Pentoxifylline/Chitosan Films on Wound Healing: In Vitro/In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Preparation
2.2. Characterization of Films
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.3. X-ray Diffraction (XRD)
2.2.4. Differential Scanning Calorimetry (DSC)
2.3. In Vitro Release Test with Franz Cells in Synthetic Membrane
- Zero-order [35]
- First-order [36]
- Higuchi Model [36]
- Korsmeyer–Peppas Model [37]
- Hixson–Crowell Model [38]
2.4. Study of Wound Healing In Vivo
2.4.1. Animals
2.4.2. Wound Healing Assay: Treatment Groups, Clinical and Morphometric Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Film Development and Characterization
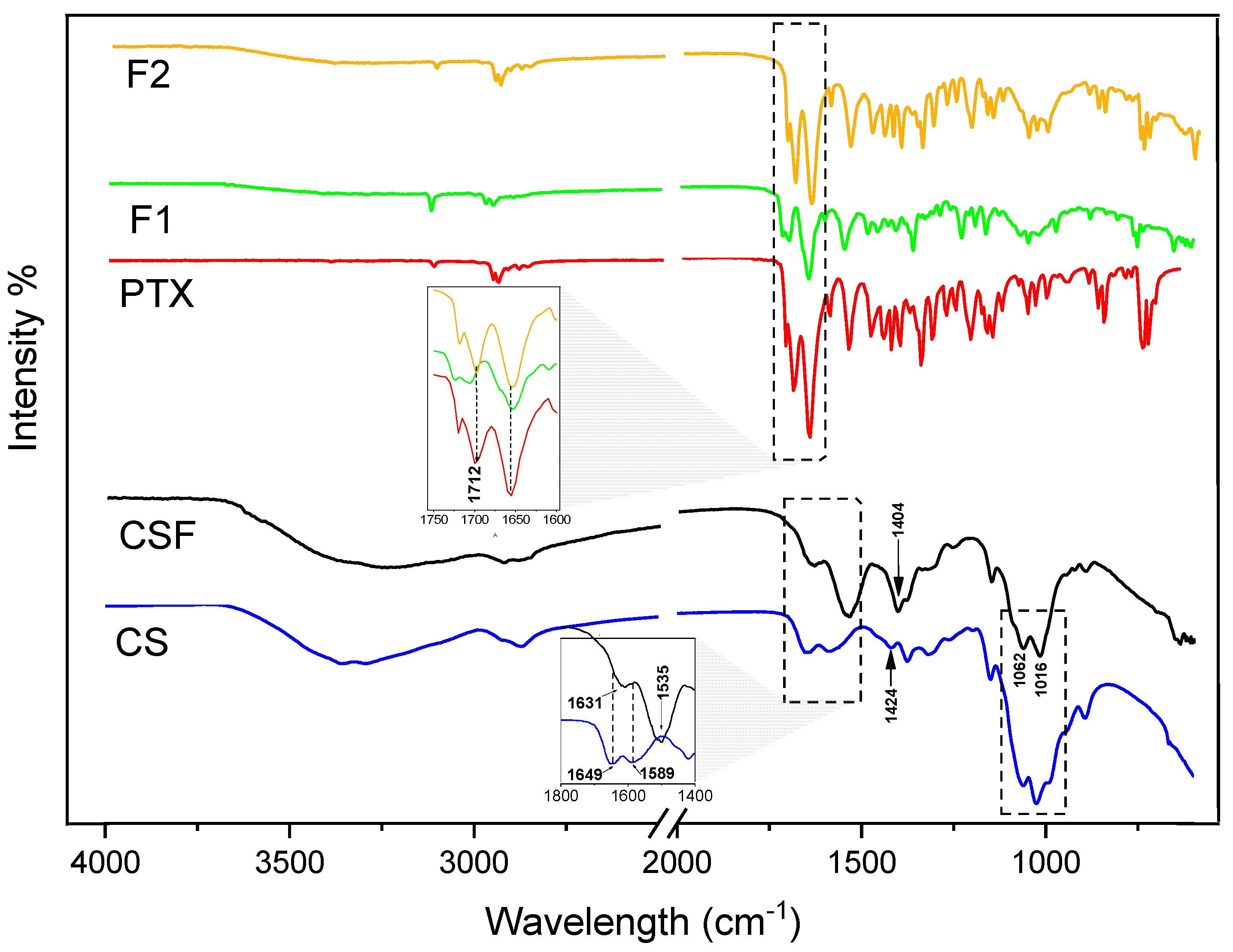
3.1.1. FTIR Spectroscopy Analysis
3.1.2. X-ray Diffraction (XRD)
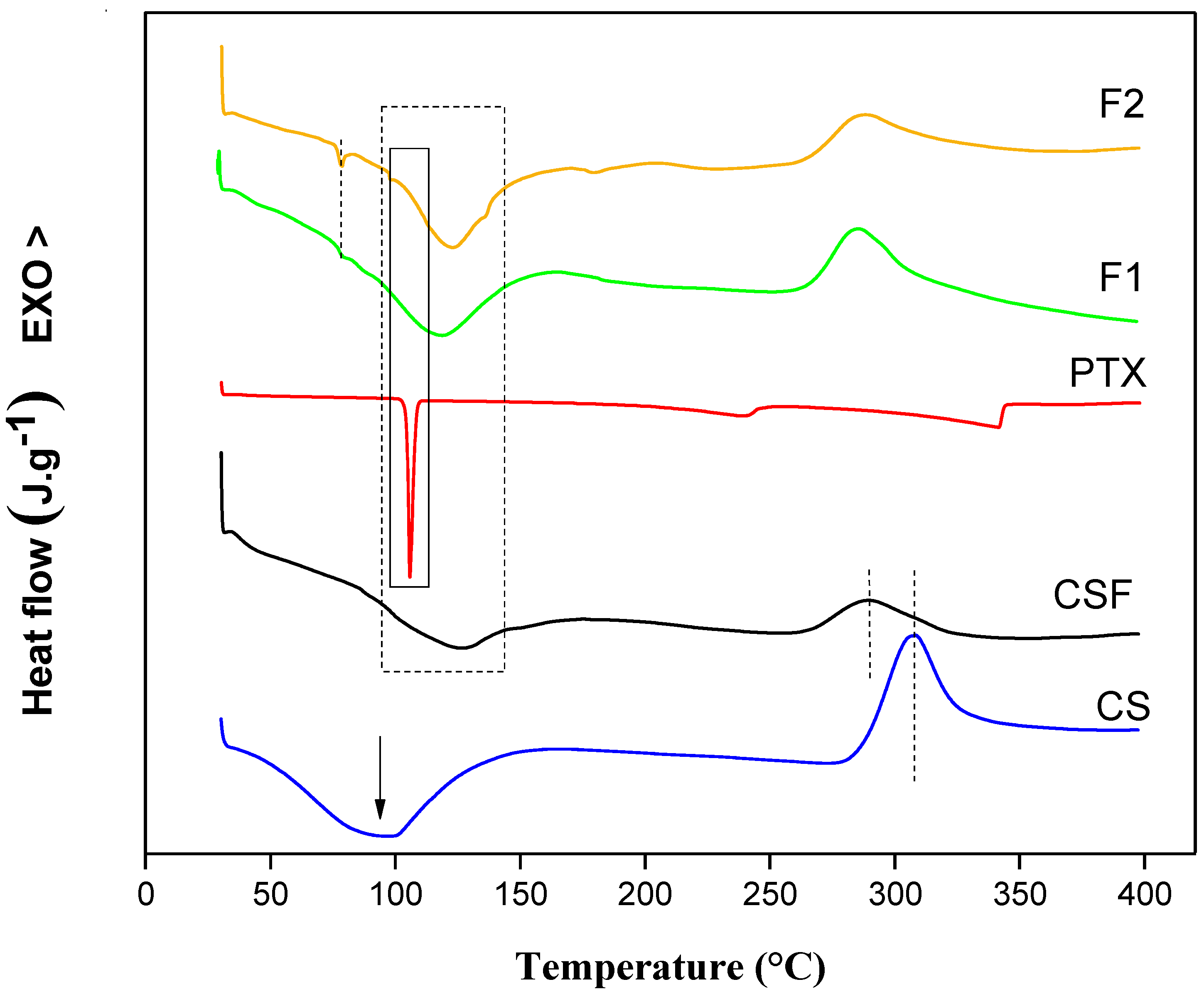
3.1.3. Differential Scanning Calorimetry
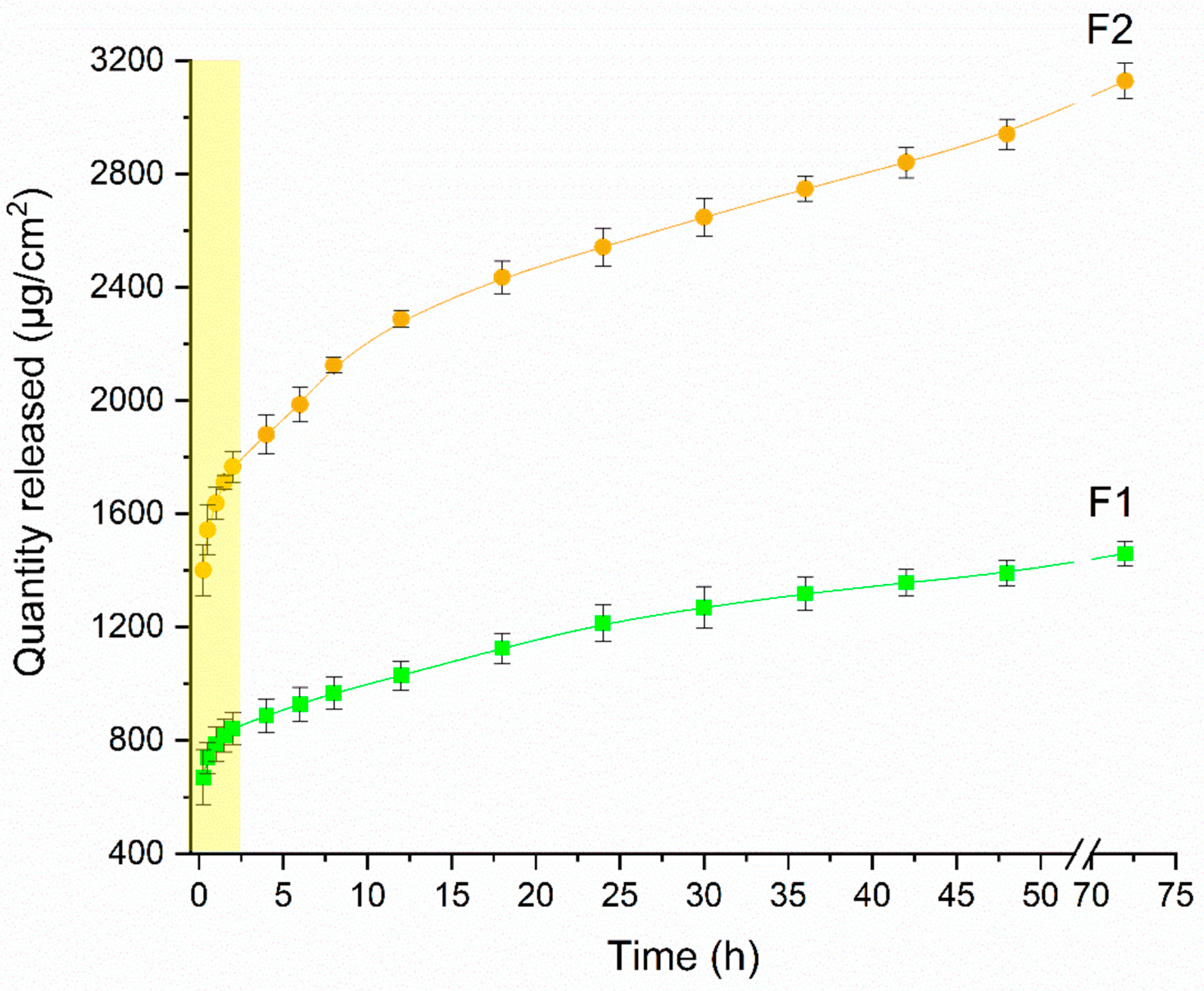
3.2. In Vitro Release Study
3.3. In Vivo Study
Clinical Aspects and Morphometric Analysis of Skin Wounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehtesabi, H.; Kalji, S.-O.; Movsesian, L. Smartphone-Based Wound Dressings: A Mini-Review. Heliyon 2022, 8, e09876. