Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems
Abstract
:1. Introduction
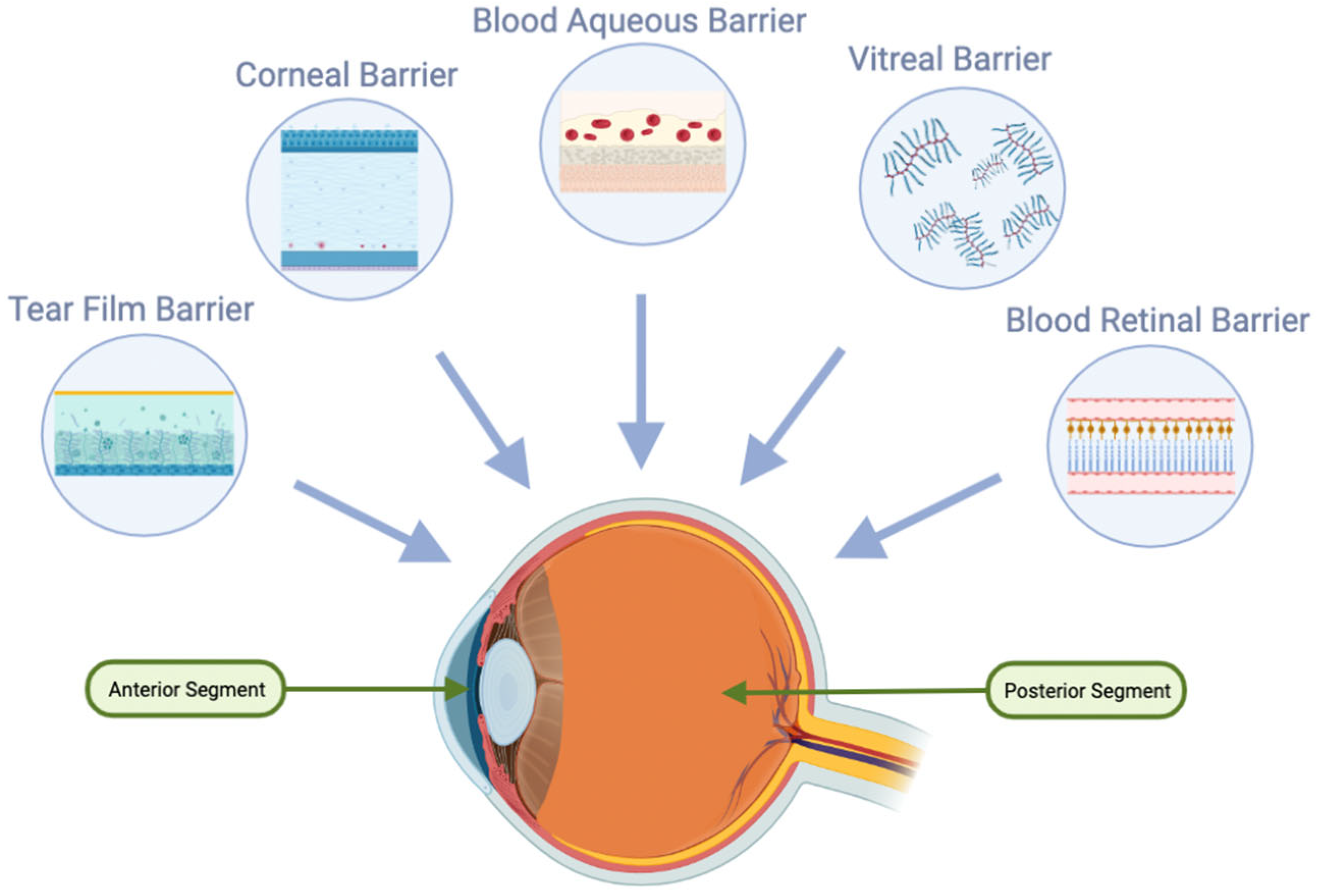
2. Anatomical Barriers in Ocular Drug Delivery
2.1. Tear Film in Ocular Drug Delivery
2.2. Nasolacrimal Drainage System in Ocular Drug Delivery
2.3. Cornea in Ocular Drug Delivery
2.4. Blood–Ocular Barrier (BOB)
2.4.1. Blood–Aqueous Barrier (BAB)
2.4.2. Blood–Retinal Barrier (BRB)
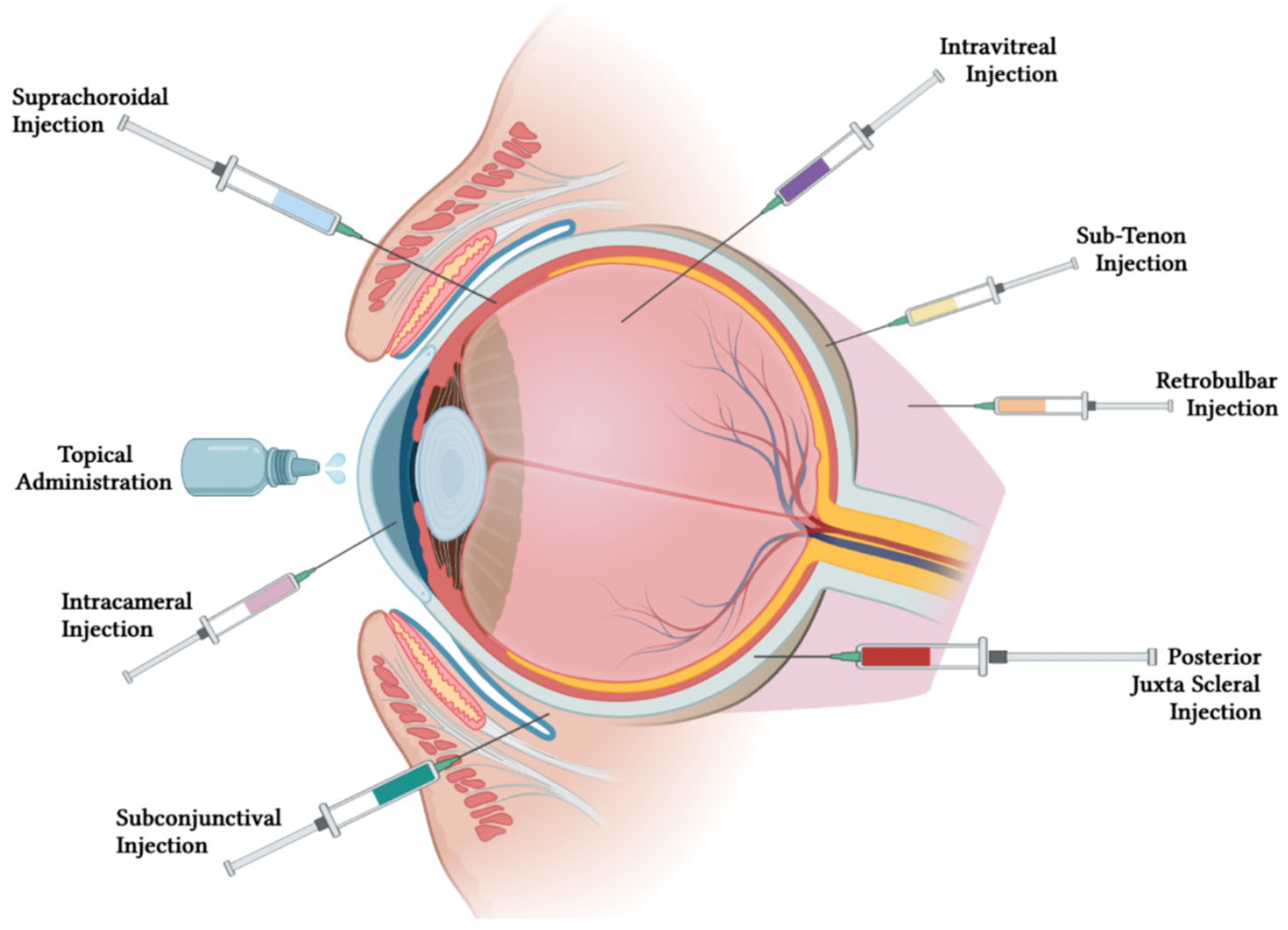
3. Routes of Administration for Treating Ocular Diseases
4. Overview of Biodegradable Nano-Based Drug Delivery System (DDS)
4.1. Biodegradable Nanocarriers for Improved Drug Delivery in Ocular Formulations
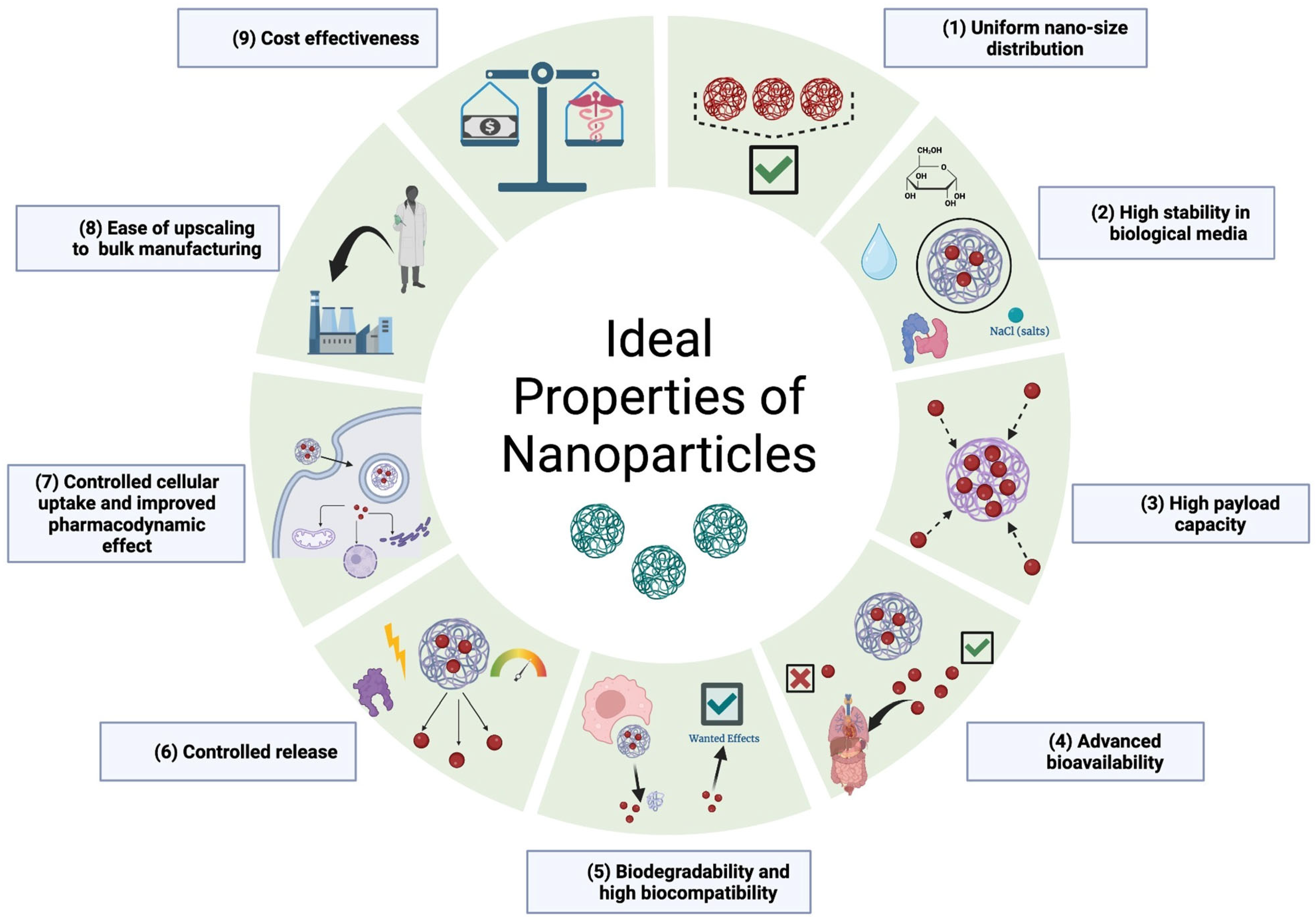
4.2. Ideal Properties of Nanocarriers
4.3. The Importance of Biodegradability and Morphology in Nano-Based DDS
4.4. The Different Biodegradable Polymers and Their Advantage
4.5. Types of Nano-Based Drug Delivery Systems: Characteristics and Enhancements
4.5.1. Nanomicelles
4.5.2. Liposomes
4.5.3. Dispersed Nanoparticles
4.5.4. Dendrimers
4.5.5. Hydrogels
4.5.6. Nanosuspensions and Nanoemulsions
4.5.7. Microneedles
5. Posterior Segment Diseases
5.1. Retinitis Pigmentosa
5.2. Age-Related Macular Degeneration and Choroidal Neovascularization
5.3. Diabetic Retinopathy
5.4. Diabetic Macular Edema (DME)
| Disease | DDS Technology | Drug | Advantages & Considerations | Administration Route | Stage | Reference |
|---|---|---|---|---|---|---|
| RP | Self-assembled PEG-based NPs | ML240 (VCP inhibitor) | Prolonged drug release Long-lasting neuroprotective effect | IVT injection | Preclinical: ex vivo, in vitro, in vivo | [74] |
| NPs | Myriocin | Effective level of myriocin at back of eye Decrease retinal sphingolipid levels | Topical | Preclinical | [75] | |
| PLGA Microspheres | GDNF and TUDCA | Sustained dual drug release Neuroprotective, cytoprotective effects Preliminary study, further confirmatory studies required | IVT injection | Preclinical: in vitro | [71] | |
| PVA/PVP/PG polymer | Progesterone | Good biocompatibility, controlled release Accumulates in sclera Delays photoreceptor cell death Further studies on therapeutic efficacy required | Ocular inserts | Preclinical: in vitro, ex vivo | [96] | |
| Wet AMD | PCL NPs | Resveratrol and Metformin | Enhanced retinal permeability Combined anti-inflammatory, antioxidant, and anti-angiogenic effects | IVT injection | Preclinical: in vitro and in vivo | [83] |
| CNV | MSC-transfected PLGA NPs | HIF-1α siRNA | Reduced HIF-1α activity in hypoxic environment Biomimetic delivery system Preliminary validation study | IVT injection | Preclinical: in vitro | [81] |
| Dry and wet-AMD | sHDL NPs | Rapamycin | High encapsulation efficiency Dual function of reducing cholesterol in tissue targeted Targeted anti-inflammatory effects on RPE | IVT injection | Preclinical: in vivo | [78] |
| Solid lipid NPs | Atorvastatin | Prolonged residence More bioavailability Improved stability | Topical | Preclinical | [97] | |
| Nanoceria | Glycol Chitosan | Decrease ROS-induced pro-angiogenic VEGF | IVT injection | Preclinical | [98] | |
| PAMAM-coated liposomes | BBH and Chrysophanol | Appreciable cellular permeability Improved BBH bioavailability | Topical | Preclinical | [54] | |
| Wet-AMD/ CNV | MRaNPs | Rapamycin | Biomimetic non-invasive DDS Improved accumulation in CNV lesions Anti-angiogenic, anti-inflammatory, enhanced autophagy effects | IVT injection | Preclinical: in vivo | [77] |
| Porous poly (PDMS) capsule | Ranibizumab | Sustained released for 16 weeks Reduced CNV area | Transscleral | Preclinical | [99] | |
| PDR/ Wet-AMD | NPs | Fenofibrate | Prolonged drug release Beneficial effect on neovascular AMD No toxicity detected | IVT injection | Preclinical | [100] |
| PDR | NPs coated with HA | Apatinib | Show size, Pdl and zeta potential High entrapment efficiency | Topical | Preclinical | [90] |
| Nanoparticles | Interleukin-12 | Sustained drug release Effective drug treatment Restore thickness | IVT injection | Preclinical | [85] | |
| NPs | Triamcinolone acetonide | Non-invasive delivery Improve structural and functional activity Reduce retinal inflammation and vascular abnormalities | Topical | Preclinical | [91] | |
| PLGA-PEG Lipid-polymer hybrid NPs | Melatonin | Confirmed in-vitro antioxidant and neuroprotective effectiveness Confirmed ocular tolerability, no cytotoxicity in vivo | Topical | Preclinical: in vitro and in vivo | [86] | |
| Nanostructured lipid carriers | Diosmin | Cytoprotective, anti-inflammatory effects Confirmed ocular tolerability and safety in vitro Preliminary study, studies regarding therapeutic efficacy further required | Topical | Preclinical: in vitro | [87] | |
| Wet-AMD/CNV/DME/ PDR | Chitosan coated liposomes | Triamcinolone Acetonide | Increased bioavailability Sustained drug release Improved biodegradability and mucoadhesion | Topical | Preclinical: in vivo | [96] |
| DME | Liposomes | Triamcinolone Aceonide | Sustained release Confirmed safety, tolerability, and therapeutic activity in humans Further long-term safety and therapeutic efficacy clinical studies required | Topical | Clinical: in vivo, Phase 1 clinical assay | [93] |
6. Anti-VEGF Agents
6.1. Novel DDS for Anti-VEGF Agents
6.1.1. Hydrogel
6.1.2. Polymers Nanoparticles and Microparticles (MPs)
6.1.3. Lipid-Based
| Disease | Drug | DDS | Advantages & Considerations | Administration Route | Stage | Reference |
|---|---|---|---|---|---|---|
| HYDROGEL | ||||||
| CNV (AMD) | Bevacizumab | Thermo-sensitive hydrogel (mPEG-PLGA-BOX) | Prolonged drug release Easy preparation Good cytocompatibility and biodegradability Sol-gel phase transition No reported adverse effect on the retina | IVT injection | Preclinical: in vitro, in vivo (rabbit model) | [115] |
| CNV | Sunitinib Acriflavine | Liposome-loaded, injectable hydrogel (cSA@Lip-HAC) | Higher prolonged drug concentration Higher drug residency time Antiangiogenic activity Enhanced anti-CNV activity Anti-CNV activity superior to commerical product injection | Periocular (Subtenon) | Preclinical: in vitro, in vivo CNV rat model) | [120] |
| Wet-AMD, PDR | Aflibercept | PGLA microspheres in a PEG-PLLA-DA/NIPAAm thermorepsonsive hydrogel | Controlled and prolonged drug release over 6 months No reported toxicity Drug bioactivity remained at therapeutic level | Intravitreal injection | Preclinical: in vitro | [117] |
| CNV (AMD) | Conbercept | Short chain peptide hydrogel | Good biocompatibility Inhibits tube formation in human retinal endothelial cells Self-assemble as a hydrogel (pH-sensitive) Ease of injection | IVT injection | Preclinical: in vitro | [119] |
| CNV | Aflibercept | PGLA microspheres in a PEG-PLLA-DA/NIPAAm thermoresponsive hydrogel | Good tolerability and biocompatibility Prolonged drug release of 6 months No reported toxicity or ocular complications | IVT injection | Preclinical: in vivo laser-induced CNV rat model | [118] |
| CNV | Ranibizumab | PGLA microspheres in a PEG-PLLA-DA/NIPAAm thermorespon-sive hydrogel | Prolonged drug release over 6 monts Ease of injection Good biodegradability | - | Preclinical: in vitro | [38] |
| Wet-AMD, PDR | Bevacizumab Aflibercept | Thermoresponsive hydrogel PEG-PPG-PCL (EPC) | No reported toxicity to endothelial cells Good biocompatibility Controlled and moduable drug release rate of 40 days | IVT injection | Preclinical: in vitro, ex vivo rat model, in vivo rabbit model | [116] |
| CNV | Ranibizumab Aflibercept | PLGA microspheres in a PEG-PLLA-DA/NIPAA hydrogel PNIPAAm-PED-DA | Controlled and prolonged durg release No reported adverse effect Less drug required to achieve therapeutic effects Biocompatible Only studies the preventive effects of CNV | IVT injection | Preclinical: in vivo laser-induced CNV rat model | [114] |
