Acyclovir Cream 5% Products: Evaluating Bioequivalence with an In Vitro Permeation Test and an Adaptation of Scaled Average Bioequivalence. Pharm. Res. 2020, 37, 210. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Draft Guidance on Acyclovir. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_018604.pdf (accessed on 15 February 2023).
- Ghosh, P.; Raney, S.G.; Luke, M.C. Evaluation of cutaneous pharmacokinetics: The past, the present and the future. In Proceedings of the Visualizing and Quantifying Drug Distribution in Tissue V, Online, 6–11 March 2021; p. 1162405. [Google Scholar]
- Yacobi, A.; Shah, V.P.; Bashaw, E.D.; Benfeldt, E.; Davit, B.; Ganes, D.; Ghosh, T.; Kanfer, I.; Kasting, G.B.; Katz, L.; et al. Current Challenges in Bioequivalence, Quality, and Novel Assessment Technologies for Topical Products. Pharm. Res. 2014, 31, 837–846. [Google Scholar] [CrossRef]
- Caspers, P.J.; Lucassen, G.W.; Bruining, H.A.; Puppels, G.J. Automated depth-scanning confocal Raman microspectrometer for rapid in vivo determination of water concentration profiles in human skin. J. Raman Spectrosc. 2000, 31, 813–818. [Google Scholar] [CrossRef]
- Caspers, P.J.; Lucassen, G.W.; Wolthuis, R.; Bruining, H.A.; Puppels, G.J. In vitro and in vivo Raman spectroscopy of human skin. Biospectroscopy 1998, 4, S31–S39. [Google Scholar] [CrossRef]
- Mateus, R.; Abdalghafor, H.; Oliveira, G.; Hadgraft, J.; Lane, M.E. A new paradigm in dermatopharmacokinetics–Confocal Raman Spectroscopy. Int. J. Pharm. 2013, 444, 106–108. [Google Scholar] [CrossRef]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In Vitro–In Vivo Correlation in Skin Permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef]
- Caspers, P.J.; Nico, C.; Bakker Schut, T.C.; de Sterke, J.; Pudney, P.D.; Curto, P.R.; Illand, A.; Puppels, G.J. Method to quantify the in vivo skin penetration of topically applied materials based on Confocal Raman Spectroscopy. Transl. Biophotonics 2019, 1, e201900004. [Google Scholar] [CrossRef] [Green Version]
- Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. Franz Cell Diffusion Testing and Quantitative Confocal Raman Spectroscopy: In Vitro–In Vivo Correlation. Pharmaceutics 2020, 12, 887. [Google Scholar] [CrossRef]
- Patel, A.; Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. In Vitro–In Vivo Correlation in Dermal Delivery: The Role of Excipients. Pharmaceutics 2021, 13, 542. [Google Scholar] [CrossRef]
- Patel, A.; Bell, M.; O’Connor, C.; Inchley, A.; Wibawa, J.; Lane, M.E. Delivery of ibuprofen to the skin. Int. J. Pharm. 2013, 457, 9–13. [Google Scholar] [CrossRef]
- The Medicines and Healthcare Products Regulatory Agency. Public Assessment Report, UKPAR Ibuprofen 10% w/w Gel. Available online: https://mhraproducts4853.blob.core.windows.net/docs/170bdca5819db72058f65980fe718b14915b4693 (accessed on 25 July 2022).
- European Medicines Agency. List of Nationally Authorised Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/psusa/ibuprofen-ibuprofen-lysine-not-indicated-ductus-arteriosus-ibuprofen/caffeine-list-nationally-authorised-medicinal-products-psusa/00010649/202002_en.pdf (accessed on 25 July 2022).
- Compendium, E.M. Nurofen Maximum Strength 10% Gel. Available online: https://www.medicines.org.uk/emc/product/13002/smpc#gref (accessed on 14 February 2023).
- Compendium, E.M. Ibuleve Maximum Strength Gel 50 g. Available online: https://www.medicines.org.uk/emc/product/8532/smpc#gref (accessed on 14 February 2023).
- ICH Harmonised Tripartite. Validation of analytical procedures: Text and methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 6 November 2005. [Google Scholar]
- Organization for Economic Cooperation Development. Test No. 428: Skin Absorption: In Vitro Method; Section 4; OECD Publishing: Paris, France, 2004. [CrossRef]
- Organization for Economic Cooperation Development. OECD Guidance Document for the Conduct of Skin Absorption Studies; Organization for Economic Cooperation Development: Paris, France, 2004.
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The influence of volatile solvents on transport across model membranes and human skin. Int. J. Pharm. 2012, 435, 38–49. [Google Scholar] [CrossRef]
- Andersen, F.A. Final Report on the Safety Assessment of Oleth-2, -3, -4, -5, -6, -7, -8, -9, -10, -11, -12, -15, -16, -20, -23, -25, -30, -40, -44, and -501. Int. J. Toxicol. 1999, 18, 17–24. [Google Scholar] [CrossRef]
- Caspers, P.J.; Bruining, H.A.; Puppels, G.J.; Lucassen, G.W.; Carter, E.A. In Vivo Confocal Raman Microspectroscopy of the Skin: Noninvasive Determination of Molecular Concentration Profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Pradal, J. Comparison of skin permeation and putative anti-inflammatory activity of commercially available topical products containing ibuprofen and diclofenac. J. Pain Res. 2020, 13, 2805–2814. [Google Scholar] [CrossRef]
- UK Legislation. The Methylated Spirits Regulations 1987. Available online: https://www.legislation.gov.uk/uksi/1987/2009/body/made (accessed on 15 June 2022).