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Aguzzi, C.; Mori, M.; Grisoli, P.; Cerezo, P.; Tenci, M.; Viseras, C.; et al. Montmorillonite-Chitosan-Silver Sulfadiazine Nanocomposites for Topical Treatment of Chronic Skin Lesions: In vitro Biocompatibility, Antibacterial Efficacy, and Gap Closure Cell Motility Properties. Carbohydr. Polym. 2014, 102, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nímia, H.H.; Carvalho, V.F.; Isaac, C.; Souza, F.Á.; Gemperli, R.; Paggiaro, A.O. Comparative Study of Silver Sulfadiazine with Other Materials for Healing and Infection Prevention in Burns: A Systematic Review and Meta-Analysis. Burns 2019, 45, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Jangra, S.; Devi, S.; Tomer, V.K.; Chhokar, V.; Duhan, S. Improved Antimicrobial Property and Controlled Drug Release Kinetics of Silver Sulfadiazine Loaded Ordered Mesoporous Silica. J. Asian Ceram. Soc. 2016, 4, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Alvarado-Gomez, E.; Martínez-Castañon, G.; Sanchez-Sanchez, R.; Ganem-Rondero, A.; Yacaman, M.J.; Martinez-Gutierrez, F. Evaluation of Anti-Biofilm and Cytotoxic Effect of a Gel Formulation with Pluronic F-127 and Silver Nanoparticles as a Potential Treatment for Skin Wounds. Mater. Sci. Eng. C 2018, 92, 621–630. [Google Scholar] [CrossRef]