| CNV (AMD) | Ranibizumab Aflibercept | PGLA in a PNIPAAm-PEG-diacrylate-thermoresponsive based hydrogel | Controlled and prolonged drug release for 196 days in vitro Bioactivity remained | IVT injection | Preclinical: in vitro | [113] |
| POLYMER: SYNTHETIC | ||||||
| CNV (AMD), RNV (PDR, RoP) | Bevacizumab | PLGA nanoparticles | Higher drug bioavailability Prolonged drug release and drug half-life in vitreous and asqueous humor Increased antiangiogenic effects (HUVEC + OIR mice and CNV models) Decrease bevacizumab toxicity No reported toxicity on endothelial and retinalcells | IVT Subconjunctival | Preclinical: in vitro, in vivo alkali-induced CNV/OIR mouse model | [124] |
| CNV (AMD) | Bevacizumab Dexamethason | PLGA nanoparticles PVA Polyethilenimine | Stable in vivo Significantly decreased CNV leakage area Increased efficacy of CNV inhibition Antiangiogenic effects (in vitro HUVECs) | IVT | Preclinical: in vitro, in vivo | [127] |
| Wet-AMD | Bevacizumab | PDA nanoparticles | Antiangiogeneic activity of the DDS Reduces reactive oxygen species (ROS) associated with VEGF agents Controlled drug relrease | IVT | Preclinical: in vitro, ex vivo porcine eye model | [146] |
| Ocular angiogenesis | Bevacizumab-dextran | PLGA/PCADK microspheres | Prolonged drug release over 50 days in vitro and in vivo Bioactivity remained No reported toxicity Good tolerability | IVT | Preclinical: in vitro, in vivo rabbit model | [129] |
| CNV (AMD) | Anti-VEGF agents | PEG CCS (S-PEG-ICG-RGD-RBZ nanoparticles) polymers | Decrease tube formation (HUVEC) Promising biosafety High drug bioavailability Good cellular compatibility | Intravenous | Preclinical: in vitro, in vivo laser-induced CNV mouse model | [132] |
| Wet-AMD | Bevacizumab | PLGA microspheres | Accurate prediction of drug transport and concentration | IVT injection | Preclinical: computational rabbit model | [128] |
| Wet-AMD | Aflibercept | PLGA nanoparticles | Prolonged drug release High encapsulation efficiency | - | Preclinical: in vitro | [125] |
| CNV (AMD) | Ranibizumab | Magnetic nanoparticles loaded PEG-PLGA copolymer (iron oxide (Fe3O4)/PEGylated poly lactide-co-glycolide (PEG-PLGA) | Tube formation inhibition (HUVEC) Increased antiangiogenic properties with maxtrix | - | Preclinical: in vitro | [126] |
| Wet-AMD | Bevacizumab | PGLA nanoparticules | Controlled and prolonged drug release Low release rate Drug bioactivity remained pH dependant drug release | - | Preclinical: in vitro | [122,123] |
| CNV (AMD), PDR, RVO | Sunitinib | PLGA-PEG microparticles | Non-inflammatory Nontoxic Prolonged drug release over 6 months in vivo mouse model | Intravitreous injection | Preclinical: in vitro, in vivo laser induced CNV mouse models | [130] |
| Wet-AMD | Ranibizumab biosimilar | PLGA microparticles | Prolonged and controlled drug release Good target delivery | Intravitreous | Preclinical: in vitro | [121] |
| POLYMERS: SYNTHETIC AND NATURAL | ||||||
| Wet-AMD | Bevacizumab | Chitosan PCL | Prolonged drug release and efficacy up to 6 months High loading capacity Drug potency remained up to 6 months Good biocompatibility Good injectability High physical integrity and uniform size | - | Preclinical: in vitro, ex vivo porcine eye model | [140] |
| Wet-AMD | Bevacizumab | PCL-chitosan bi-layered capsule | Good structural integrity Low toxicity Maintain drug efficacy Controlled and prolonged drug release over one year in vitro | IVT injection | Preclinical: in vitro | [139] |
| Wet-AMD, PDR | Bevacizumab | Chitosan PLGA nanoparticles | Controlled and prolonged drug release Increased surface and posterior drug bioavailability | Subconjunctival injection | Preclinical: in vitro, ex vivo pig/ goat | [136] |
| POLYMER: NATURAL | ||||||
| CNV | Bevacizumab Suramin | Human serum albumin nanoparticles | Prolonged and controlled drug release Accommodate with hydrophilic drugs | Topical | Preclinical: in vitro | [135] |
| CNV | Bevacizumab | Human serum albumin nanoparticles | Prolonged drug release Stays on the eye surface 4 h post-topical application Increased antiangiogenic efficacy (rat CNV model) Formulation stability No reported toxicity | Topical | Preclinical: in vitro, in vivo CNV rat model | [133,134] |
| CNV | Bevacizumab | Chitosan nanoparticles | Excellent biocompatibility No toxicity reported Drug structure remains | - | Preclinical: in vitro | [55] |
| CRVO, CME, CNV (AMD), PDR) | Bevacizumab | Chitosan grafted poly(ethylene glycol) methacrylate (PEGMA) nanoparticles | No reported toxicity Good hemocompatibility Ocular tolerability Conrolled and prolonged drug release between 14–30 days Lower drug quantitiy while preserving therapeutic level | Subtenon | Preclinical: in vitro, in vivo rabbit model | [138] |
| Ocular NV | Bevacizumab | Chitosan nanoparticles | Controlled and effective drug concentration High drug concetnration Maintain drug efficacy No reported toxicity adverse effect Unequal drug loading capacity Unknown stability of nanoparticles | Subtenon | Preclinical: in vitro, in vivo rabbit model | [137] |
| CNV (AMD) | VEGFR-2 siRNA | Chitosan-hyaluronic acid nano-polyplexes | No reported toxicity Prolonged drug release Low water solubility may hinder DDS stability and loading capacity | Intraviteral injection | Preclinical: in vivo laser-induced CNV rat eye and rabbit models | [141] |
| LIPID-BASED | ||||||
| Solid tumors (GB) | Bevacizumab | Chitosan-coated lipid-core nanocapsules Gold | Potential cytotoxicity activity (in vitro) Potential kinetic instability Increased antiangiogenic effect | - | Preclinical: in vitro, in vivo | [145] |
| Ocular angiogene-sis | Bevacizumab Triamcinolone acetonide | Hybrid lipid nanocapsules | Increased antiangiogenic effects (HUVEC) High drug load capacity and antibody coupling capacity | - | Preclinical: in vitro | [142] |
| CNV (AMD) | Bevacizumab | Multivesicular liposomes (MLV) | Drug structure and efficacy remain Sustained drug release High encapsulation efficiency Bioactivity remained preserved Drug structural integrity preserved after release in vitro Potentical increased drug residency time in vitreous humor | IVT injection | Preclinical: in vitro, in vivo laser-induced CNV rat eye model | [143] |
| CNV (AMD), RNV (PDR) | Bevacizumab | Liposomes | Prolonged and slow drug release for 22 weeks in vivo Increased drug availability (rabbit model) Drug bioactivity remained Drug efficacy remained Increased antiangiogenic effect (CNV rat model) | IVT injection | Preclinical: in vitro, in vivo rabbit models | [144] |
7. Pediatric Posterior Segment Diseases
Retinopathy of Prematurity
Current Landscape of Novel Nano-Based DDS
| Disease | Drug | DDS | Advantages & Considerations | Administration Route | Stage | Reference |
|---|---|---|---|---|---|---|
| ROP | Cyclosporin A (CsA) | Lipid nanocapsule (LNC) | Target cell specificity, but not exclusively (nanocarrier spreads in the entire body) High drug concentration Higher drug residency time No reported adverse effect Promising preventive treatment | Intravenous | Preclinical: in vitro, in vivo OIR mouse model | [160] |
| miRNA-223 | Folic acid–chitosan-modified mesoporous silica NPs | High stability Loading efficiency Targeted delivery Controlled drug release Reduces inflammation by transitioning to M2 phenotype | IVT injection | Preclinical: in vitro, in vivo mouse OIR model | [174] | |
| VEGF siRNA | Lipidoid NPs (1-O16B) | Inhibition of tube formation (HUVEC) In vivo results similar to ranibizumab therapy Lack of data for long-term outcomes Lack of data on side effects and immune reactions | IVT injection | Preclinical: in vitro, in vivo rat OIR model | [155] | |
| VEGF-A165 | PLGA | Potential RoP Phase I treatment to avoid systemic effects of drugs targetting Phase II Controllable microparticle morphology Sustained drug release Reduction of retinal vaso-obliteration Prompt recovery of vein dilatation and arterial tortuosity Moderate protein loading capacity No reported systemic effects | IVT injection | Preclinical: in vitro, in vivo mouse OIR model | [175] | |