- Watkinson, R.M.; Herkenne, C.; Guy, R.H.; Hadgraft, J.; Oliveira, G.; Lane, M.E. Influence of Ethanol on the Solubility, Ionization and Permeation Characteristics of Ibuprofen in Silicone and Human Skin. Ski. Pharmacol. Physiol. 2009, 22, 15–21. [Google Scholar] [CrossRef]
- Goh, C.F.; Boyd, B.J.; Craig, D.Q.M.; Lane, M.E. Profiling of drug crystallization in the skin. Expert Opin. Drug Deliv. 2020, 17, 1321–1334. [Google Scholar] [CrossRef]
- Goh, C.F.; Moffat, J.G.; Craig, D.Q.M.; Hadgraft, J.; Lane, M.E. Monitoring Drug Crystallization in Percutaneous Penetration Using Localized Nanothermal Analysis and Photothermal Microspectroscopy. Mol. Pharm. 2019, 16, 359–370. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Drug crystallization–implications for topical and transdermal delivery. Expert Opin. Drug Deliv. 2016, 13, 817–830. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Goh, C.F.; Haque, T.; Rahma, A.; Lane, M.E. Dermal Delivery of Diclofenac Sodium—In Vitro and In Vivo Studies. Pharmaceutics 2022, 14, 2106. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. Novel use of Confocal Raman Spectroscopy for in vivo quantification of skin permeants in real time: A non-invasive method for assessing bioequivalence. In Proceedings of the Visualizing and Quantifying Drug Distribution in Tissue V, Online, 6–11 March 2021; p. 1162407. [Google Scholar]
- Mateus, R.; Moore, D.J.; Hadgraft, J.; Lane, M.E. Percutaneous absorption of salicylic acid–in vitro and in vivo studies. Int. J. Pharm. 2014, 475, 471–474. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use, European Medicines Agency. Guideline on the Investigation of Bioequivalence. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 25 July 2022).
- US Food and Drug Administration. FDA Guidance for Industry Statistical Approaches to Establishing Bioequivalence. Available online: https://www.fda.gov/media/70958/download (accessed on 14 October 2022).
- US Food and Drug Administration. Draft Guidance on Acyclovir. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Acyclovir_topical%20cream_RLD%2021478_RV12-16.pdf (accessed on 14 October 2022).
- Yang, Y.; Ako-Adounvo, A.-M.; Wang, J.; Coelho, S.G.; Adah, S.A.; Matta, M.K.; Strauss, D.; Michele, T.M.; Wang, J.; Faustino, P.J.; et al. In Vitro Testing of Sunscreens for Dermal Absorption: Method Comparison and Rank Order Correlation with In Vivo Absorption. AAPS PharmSciTech 2022, 23, 121. [Google Scholar] [CrossRef]
- Benfeldt, E.; Hansen, S.H.; Vølund, A.; Menné, T.; Shah, V.P. Bioequivalence of Topical Formulations in Humans: Evaluation by Dermal Microdialysis Sampling and the Dermatopharmacokinetic Method. J. Investig. Dermatol. 2007, 127, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Bodenlenz, M.; Augustin, T.; Birngruber, T.; Tiffner, K.I.; Boulgaropoulos, B.; Schwingenschuh, S.; Raney, S.G.; Rantou, E.; Sinner, F. Variability of Skin Pharmacokinetic Data: Insights from a Topical Bioequivalence Study Using Dermal Open Flow Microperfusion. Pharm. Res. 2020, 37, 204. [Google Scholar] [CrossRef]
- van de Sandt, J.J.M.; van Burgsteden, J.A.; Cage, S.; Carmichael, P.L.; Dick, I.; Kenyon, S.; Korinth, G.; Larese, F.; Limasset, J.C.; Maas, W.J.M.; et al. In vitro predictions of skin absorption of caffeine, testosterone, and benzoic acid: A multi-centre comparison study. Regul. Toxicol. Pharmacol. 2004, 39, 271–281. [Google Scholar] [CrossRef]




| Ibuleve Speed Relief Max Strength 10% Gel | Nurofen Max Strength 10% Gel |
|---|---|
| IBU | IBU |
| Industrial methylated spirit | Hydroxyethyl cellulose |
| Carbomers | Sodium hydroxide |
| Diethylamine | Benzyl alcohol |
| Water | Isopropyl alcohol |
| Water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliopoulos, F.; Tang, C.F.; Li, Z.; Rahma, A.; Lane, M.E. Confocal Raman Spectroscopy for Assessing Bioequivalence of Topical Formulations. Pharmaceutics 2023, 15, 1075. https://doi.org/10.3390/pharmaceutics15041075
Iliopoulos F, Tang CF, Li Z, Rahma A, Lane ME. Confocal Raman Spectroscopy for Assessing Bioequivalence of Topical Formulations. Pharmaceutics. 2023; 15(4):1075. https://doi.org/10.3390/pharmaceutics15041075
Chicago/Turabian StyleIliopoulos, Fotis, Chun Fung Tang, Ziyue Li, Annisa Rahma, and Majella E. Lane. 2023. "Confocal Raman Spectroscopy for Assessing Bioequivalence of Topical Formulations" Pharmaceutics 15, no. 4: 1075. https://doi.org/10.3390/pharmaceutics15041075
APA StyleIliopoulos, F., Tang, C. F., Li, Z., Rahma, A., & Lane, M. E. (2023). Confocal Raman Spectroscopy for Assessing Bioequivalence of Topical Formulations. Pharmaceutics, 15(4), 1075. https://doi.org/10.3390/pharmaceutics15041075