- Cho, J.R.; Lee, M.H.; Oh, H.K.; Kim, H.; Kweon, D.K.; Kang, S.M.; Kim, B.K.; Heo, C.Y.; Kim, D.W.; Kang, S.B. Efficacy of Hyaluronic Acid Film on Perianal Wound Healing in a Rat Model. Ann. Surg. Treat Res. 2021, 101, 206–213. [Google Scholar] [CrossRef]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; da Silva Almeida, J.R.G. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef] [Green Version]
- El-Feky, G.S.; El-Banna, S.T.; El-Bahy, G.S.; Abdelrazek, E.M.; Kamal, M. Alginate Coated Chitosan Nanogel for the Controlled Topical Delivery of Silver Sulfadiazine. Carbohydr. Polym. 2017, 177, 194–202. [Google Scholar] [CrossRef]
- He, S.; Liu, J.; He, S.; Liu, A.; Shao, W. Double Crosslinked Polyvinyl Alcohol/Gelatin/Silver Sulfadiazine Sponges with Excellent Antibacterial Performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128737. [Google Scholar] [CrossRef]
- Ma, X.; Bian, Q.; Hu, J.; Gao, J. Stem from Nature: Bioinspired Adhesive Formulations for Wound Healing. J. Control. Release 2022, 345, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Zandraa, O.; Ngwabebhoh, F.A.; Patwa, R.; Nguyen, H.T.; Motiei, M.; Saha, N.; Saha, T.; Saha, P. Development of Dual Crosslinked Mumio-Based Hydrogel Dressing for Wound Healing Application: Physico-Chemistry and Antimicrobial Activity. Int. J. Pharm. 2021, 607, 120952. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, R.; Sugumar, M.; Karuppasamy, S.; Prasad, P.; Dharmalingam, S. Fabrication of Wheatgrass Incorporated PCL/Chitosan Biomimetic Nanoscaffold for Skin Wound Healing: In vitro and in silico Analysis. J. Drug Deliv. Sci. Technol. 