| miR-24-3p | Microglia-derived exosome | Low toxicity Inhibition of VEGF (in vitro and in vivo) Difficulty to identify and isolate human microglial cells | IVT injection | Preclinical: in vitro, in vivo mouse OIR model | [176] | |
| Anti-VEGF oligonucleotides (VEGFR-2 ODN17) | DOTAP blank cationic nanoemulsion | Potential drug stabilization with the nanoemulsion Inflammation reduction Enhanced efficacy in inhibiting neovascularization | IVT injection | Preclinical: in vivo ROP mouse and rat CNV models | [163,164,165] |
8. Clinical Advancements in Posterior Segment Diseases
9. Translation of DDS from Bench to Bedside: Preclinical and Clinical Considerations
9.1. Barrier to Clinical Translation: Anatomical Differences across Animals and Humans and Their Implications for DDS in Posterior Segment Disease Treatment
9.2. Implications of Artificially Induced Disease Models in Clinical Translation
9.3. Advancing Biodegradable Drug Delivery Systems for Ocular Applications: Insights and Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urtti, A. Challenges and Obstacles of Ocular Pharmacokinetics and Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef]
- Addo, R.T. Ocular Drug Delivery: Advances, Challenges and Applications. In Ocular Drug Delivery: Advances, Challenges and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–185. [Google Scholar] [CrossRef]
- Chang, A.Y.; Purt, B. Biochemistry, Tear Film. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Wu, K.Y.; Kulbay, M.; Tanasescu, C.; Jiao, B.; Nguyen, B.H.; Tran, S.D. An Overview of the Dry Eye Disease in Sjögren’s Syndrome Using Our Current Molecular Understanding. Int. J. Mol. Sci. 2023, 24, 1580. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-S.; Hou, P.-K.; Tai, T.-Y.; Lin, B.J. Blood-Ocular Barriers. Tzu Chi Med. J. 2008, 20, 25–34. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-Retinal Barrier. Eur. J. Ophthalmol. 2011, 21, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bertens, C.J.F.; Gijs, M.; van den Biggelaar, F.J.H.M.; Nuijts, R.M.M.A. Topical Drug Delivery Devices: A Review. Exp. Eye Res. 2018, 168, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Singh Malik, D.; Mital, N.; Kaur, G. Topical Drug Delivery Systems: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef]
- Farkouh, A.; Frigo, P.; Czejka, M. Systemic Side Effects of Eye Drops: A Pharmacokinetic Perspective. Clin. Ophthalmol. 2016, 10, 2433–2441. [Google Scholar] [CrossRef]
- Wu, W.; He, Z.; Zhang, Z.; Yu, X.; Song, Z.; Li, X. Intravitreal Injection of Rapamycin-Loaded Polymeric Micelles for Inhibition of Ocular Inflammation in Rat Model. Int. J. Pharm. 2016, 513, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.; Shariati, M.M.; Malaekeh-Nikouei, B.; Tajani, A.S.; Mahmoudi, A.; Abrishami, M.; Khameneh, B. Preparation and in Vivo Evaluation of Nanoliposomes Containing Vancomycin after Intravitreal Injection in Albino Rabbits. Iran. J. Basic Med. Sci. 2020, 23, 551–555. [Google Scholar] [CrossRef] [PubMed]
- García-Caballero, C.; Prieto-Calvo, E.; Checa-Casalengua, P.; García-Martín, E.; Polo-Llorens, V.; García-Feijoo, J.; Molina-Martínez, I.T.; Bravo-Osuna, I.; Herrero-Vanrell, R. Six Month Delivery of GDNF from PLGA/Vitamin E Biodegradable Microspheres after Intravitreal Injection in Rabbits. Eur. J. Pharm. Sci. 2017, 103, 19–26. [Google Scholar] [CrossRef]
- Park, J.G.; Callaway, N.F.; Ludwig, C.A.; Mahajan, V.B. Intravitreal Methotrexate and Fluocinolone Acetonide Implantation for Vogt-Koyanagi-Harada Uveitis. Am. J. Ophthalmol. Case Rep. 2020, 19, 100859. [Google Scholar] [CrossRef] [PubMed]
- Glendenning, A.; Crews, K.; Sturdivant, J.; deLong, M.A.; Kopczynski, C.; Lin, C.-W. Sustained Release, Biodegradable PEA Implants for Intravitreal Delivery of the ROCK/PKC Inhibitor AR-13503. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5672. [Google Scholar]
- Blazaki, S.; Pachis, K.; Tzatzarakis, M.; Tsilimbaris, M.; Antimisiaris, S.G. Novel Liposome Aggregate Platform (LAP) System for Sustained Retention of Drugs in the Posterior Ocular Segment Following Intravitreal Injection. Int. J. Pharm. 2020, 576, 118987. [Google Scholar] [CrossRef]
- Agban, Y.; Thakur, S.S.; Mugisho, O.O.; Rupenthal, I.D. Depot Formulations to Sustain Periocular Drug Delivery to the Posterior Eye Segment. Drug Discov. Today 2019, 24, 1458–1469. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Vincent, A.; Joseph, R.R.; Moreno, M.; Dickescheid, A.; Agrawal, R.; Venkatraman, S. Hollow Microcapsules as Periocular Drug Depot for Sustained Release of Anti-VEGF Protein. Pharmaceutics 2019, 11, 330. [Google Scholar] [CrossRef]
- Yiu, G.; Chung, S.H.; Mollhoff, I.N.; Nguyen, U.T.; Thomasy, S.M.; Yoo, J.; Taraborelli, D.; Noronha, G. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol. Ther. Methods Clin. Dev. 2020, 16, 179–191. [Google Scholar] [CrossRef]
- Weijtens, O.; Feron, E.J.; Schoemaker, R.C.; Cohen, A.F.; Lentjes, E.G.W.M.; Romijn, F.P.H.T.M.; Van Meurs, J.C. High Concentration of Dexamethasone in Aqueous and Vitreous after Subconjunctival Injection. Am. J. Ophthalmol. 1999, 128, 192–197. [Google Scholar] [CrossRef]
- Salama, H.A.; Ghorab, M.; Mahmoud, A.A.; Abdel Hady, M. PLGA Nanoparticles as Subconjunctival Injection for Management of Glaucoma. AAPS PharmSciTech 2017, 18, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sun, F.; Liu, W.; Liu, Y.; Gedam, M.; Hu, Q.; Fridley, C.; Quigley, H.A.; Hanes, J.; Pitha, I. Subconjunctival Delivery of Dorzolamide-Loaded Poly(Ether-Anhydride) Microparticles Produces Sustained Lowering of Intraocular Pressure in Rabbits. Mol. Pharm. 2016, 13, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Cuming, R.S.; Abarca, E.M.; Duran, S.; Wooldridge, A.A.; Stewart, A.J.; Ravis, W.; Babu, R.J.; Lin, Y.J.; Hathcock, T. Development of a Sustained-Release Voriconazole-Containing Thermogel for Subconjunctival Injection in Horses. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrasco, R.; Sánchez-Abarca, L.I.; Nieto-Gómez, C.; Martín García, E.; Sánchez-Guijo, F.; Argüeso, P.; Aijón, J.; Hernández-Galilea, E.; Velasco, A. Subconjunctival Injection of Mesenchymal Stromal Cells Protects the Cornea in an Experimental Model of GVHD. Ocul. Surf. 2019, 17, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, J.M.; Taipale, C.; Ylinen, P.; Tuuminen, R. Perioperative Subconjunctival Triamcinolone Acetonide Injection for Prevention of Inflammation and Macular Oedema after Cataract Surgery. Acta Ophthalmol. 2020, 98, 36–42. [Google Scholar] [CrossRef]
- Schneider, M.E.; Milstein, D.E.; Oyakawa, R.T.; Ober, R.R.; Campo, R. Ocular Perforation from a Retrobulbar Injection. Am. J. Ophthalmol. 1988, 106, 35–40. [Google Scholar] [CrossRef]
- Safi, M.; Ang, M.J.; Patel, P.; Silkiss, R.Z. Rhino-Orbital-Cerebral Mucormycosis (ROCM) and Associated Cerebritis Treated with Adjuvant Retrobulbar Amphotericin B. Am. J. Ophthalmol. Case Rep. 2020, 19, 100771. [Google Scholar] [CrossRef]
- Arbisser, L.B. Safety of Intracameral Moxifloxacin for Prophylaxis of Endophthalmitis after Cataract Surgery. J. Cataract. Refract. Surg. 2008, 34, 1114–1120. [Google Scholar] [CrossRef]
- Christopher, K.; Chauhan, A. Contact Lens Based Drug Delivery to the Posterior Segment Via Iontophoresis in Cadaver Rabbit Eyes. Pharm. Res. 2019, 36. [Google Scholar] [CrossRef]
- Jung, J.H.; Chiang, B.; Grossniklaus, H.E.; Prausnitz, M.R. Ocular Drug Delivery Targeted by Iontophoresis in the Suprachoroidal Space Using a Microneedle. J. Control. Release 2018, 277, 14–22. [Google Scholar] [CrossRef]
- See, G.L.; Sagesaka, A.; Torizuka, H.; Todo, H.; Sugibayashi, K. Iontophoresis-Aided Drug Delivery into the Eyeball via Eyelid Skin. J. Drug Deliv. Sci. Technol. 2018, 47, 380–385. [Google Scholar] [CrossRef]
- dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; dos Santos Ré, A.C.; Anjos, J.L.V.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin Liposomes with Positively Charged Additives for an Improved Topical Ocular Delivery. Sci. Rep. 2020, 10, 19285. [Google Scholar] [CrossRef] [PubMed]
- Habot-Wilner, Z.; Noronha, G.; Wykoff, C.C. Suprachoroidally Injected Pharmacological Agents for the Treatment of Chorio-Retinal Diseases: A Targeted Approach. Acta Ophthalmol. 2019, 97, 460–472. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic Block Copolymers in Drug Delivery: Advances in Formulation Structure and Performance. Expert. Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic Acid in Ocular Drug Delivery. Carbohydr. Polym. 2021, 264, 118006. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Dutta, S.; Das, D.; Nath, J.; Pal, C.; Chattopadhyay, D. Utilization of Cellulose Nanocrystals (CNC) Biopolymer Nanocomposites in Ophthalmic Drug Delivery System (ODDS). J. Nanotechnol. Res. 2019, 1, 75–87. [Google Scholar]
- Gupta, B.; Mishra, V.; Gharat, S.; Momin, M.; Omri, A. Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems. Pharmaceuticals 2021, 14, 1201. [Google Scholar] [CrossRef]
- Tavakolian, M.; Jafari, S.M.; van de Ven, T.G.M. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nanomicro Lett. 2020, 12, 73. [Google Scholar] [CrossRef]
- Junnuthula, V.; Boroujeni, A.S.; Cao, S.; Tavakoli, S.; Ridolfo, R.; Toropainen, E.; Ruponen, M.; van Hest, J.C.M.; Urtti, A. Intravitreal Polymeric Nanocarriers with Long Ocular Retention and Targeted Delivery to the Retina and Optic Nerve Head Region. Pharmaceutics 2021, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, S.; Solouk, A.; Saber-Samandari, S.; Keshel, S.H.; Pasbakhsh, P. Alginate Nanoparticles as Ocular Drug Delivery Carriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102889. [Google Scholar] [CrossRef]
- Wong, F.S.Y.; Tsang, K.K.; Chu, A.M.W.; Chan, B.P.; Yao, K.M.; Lo, A.C.Y. Injectable Cell-Encapsulating Composite Alginate-Collagen Platform with Inducible Termination Switch for Safer Ocular Drug Delivery. Biomaterials 2019, 201, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Jia, H.; Qiu, J.; Mo, Z.; Wen, Y.; Zhang, Y.; Wen, Y.; Xie, Q.; Ban, J.; Lu, Z.; et al. PLGA Nanoparticle Platform for Trans-Ocular Barrier to Enhance Drug Delivery: A Comparative Study Based on the Application of Oligosaccharides in the Outer Membrane of Carriers. Int. J. Nanomed. 2020, 15, 9373–9387. [Google Scholar] [CrossRef] [PubMed]
- Swetledge, S.; Carter, R.; Stout, R.; Astete, C.E.; Jung, J.P.; Sabliov, C.M. Stability and Ocular Biodistribution of Topically Administered PLGA Nanoparticles. Sci. Rep. 2021, 11, 12270. [Google Scholar] [CrossRef]
- Weng, Y.H.; Ma, X.W.; Che, J.; Li, C.; Liu, J.; Chen, S.Z.; Wang, Y.Q.; Gan, Y.L.; Chen, H.; Hu, Z.B.; et al. Nanomicelle-Assisted Targeted Ocular Delivery with Enhanced Antiinflammatory Efficacy In Vivo. Adv. Sci. 2017, 5, 1700455. [Google Scholar] [CrossRef]
- Dai, L.; Li, X.; Yao, M.; Niu, P.; Yuan, X.; Li, K.; Chen, M.; Fu, Z.; Duan, X.; Liu, H.; et al. Programmable Prodrug Micelle with Size-Shrinkage and Charge-Reversal for Chemotherapy-Improved IDO Immunotherapy. Biomaterials 2020, 241, 119901. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric Micelles for the Delivery of Poorly Soluble Drugs: From Nanoformulation to Clinical Approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Reduction-Responsive Polymeric Micelles and Vesicles for Triggered Intracellular Drug Release. Antioxid. Redox Signal. 2014, 21, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, D.; Li, Y.; Yang, W.; Tu, J.; Shen, Y. Improving the Topical Ocular Pharmacokinetics of Lyophilized Cyclosporine A-Loaded Micelles: Formulation, in Vitro and in Vivo Studies. Drug Deliv. 2018, 25, 888–899. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional Chitosan Oligosaccharide Nanomicelles for Topical Ocular Drug Delivery of Dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wei, Y.; Wu, Q.; Zhou, K.; Liu, T.; Zhang, Y.; Jiang, N.; Xiao, W.; Chen, J.; Liu, Q.; et al. Liposomes for Effective Drug Delivery to the Ocular Posterior Chamber. J. Nanobiotechnol. 2019, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Arkan, E.; Mansouri, K.; Shekarbeygi, Z.; Barzegari, E. Interactions of Bevacizumab with Chitosan Biopolymer Nanoparticles: Molecular Modeling and Spectroscopic Study. J. Mol. Liq. 2021, 339, 116655. [Google Scholar] [CrossRef]
- Ishida, T.; Harashima, H.; Kiwada, H. Liposome Clearance. Biosci. Rep. 2002, 22, 197–224. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, L.; Xue, B.; Zhu, X.; Yan, D. Supramolecular Nanoscale Drug-Delivery System with Ordered Structure. Natl. Sci. Rev. 2019, 6, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Kohane, D.S. Nanoscale Systems for Local Drug Delivery. Nano Today 2019, 28, 100765. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef]
- Rebibo, L.; Tam, C.; Sun, Y.; Shoshani, E.; Badihi, A.; Nassar, T.; Benita, S. Topical Tacrolimus Nanocapsules Eye Drops for Therapeutic Effect Enhancement in Both Anterior and Posterior Ocular Inflammation Models. J. Control. Release 2021, 333, 283–297. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A Brief Review on Solid Lipid Nanoparticles: Part and Parcel of Contemporary Drug Delivery Systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Lancina, M.G.; Yang, H. Dendrimers for Ocular Drug Delivery. Can. J. Chem. 2017, 95, 897–902. [Google Scholar] [CrossRef]
- Lin, D.; Lei, L.; Shi, S.; Li, X. Stimulus-Responsive Hydrogel for Ophthalmic Drug Delivery. Macromol. Biosci. 2019, 19, e1900001. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Otake, H. Novel Drug Delivery Systems for the Management of Dry Eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging Role of Nanosuspensions in Drug Delivery Systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef]
- Xie, J.; Luo, Y.; Liu, Y.; Ma, Y.; Yue, P.; Yang, M. Novel Redispersible Nanosuspensions Stabilized by Co-Processed Nanocrystalline Cellulose-Sodium Carboxymethyl Starch for Enhancing Dissolution and Oral Bioavailability of Baicalin. Int. J. Nanomed. 2019, 14, 353–369. [Google Scholar] [CrossRef]
- Gade, S.S.; Pentlavalli, S.; Mishra, D.; Vora, L.K.; Waite, D.; Alvarez-Lorenzo, C.I.; Herrero Vanrell, M.R.; Laverty, G.; Larraneta, E.; Donnelly, R.F.; et al. Injectable Depot Forming Thermoresponsive Hydrogel for Sustained Intrascleral Delivery of Sunitinib Using Hollow Microneedles. J. Ocul. Pharmacol. Ther. 2022, 38, 433–448. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernández-Sánchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular Responses Following Retinal Injuries and Therapeutic Approaches for Neurodegenerative Diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Arranz-Romera, A.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Bravo-Osuna, I.; Herrero-Vanrell, R. Co-Delivery of Glial Cell-Derived Neurotrophic Factor (GDNF) and Tauroursodeoxycholic Acid (TUDCA) from PLGA Microspheres: Potential Combination Therapy for Retinal Diseases. Drug Deliv. Transl. Res. 2021, 11, 566–580. [Google Scholar] [CrossRef]
- Martín-Sabroso, C.; Fraguas-Sánchez, A.I.; Aparicio-Blanco, J.; Cano-Abad, M.F.; Torres-Suárez, A.I. Critical Attributes of Formulation and of Elaboration Process of PLGA-Protein Microparticles. Int. J. Pharm. 2015, 480, 27–36. [Google Scholar] [CrossRef]
- Sen, M.; Bassetto, M.; Poulhes, F.; Zelphati, O.; Ueffing, M.; Arango-Gonzalez, B. Efficient Ocular Delivery of VCP SiRNA via Reverse Magnetofection in RHO P23H Rodent Retina Explants. Pharmaceutics 2021, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Al-Amin, M.; Kicková, E.; Sadeghi, A.; Puranen, J.; Urtti, A.; Caliceti, P.; Salmaso, S.; Arango-Gonzalez, B.; Ueffing, M. Retinal Neuroprotection by Controlled Release of a VCP Inhibitor from Self-Assembled Nanoparticles. J. Control. Release 2021, 339, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Dei Cas, M.; Cianciolo, S.; Fidilio, A.; Lazzara, F.; Paroni, R.; Pignatello, R.; Strettoi, E.; Ghidoni, R.; Drago, F.; et al. Novel Ophthalmic Formulation of Myriocin: Implications in Retinitis Pigmentosa. Drug Deliv. 2019, 26, 237–243. [Google Scholar] [CrossRef]
- Strettoi, E.; Gargini, C.; Novelli, E.; Sala, G.; Piano, I.; Gasco, P.; Ghidoni, R. Inhibition of Ceramide Biosynthesis Preserves Photoreceptor Structure and Function in a Mouse Model of Retinitis Pigmentosa. Proc. Natl. Acad. Sci. USA 2010, 107, 18706–18711. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Li, C.; Chen, Q.; Huang, J.; Zhao, Z.; Liu, P.; Xu, K.; Li, L.; Hu, F.; Zhang, S.; et al. Intravenous Route to Choroidal Neovascularization by Macrophage-Disguised Nanocarriers for MTOR Modulation. Acta Pharm. Sin. B 2022, 12, 2506–2521. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Yu, M.; Liu, Y.; Weh, E.; Pawar, M.; Li, L.; Besirli, C.G.; Schwendeman, A.A. Synthetic High-Density Lipoprotein Nanoparticles Delivering Rapamycin for the Treatment of Age-Related Macular Degeneration. Nanomedicine 2022, 44, 102571. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L. Ranibizumab and Bevacizumab for Treatment of Neovascular Age-Related Macular Degeneration: Two-Year Results. Ophthalmology 2020, 127, S135–S145. [Google Scholar] [CrossRef]
- Feng, L.; Ju, M.; Lee, K.Y.V.; Mackey, A.; Evangelista, M.; Iwata, D.; Adamson, P.; Lashkari, K.; Foxton, R.; Shima, D.; et al. A Proinflammatory Function of Toll-Like Receptor 2 in the Retinal Pigment Epithelium as a Novel Target for Reducing Choroidal Neovascularization in Age-Related Macular Degeneration. Am. J. Pathol. 2017, 187, 2208–2221. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.J.; Wang, H.Y.; Li, M.Q.; Wang, X.X.; Fan, L.; Wang, Y.S. A Trojan Horse Biomimetic Delivery System Using Mesenchymal Stem Cells for HIF-1α SiRNA-Loaded Nanoparticles on Retinal Pigment Epithelial Cells under Hypoxia Environment. Int. J. Ophthalmol. 2022, 15, 1743–1751. [Google Scholar] [CrossRef]
- Kimbrel, E.A.; Lanza, R. Next-Generation Stem Cells—Ushering in a New Era of Cell-Based Therapies. Nat. Rev. Drug Discov. 2020, 19, 463–479. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.J.; Yang, C.J.; Lai, J.Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef]
- Oshitari, T. Neurovascular Impairment and Therapeutic Strategies in Diabetic Retinopathy. Int. J. Environ. Res. Public Health 2021, 19, 439. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, W.; Shi, L.; Chen, X.; Wu, R.; Zhang, Y.; Chen, H.; Chen, H. Poly(Lactic-Co-Glycolic Acid) Nanoparticle-Mediated Interleukin-12 Delivery for the Treatment of Diabetic Retinopathy. Int. J. Nanomed. 2019, 14, 6357–6369. [Google Scholar] [CrossRef]
- Romeo, A.; Bonaccorso, A.; Carbone, C.; Lupo, G.; Daniela Anfuso, C.; Giurdanella, G.; Caggia, C.; Randazzo, C.; Russo, N.; Luca Romano, G.; et al. Melatonin Loaded Hybrid Nanomedicine: DoE Approach, Optimization and in Vitro Study on Diabetic Retinopathy Model. Int. J. Pharm. 2022, 627, 122195. [Google Scholar] [CrossRef]
- Zingale, E.; Rizzo, S.; Bonaccorso, A.; Consoli, V.; Vanella, L.; Musumeci, T.; Spadaro, A.; Pignatello, R. Optimization of Lipid Nanoparticles by Response Surface Methodology to Improve the Ocular Delivery of Diosmin: Characterization and In-Vitro Anti-Inflammatory Assessment. Pharmaceutics 2022, 14, 1961. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Müller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN and NLC): Present State of Development and Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.E.S.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [Google Scholar] [CrossRef]
- Mahaling, B.; Srinivasarao, D.A.; Raghu, G.; Kasam, R.K.; Bhanuprakash Reddy, G.; Katti, D.S. A Non-Invasive Nanoparticle Mediated Delivery of Triamcinolone Acetonide Ameliorates Diabetic Retinopathy in Rats. Nanoscale 2018, 10, 16485–16498. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.C.; Dawson, M.; Lai, S.K.; Wang, Y.Y.; Suk, J.S.; Yang, M.; Zeitlin, P.; Boyle, M.P.; Fu, J.; Hanes, J. Biodegradable Polymer Nanoparticles That Rapidly Penetrate the Human Mucus Barrier. Proc. Natl. Acad. Sci. USA 2009, 106, 19268–19273. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Partida, J.; Altamirano-Vallejo, J.C.; la Rosa, A.G.D.; Armendariz-Borunda, J.; Castro-Castaneda, C.R.; Santos, A. Safety and Tolerability of Topical Ophthalmic Triamcinolone Acetonide-Loaded Liposomes Formulation and Evaluation of Its Biologic Activity in Patients with Diabetic Macular Edema. Pharmaceutics 2021, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.M.; Fan, D.S.P.; Chan, W.M.; Lai, W.W.; Lee, V.Y.W.; Lam, D.S.C. Ocular-Hypertensive Response and Corneal Endothelial Changes after Intravitreal Triamcinolone Injections in Chinese Subjects: A 6-Month Follow-up Study. Eye 2005, 19, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Hashmi, U.; Riaz, R.; Rukh Abbas, S. Chitosan Coated Liposomes (CCL) Containing Triamcinolone Acetonide for Sustained Delivery: A Potential Topical Treatment for Posterior Segment Diseases. Int. J. Biol. Macromol. 2020, 143, 483–491. [Google Scholar] [CrossRef]
- Alambiaga-Caravaca, A.M.; Domenech-Monsell, I.M.; Sebastián-Morelló, M.; Calatayud-Pascual, M.A.; Merino, V.; Rodilla, V.; López-Castellano, A. Development, Characterization, and Ex Vivo Evaluation of an Insert for the Ocular Administration of Progesterone. Int. J. Pharm. 2021, 606, 120921. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Schiavone, N.; Guzman-Aranguez, A.; Giansanti, F.; Papucci, L.; Perez de Lara, M.J.; Singh, M.; Kaur, I.P. Correction to: Atorvastatin-Loaded Solid Lipid Nanoparticles as Eye Drops: Proposed Treatment Option for Age-Related Macular Degeneration (AMD). Drug Deliv. Transl. Res. 2020, 10, 1531. [Google Scholar] [CrossRef]
- Mitra, R.N.; Gao, R.; Zheng, M.; Wu, M.J.; Voinov, M.A.; Smirnov, A.I.; Smirnova, T.I.; Wang, K.; Chavala, S.; Han, Z. Glycol Chitosan Engineered Autoregenerative Antioxidant Significantly Attenuates Pathological Damages in Models of Age-Related Macular Degeneration. ACS Nano 2017, 11, 4669–4685. [Google Scholar] [CrossRef]
- Nagai, N.; Daigaku, R.; Motoyama, R.; Kaji, H.; Abe, T. Release of Ranibizumab Using a Porous Poly(Dimethylsiloxane) Capsule Suppressed Laser-Induced Choroidal Neovascularization via the Transscleral Route. J. Mater. Sci. Mater. Med. 2022, 34, 5. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X.; et al. Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration. Mol. Pharm. 2019, 16, 1958–1970. [Google Scholar] [CrossRef]
- Sun, Q.; Shen, Y.; Su, L.; Xu, X. Inhibition of Pathological Retinal Neovascularization by a Small Peptide Derived from Human Tissue-Type Plasminogen Kringle 2. Front. Pharmacol. 2020, 10, 1639. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Takahashi, A.; Omae, T.; Song, Y.S.; Takahashi, T.; Yoshida, A. Characteristics of Retinal Neovascularization in Proliferative Diabetic Retinopathy Imaged by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6247–6255. [Google Scholar] [CrossRef]
- Sacconi, R.; Fragiotta, S.; Sarraf, D.; Sadda, S.V.R.; Freund, K.B.; Parravano, M.; Corradetti, G.; Cabral, D.; Capuano, V.; Miere, A.; et al. Towards a Better Understanding of Non-Exudative Choroidal and Macular Neovascularization. Prog. Retin. Eye Res. 2023, 92, 101113. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; dos Santos, T.; Granja, P.L.; Sanchez-Lopez, E.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Permeability, Anti-Inflammatory and Anti-VEGF Profiles of Steroidal-Loaded Cationic Nanoemulsions in Retinal Pigment Epithelial Cells under Oxidative Stress. Int. J. Pharm. 2022, 617, 121615. [Google Scholar] [CrossRef] [PubMed]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, M.; Santos-Ramos, P.; Fernández-Rodríguez, M.; Abraldes, M.J.; Rodríguez-Cid, M.J.; Santiago-Varela, M.; Fernández-Ferreiro, A.; Gómez-Ulla, F. Pharmacological Advances in the Treatment of Age-Related Macular Degeneration. Curr. Med. Chem. 2020, 27, 583–598. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten Years of Anti-Vascular Endothelial Growth Factor Therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Klettner, A.; Recber, M.; Roider, J. Comparison of the Efficacy of Aflibercept, Ranibizumab, and Bevacizumab in an RPE/Choroid Organ Culture. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Oo, C.; Kalbag, S.S. Leveraging the Attributes of Biologics and Small Molecules, and Releasing the Bottlenecks: A New Wave of Revolution in Drug Development. Expert. Rev. Clin. Pharmacol. 2016, 9, 747–749. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Galante, R.; Pinto, T.J.A.; Colaço, R.; Serro, A.P. Sterilization of Hydrogels for Biomedical Applications: A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2472–2492. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and Extended In Vitro Release of Bioactive Anti-Vascular Endothelial Growth Factors from a Microsphere-Hydrogel Drug Delivery System. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Osswald, C.R.; Guthrie, M.J.; Avila, A.; Valio, J.A.; Mieler, W.F.; Kang-Mieler, J.J. In Vivo Efficacy of an Injectable Microsphere-Hydrogel Ocular Drug Delivery System. Curr. Eye Res. 2017, 42, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Chiu, Y.C.; Chaw, J.R.; Chen, C.F.; Liu, H.W. Thermo-Responsive Hydrogel as an Anti-VEGF Drug Delivery System to Inhibit Retinal Angiogenesis in Rex Rabbits. Technol. Health Care 2019, 27, S153–S163. [Google Scholar] [CrossRef]
- Xue, K.; Zhao, X.; Zhang, Z.; Qiu, B.; Tan, Q.S.W.; Ong, K.H.; Liu, Z.; Parikh, B.H.; Barathi, V.A.; Yu, W.; et al. Sustained Delivery of Anti-VEGFs from Thermogel Depots Inhibits Angiogenesis without the Need for Multiple Injections. Biomater. Sci. 2019, 7, 4603–4614. [Google Scholar] [CrossRef]
- Liu, W.; Lee, B.S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274. [Google Scholar] [CrossRef]
- Liu, W.; Tawakol, A.P.; Rudeen, K.M.; Mieler, W.F.; Kang-Mieler, J.J. Treatment Efficacy and Biocompatibility of a Biodegradable Aflibercept-Loaded Microsphere-Hydrogel Drug Delivery System. Transl. Vis. Sci. Technol. 2020, 9, 13. [Google Scholar] [CrossRef]
- Fan, W.; Li, S.; Tao, J.; Yu, C.; Sun, M.; Xie, Z.; Wu, X.; Ge, L.; Wu, Y.; Liu, Y. Anti-Vascular Endothelial Growth Factor Drug Conbercept-Loaded Peptide Hydrogel Reduced Angiogenesis in the Neovascular Age-Related Macular Degeneration. J. Biomed. Nanotechnol. 2022, 18, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, Q.; Sun, H.; Zhang, Y.; Yang, X.; Kaur, P.; Wang, R.; Fang, Y.; Yan, H.; Du, X.; et al. A Novel, Liposome-Loaded, Injectable Hydrogel for Enhanced Treatment of Choroidal Neovascularization by Sub-Tenon’s Injection. Mater. Today Nano 2022, 20, 100264. [Google Scholar] [CrossRef]
- Tanetsugu, Y.; Tagami, T.; Terukina, T.; Ogawa, T.; Ohta, M.; Ozeki, T. Development of a Sustainable Release System for a Ranibizumab Biosimilar Using Poly(Lactic-Co-Glycolic Acid) Biodegradable Polymer-Based Microparticles as a Platform. Biol. Pharm. Bull. 2017, 40, 145–150. [Google Scholar] [CrossRef]
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A New Paradigm for Antiangiogenic Therapy through Controlled Release of Bevacizumab from PLGA Nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef]
- Sousa, F.; Cruz, A.; Pinto, I.M.; Sarmento, B. Nanoparticles Provide Long-Term Stability of Bevacizumab Preserving Its Antiangiogenic Activity. Acta Biomater. 2018, 78, 285–295. [Google Scholar] [CrossRef]
- Zhang, X.P.; Sun, J.G.; Yao, J.; Shan, K.; Liu, B.H.; Yao, M.D.; Ge, H.M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of Nanoencapsulation Using Poly (Lactide-Co-Glycolide) (PLGA) on Anti-Angiogenic Activity of Bevacizumab for Ocular Angiogenesis Therapy. Biomed. Pharmacother. 2018, 107, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.J.; Hirani, A.; Shahidadpury, V.; Solanki, A.; Halasz, K.; Gupta, S.V.; Madow, B.; Sutariya, V. Aflibercept Nanoformulation Inhibits VEGF Expression in Ocular In Vitro Model: A Preliminary Report. Biomedicines 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Peng, X.; Cai, Y.; Cong, W. Development of Facile Drug Delivery Platform of Ranibizumab Fabricated PLGA-PEGylated Magnetic Nanoparticles for Age-Related Macular Degeneration Therapy. J. Photochem. Photobiol. B 2018, 183, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Li, G.; Xu, F.; Li, S.; Teng, L.; Li, Y.; Sun, F. Anti-Angiogenic Activity Of Bevacizumab-Bearing Dexamethasone-Loaded PLGA Nanoparticles For Potential Intravitreal Applications. Int. J. Nanomed. 2019, 14, 8819–8834. [Google Scholar] [CrossRef]
- Heljak, M.K.; Swieszkowski, W. In Silico Model of Bevacizumab Sustained Release from Intravitreal Administrated PLGA Drug-Loaded Microspheres. Mater. Lett. 2021, 307, 131080. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Li, G.; Li, X.; Yu, C.; Fu, Z.; Li, X.; Teng, L.; Li, Y.; Sun, F. Highly Bioactive, Bevacizumab-Loaded, Sustained-Release PLGA/PCADK Microspheres for Intravitreal Therapy in Ocular Diseases. Int. J. Pharm. 2019, 563, 228–236. [Google Scholar] [CrossRef]
- Tsujinaka, H.; Fu, J.; Shen, J.; Yu, Y.; Hafiz, Z.; Kays, J.; McKenzie, D.; Cardona, D.; Culp, D.; Peterson, W.; et al. Sustained Treatment of Retinal Vascular Diseases with Self-Aggregating Sunitinib Microparticles. Nat. Commun. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yang, J.; Oh, Y.; Hartsock, M.J.; Xia, S.; Kim, Y.C.; Ding, Z.; Meng, T.; Eberhart, C.G.; Ensign, L.M.; et al. Controlled Release of Corticosteroid with Biodegradable Nanoparticles for Treating Experimental Autoimmune Uveitis. J. Control. Release 2019, 296, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, Q.; Shen, T.; Yang, Q.; Hu, W.; Zhao, P.; Yu, J. Intravenous Anti-VEGF Agents with RGD Peptide-Targeted Core Cross-Linked Star (CCS) Polymers Modified with Indocyanine Green for Imaging and Treatment of Laser-Induced Choroidal Neovascularization. Biomater. Sci. 2020, 8, 4481–4491. [Google Scholar] [CrossRef]
- Luis de Redín, I.; Boiero, C.; Martínez-Ohárriz, M.C.; Agüeros, M.; Ramos, R.; Peñuelas, I.; Allemandi, D.; Llabot, J.M.; Irache, J.M. Human Serum Albumin Nanoparticles for Ocular Delivery of Bevacizumab. Int. J. Pharm. 2018, 541, 214–223. [Google Scholar] [CrossRef]
- Luis de Redín, I.; Boiero, C.; Recalde, S.; Agüeros, M.; Allemandi, D.; Llabot, J.M.; García-Layana, A.; Irache, J.M. In Vivo Effect of Bevacizumab-Loaded Albumin Nanoparticles in the Treatment of Corneal Neovascularization. Exp. Eye Res. 2019, 185, 107697. [Google Scholar] [CrossRef]
- Llabot, J.M.; Luis de Redin, I.; Agüeros, M.; Dávila Caballero, M.J.; Boiero, C.; Irache, J.M.; Allemandi, D. In Vitro Characterization of New Stabilizing Albumin Nanoparticles as a Potential Topical Drug Delivery System in the Treatment of Corneal Neovascularization (CNV). J. Drug Deliv. Sci. Technol. 2019, 52, 379–385. [Google Scholar] [CrossRef]
- Pandit, J.; Sultana, Y.; Aqil, M. Chitosan-Coated PLGA Nanoparticles of Bevacizumab as Novel Drug Delivery to Target Retina: Optimization, Characterization, and in Vitro Toxicity Evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1397–1407. [Google Scholar] [CrossRef]
- Ugurlu, N.; Aşık, M.D.; Çakmak, H.B.; Tuncer, S.; Turk, M.; Çagıl, N.; Denkbas, E.B. Transscleral Delivery of Bevacizumab-Loaded Chitosan Nanoparticles. J. Biomed. Nanotechnol. 2019, 15, 830–838. [Google Scholar] [CrossRef]
- Savin, C.L.; Popa, M.; Delaite, C.; Costuleanu, M.; Costin, D.; Peptu, C.A. Chitosan Grafted-Poly(Ethylene Glycol) Methacrylate Nanoparticles as Carrier for Controlled Release of Bevacizumab. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chaparro, F.J.; Cuddington, C.T.; Palmer, A.F.; Ohr, M.P.; Lannutti, J.J.; Swindle-Reilly, K.E. Injectable Biodegradable Bi-Layered Capsule for Sustained Delivery of Bevacizumab in Treating Wet Age-Related Macular Degeneration. J. Control. Release 2020, 320, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Jacobs, K.M.; Ohr, M.P.; Swindle-Reilly, K.E. Chitosan-Polycaprolactone Core-Shell Microparticles for Sustained Delivery of Bevacizumab. Mol. Pharm. 2020, 17, 2570–2584. [Google Scholar] [CrossRef]
- Chaharband, F.; Daftarian, N.; Kanavi, M.R.; Varshochian, R.; Hajiramezanali, M.; Norouzi, P.; Arefian, E.; Atyabi, F.; Dinarvand, R. Trimethyl Chitosan-Hyaluronic Acid Nano-Polyplexes for Intravitreal VEGFR-2 SiRNA Delivery: Formulation and in Vivo Efficacy Evaluation. Nanomedicine 2020, 26, 102181. [Google Scholar] [CrossRef]
- Formica, M.L.; Legeay, S.; Bejaud, J.; Montich, G.G.; Ullio Gamboa, G.V.; Benoit, J.P.; Palma, S.D. Novel Hybrid Lipid Nanocapsules Loaded with a Therapeutic Monoclonal Antibody—Bevacizumab—And Triamcinolone Acetonide for Combined Therapy in Neovascular Ocular Pathologies. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111398. [Google Scholar] [CrossRef]
- Mu, H.; Wang, Y.; Chu, Y.; Jiang, Y.; Hua, H.; Chu, L.; Wang, K.; Wang, A.; Liu, W.; Li, Y.; et al. Multivesicular Liposomes for Sustained Release of Bevacizumab in Treating Laser-Induced Choroidal Neovascularization. Drug Deliv. 2018, 25, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Karumanchi, D.K.; Skrypai, Y.; Thomas, A.; Gaillard, E.R. Rational Design of Liposomes for Sustained Release Drug Delivery of Bevacizumab to Treat Ocular Angiogenesis. J. Drug Deliv. Sci. Technol. 2018, 47, 275–282. [Google Scholar] [CrossRef]
- de Cristo Soares Alves, A.; Lavayen, V.; Figueiró, F.; Dallemole, D.R.; de Fraga Dias, A.; Cé, R.; Battastini, A.M.O.; Guterres, S.S.; Pohlmann, A.R. Chitosan-Coated Lipid-Core Nanocapsules Functionalized with Gold-III and Bevacizumab Induced In Vitro Cytotoxicity against C6 Cell Line and In Vivo Potent Antiangiogenic Activity. Pharm. Res. 2020, 37, 91. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Choi, A.; Swindle-Reilly, K.E. Controlled Release of Anti-VEGF by Redox-Responsive Polydopamine Nanoparticles. Nanoscale 2020, 12, 17298–17311. [Google Scholar] [CrossRef] [PubMed]
- Webber, A.L.; Sharwood, P. Practical Use and Prescription of Ocular Medications in Children and Infants. Clin. Exp. Optom. 2021, 104, 385–395. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Yang, H. Pediatric Ocular Nanomedicines: Challenges and Opportunities. Chin. Chem. Lett. 2017, 28, 1817–1821. [Google Scholar] [CrossRef]
- Sunita, M.; Manisha, S.; Sanjeev, M.K.; Ravi, K.S.; Aarzoo, J.; Ajai, A. Anatomical and Clinical Characteristics of Paediatric and Adult Eyes. Natl. J. Clin. Anat. 2021, 10, 5. [Google Scholar] [CrossRef]
- Mudgil, P.; Borchman, D.; Ramasubramanian, A. Insights into Tear Film Stability from Babies and Young Adults: A Study of Human Meibum Lipid Conformation and Rheology. Int. J. Mol. Sci. 2018, 19, 3502. [Google Scholar] [CrossRef]
- Chidi-Egboka, N.C.; Briggs, N.E.; Jalbert, I.; Golebiowski, B. The Ocular Surface in Children: A Review of Current Knowledge and Meta-Analysis of Tear Film Stability and Tear Secretion in Children. Ocul. Surf. 2019, 17, 28–39. [Google Scholar] [CrossRef]
- Mutlu, F.M.; Sarici, S.U. Treatment of Retinopathy of Prematurity: A Review of Conventional and Promising New Therapeutic Options. Int. J. Ophthalmol. 2013, 6, 228–236. [Google Scholar] [CrossRef]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic Properties of Gold Nanoparticles. Clin. Cancer Res. 2005, 11, 3530–3534. [Google Scholar] [CrossRef]
- Saeed, B.A.; Lim, V.; Yusof, N.A.; Khor, K.Z.; Rahman, H.S.; Samad, N.A. Antiangiogenic Properties of Nanoparticles: A Systematic Review. Int. J. Nanomed. 2019, 14, 5135–5146. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.H.; Jo, D.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. The Inhibition of Retinal Neovascularization by Gold Nanoparticles via Suppression of VEGFR-2 Activation. Biomaterials 2011, 32, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Volatron, J.; Gazeau, F. Basic Principles of in Vivo Distribution, Toxicity, and Degradation of Prospective Inorganic Nanoparticles for Imaging. In Design and Applications of Nanoparticles in Biomedical Imaging; Springer: Cham, Switzerland, 2016; pp. 9–41. [Google Scholar] [CrossRef]
- Radomska, A.; Leszczyszyn, J.; Radomski, M.W. The Nanopharmacology and Nanotoxicology of Nanomaterials: New Opportunities and Challenges. Adv. Clin. Exp. Med. 2016, 25, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Higbee-Dempsey, E.M.; Amirshaghaghi, A.; Case, M.J.; Bouché, M.; Kim, J.; Cormode, D.P.; Tsourkas, A. Biodegradable Gold Nanoclusters with Improved Excretion Due to PH-Triggered Hydrophobic-to-Hydrophilic Transition. J. Am. Chem. Soc. 2020, 142, 7783–7794. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Bohley, M.; Dillinger, A.E.; Schweda, F.; Ohlmann, A.; Braunger, B.M.; Tamm, E.R.; Goepferich, A. A Single Intravenous Injection of Cyclosporin A-Loaded Lipid Nanocapsules Prevents Retinopathy of Prematurity. Sci. Adv. 2022, 8. [Google Scholar] [CrossRef]
- Li, Q.; Weng, J.; Wong, S.N.; Thomas Lee, W.Y.; Chow, S.F. Nanoparticulate Drug Delivery to the Retina. Mol. Pharm. 2021, 18, 506–521. [Google Scholar] [CrossRef]
- Bertrand, N.; Leroux, J.C. The Journey of a Drug-Carrier in the Body: An Anatomo-Physiological Perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef]
- Hagigit, T.; Abdulrazik, M.; Valamanesh, F.; Behar-Cohen, F.; Benita, S. Ocular Antisense Oligonucleotide Delivery by Cationic Nanoemulsion for Improved Treatment of Ocular Neovascularization: An in-Vivo Study in Rats and Mice. J. Control. Release 2012, 160, 225–231. [Google Scholar] [CrossRef]
- Hagigit, T.; Nassar, T.; Behar-Cohen, F.; Lambert, G.; Benita, S. The Influence of Cationic Lipid Type on In-Vitro Release Kinetic Profiles of Antisense Oligonucleotide from Cationic Nanoemulsions. Eur. J. Pharm. Biopharm. 2008, 70, 248–259. [Google Scholar] [CrossRef]
- Hagigit, T.; Abdulrazik, M.; Orucov, F.; Valamanesh, F.; Lambert, M.; Lambert, G.; Behar-Cohen, F.; Benita, S. Topical and Intravitreous Administration of Cationic Nanoemulsions to Deliver Antisense Oligonucleotides Directed towards VEGF KDR Receptors to the Eye. J. Control. Release 2010, 145, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wu, Y.; Hu, Z.; Sun, L.; Dou, G.; Zhang, Z.; Wang, H.; Guo, C.; Wang, Y. Exosomes from Microglia Attenuate Photoreceptor Injury and Neovascularization in an Animal Model of Retinopathy of Prematurity. Mol. Ther. Nucleic Acids 2019, 16, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, E.; Anderson, J.D.; Oltjen, S.; Goswami, M.; Zawadzki, R.J.; Nolta, J.A.; Park, S.S. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr. Eye Res. 2017, 42, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Ebneter, A.; Kokona, D.; Schneider, N.; Zinkernagel, M.S. Microglia Activation and Recruitment of Circulating Macrophages During Ischemic Experimental Branch Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2017, 58, 944–953. [Google Scholar] [CrossRef]
- Deliyanti, D.; Talia, D.M.; Zhu, T.; Maxwell, M.J.; Agrotis, A.; Jerome, J.R.; Hargreaves, E.M.; Gerondakis, S.; Hibbs, M.L.; Mackay, F.; et al. Foxp3+ Tregs Are Recruited to the Retina to Repair Pathological Angiogenesis. Nat. Commun. 2017, 8, 748. [Google Scholar] [CrossRef]
- Muffat, J.; Li, Y.; Yuan, B.; Mitalipova, M.; Omer, A.; Corcoran, S.; Bakiasi, G.; Tsai, L.H.; Aubourg, P.; Ransohoff, R.M.; et al. Efficient Derivation of Microglia-like Cells from Human Pluripotent Stem Cells. Nat. Med. 2016, 22, 1358–1367. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, A.; Zhang, H.; Wang, M.; Tang, Q.; Huang, Y.; Wang, L. Inhibition of Retinal Neovascularization by VEGF SiRNA Delivered via Bioreducible Lipid-like Nanoparticles. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2407–2418. [Google Scholar] [CrossRef]
- Singerman, L. Combination Therapy Using the Small Interfering RNA Bevasiranib. Retina 2009, 29, S49–S50. [Google Scholar] [CrossRef]
- Jiang, Y.; Huo, S.; Hardie, J.; Liang, X.J.; Rotello, V.M. Progress and Perspective of Inorganic Nanoparticle-Based SiRNA Delivery Systems. Expert Opin. Drug Deliv. 2016, 13, 547–559. [Google Scholar] [CrossRef]
- Huang, K.; Lin, Z.; Ge, Y.; Chen, X.; Pan, Y.; Lv, Z.; Sun, X.; Yu, H.; Chen, J.; Yao, Q. Immunomodulation of MiRNA-223-Based Nanoplatform for Targeted Therapy in Retinopathy of Prematurity. J. Control. Release 2022, 350, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.S.; Moon, M.J.; Thomas, R.G.; Kim, S.Y.; Lee, J.S.; Jeong, Y.Y.; Park, I.K.; Park, S.W. Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush. Int. J. Mol. Sci. 2020, 21, 9297. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Guo, M.; Dong, X.; Liao, M.; Du, M.; Wang, X.; Yin, H.; Yan, H. Exosome-Mediated Delivery of the Neuroprotective Peptide PACAP38 Promotes Retinal Ganglion Cell Survival and Axon Regeneration in Rats With Traumatic Optic Neuropathy. Front. Cell Dev. Biol. 2021, 9, 659783. [Google Scholar] [CrossRef] [PubMed]
- Mezu-Ndubuisi, O.J.; Wang, Y.; Schoephoerster, J.; Falero-Perez, J.; Zaitoun, I.S.; Sheibani, N.; Gong, S. Intravitreal Delivery of VEGF-A165-Loaded PLGA Microparticles Reduces Retinal Vaso-Obliteration in an In Vivo Mouse Model of Retinopathy of Prematurity. Curr. Eye Res. 2019, 44, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.H.; Shin, Y.U.; Cho, H. Retinopathy of Prematurity: A Review of Epidemiology and Current Treatment Strategies. Clin. Exp. Pediatr. 2022, 65, 115–126. [Google Scholar] [CrossRef]
- Desjarlais, M.; Rivera, J.C.; Lahaie, I.; Cagnone, G.; Wirt, M.; Omri, S.; Chemtob, S. MicroRNA Expression Profile in Retina and Choroid in Oxygen-Induced Retinopathy Model. PLoS ONE 2019, 14, e0218282. [Google Scholar] [CrossRef]
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable Implants for Sustained Drug Release in the Eye. Pharm. Res. 2010, 27, 2043–2053. [Google Scholar] [CrossRef]
- Castro-Navarro, V.; Cervera-Taulet, E.; Navarro-Palop, C.; Monferrer-Adsuara, C.; Hernández-Bel, L.; Montero-Hernández, J. Intravitreal Dexamethasone Implant Ozurdex® in Naïve and Refractory Patients with Different Subtypes of Diabetic Macular Edema. BMC Ophthalmol. 2019, 19, 15. [Google Scholar] [CrossRef]
- Kuno, N.; Fujii, S. Biodegradable Intraocular Therapies for Retinal Disorders: Progress to Date. Drugs Aging 2010, 27, 117–134. [Google Scholar] [CrossRef]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L.; et al. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef]
- Wong, J.G.; Chang, A.; Guymer, R.H.; Wickremasinghe, S.; Reilly, E.; Bell, N.; Vantipalli, S.; Moshfeghi, A.A.; Goldstein, M.H. Phase 1 Study of an Intravitreal Axitinib Hydrogel-Based Implant for the Treatment of Neovascular Age-Related Macular Degeneration (NAMD). Investig. Ophthalmol. Vis. Sci. 2021, 62, 218. [Google Scholar]
- Alshaikh, R.A.; Waeber, C.; Ryan, K.B. Polymer Based Sustained Drug Delivery to the Ocular Posterior Segment: Barriers and Future Opportunities for the Treatment of Neovascular Pathologies. Adv. Drug Deliv. Rev. 2022, 187, 114342. [Google Scholar] [CrossRef] [PubMed]
- Vinores, S.A. Pegaptanib in the Treatment of Wet, Age-Related Macular Degeneration. Int. J. Nanomed. 2006, 1, 263–268. [Google Scholar]
- Yang, M.; Peterson, W.M.; Yu, Y.; Kays, J.; Cardona, D.; Culp, D.; Gilger, B.C.; Cleland, J. GB-102 for Wet AMD: A Novel Injectable Formulation That Safely Delivers Active Levels of Sunitinib to the Retina and RPE/Choroid for Over Four Months. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5037. [Google Scholar]
- Cheng, Y.; Burda, C. 2.01—Nanoparticles for Photodynamic Therapy. In Comprehensive Nanoscience and Technology; Andrews, D.L., Scholes, G.D., Wiederrecht, G.P., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 1–28. ISBN 978-0-12-374396-1. [Google Scholar]
- Rodrigues, G.A.; Lutz, D.; Shen, J.; Yuan, X.; Shen, H.; Cunningham, J.; Rivers, H.M. Topical Drug Delivery to the Posterior Segment of the Eye: Addressing the Challenge of Preclinical to Clinical Translation. Pharm. Res. 2018, 35, 245. [Google Scholar] [CrossRef] [PubMed]
- Doukas, J.; Mahesh, S.; Umeda, N.; Kachi, S.; Akiyama, H.; Yokoi, K.; Cao, J.; Chen, Z.; Dellamary, L.; Tam, B.; et al. Topical Administration of a Multi-Targeted Kinase Inhibitor Suppresses Choroidal Neovascularization and Retinal Edema. J. Cell. Physiol. 2008, 216, 29–37. [Google Scholar] [CrossRef]
- Yafai, Y.; Yang, X.M.; Niemeyer, M.; Nishiwaki, A.; Lange, J.; Wiedemann, P.; King, A.G.; Yasukawa, T.; Eichler, W. Anti-Angiogenic Effects of the Receptor Tyrosine Kinase Inhibitor, Pazopanib, on Choroidal Neovascularization in Rats. Eur. J. Pharmacol. 2011, 666, 12–18. [Google Scholar] [CrossRef]
- Adams, C.M.; Anderson, K.; Artman, G.; Bizec, J.C.; Cepeda, R.; Elliott, J.; Fassbender, E.; Ghosh, M.; Hanks, S.; Hardegger, L.A.; et al. The Discovery of N-(1-Methyl-5-(Trifluoromethyl)-1H-Pyrazol-3-Yl)-5-((6- ((Methylamino)Methyl)Pyrimidin-4-Yl)Oxy)-1H-Indole-1-Carboxamide (Acrizanib), a VEGFR-2 Inhibitor Specifically Designed for Topical Ocular Delivery, as a Therapy for Neovascular Age-Related Macular Degeneration. J. Med. Chem. 2018, 61, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Zernii, E.Y.; Baksheeva, V.E.; Iomdina, E.N.; Averina, O.A.; Permyakov, S.E.; Philippov, P.P.; Zamyatnin, A.A.; Senin, I.I. Rabbit Models of Ocular Diseases: New Relevance for Classical Approaches. CNS Neurol. Disord. Drug Targets 2016, 15, 267–291. [Google Scholar] [CrossRef]
- Owen, G.R.; Brooks, A.C.; James, O.; Robertson, S.M. A Novel in Vivo Rabbit Model That Mimics Human Dosing to Determine the Distribution of Antibiotics in Ocular Tissues. J. Ocul. Pharmacol. Ther. 2007, 23, 335–342. [Google Scholar] [CrossRef]
- Shen, J.; Durairaj, C.; Lin, T.; Liu, Y.; Burke, J. Ocular Pharmacokinetics of Intravitreally Administered Brimonidine and Dexamethasone in Animal Models with and without Blood-Retinal Barrier Breakdown. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1056–1066. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in Ocular Drug Delivery Systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A Review of Drug Delivery Systems Based on Nanotechnology and Green Chemistry: Green Nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Joly-Chevrier, M.; Akbar, D.; Tran, S.D. Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1094. https://doi.org/10.3390/pharmaceutics15041094
Wu KY, Joly-Chevrier M, Akbar D, Tran SD. Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics. 2023; 15(4):1094. https://doi.org/10.3390/pharmaceutics15041094
Chicago/Turabian StyleWu, Kevin Y., Maxine Joly-Chevrier, Dania Akbar, and Simon D. Tran. 2023. "Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems" Pharmaceutics 15, no. 4: 1094. https://doi.org/10.3390/pharmaceutics15041094
APA StyleWu, K. Y., Joly-Chevrier, M., Akbar, D., & Tran, S. D. (2023). Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics, 15(4), 1094. https://doi.org/10.3390/pharmaceutics15041094