2022, 71, 103286. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Ren, D.-Y.; Feng, Z.-X.; Zhang, L.-Y.; Zhong, Y.-F.; Jin, M.-Y.; Xu, F.-W.; Feng, C.-Y.; Du, Y.-Z.; et al. Mussel-Inspired Collagen-Hyaluronic Acid Composite Scaffold with Excellent Antioxidant Properties and Sustained Release of a Growth Factor for Enhancing Diabetic Wound Healing. Mater. Today Bio 2022, 15, 100320. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, D.; Cheng, K.; Li, W.; Yu, Q.; Wang, L. Photothermal-Responsive Fiber Dressing with Enhanced Antibacterial Activity and Cell Manipulation towards Promoting Wound-healing. J. Colloid Interface Sci. 2022, 623, 21–33. [Google Scholar] [CrossRef]
- Sun, W.; Mu, C.; Zhang, X.; Shi, H.; Yan, Q.; Luan, S. Mussel-Inspired Polysaccharide-Based Sponges for Hemostasis and Bacteria Infected Wound Healing. Carbohydr. Polym. 2022, 295, 119868. [Google Scholar] [CrossRef]
- Wang, G.; Ye, J.; Wang, M.; Qi, Y.; Zhang, S.; Shi, L.; Fang, Y.; Tian, Y.; Ning, G. Copper Boron–Imidazolate Framework Incorporated Chitosan Membranes for Bacterial-Infected Wound Healing Dressing. Carbohydr. Polym. 2022, 291, 119588. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic Acid/Alginate and an Assorted Polymers Loaded with Honey, Plant, and Marine Compounds for Progressive Wound Healing—Know-How. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Liang, L.; Liu, T.; Ouyang, Q.; Li, S.; Li, C. Solid Phase Synthesis of Oxidized Sodium Alginate-Tobramycin Conjugate and Its Application for Infected Wound Healing. Carbohydr. Polym. 2022, 295, 119843. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, S.; Wu, Z.; Li, Q.; Ren, S.; Chen, J.; Xu, X.; Wang, C.; Lu, C.; Yang, X.; et al. ADSC-Exo@MMP-PEG Smart Hydrogel Promotes Diabetic Wound Healing by Optimizing Cellular Functions and Relieving Oxidative Stress. Mater. Today Bio 2022, 16, 100365. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, L.; Zhu, W.; Hu, C.; Jin, R.; Sun, B.; Shi, Y.; Zhang, Y.; Cui, W. Use of Ginsenoside Rg3-Loaded Electrospun PLGA Fibrous Membranes as Wound Cover Induces Healing and Inhibits Hypertrophic Scar Formation of the Skin. Colloids Surf. B Biointerfaces 2014, 115, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, J.; Mao, X.; Tang, S. A γ-PGA/KGM-Based Injectable Hydrogel as Immunoactive and Antibacterial Wound Dressing for Skin Wound Repair. Mater. Sci. Eng. C 2021, 129, 112374. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Jia, W.; Li, M.; Chen, Z. New Injectable Chitosan-Hyaluronic Acid Based Hydrogels for Hemostasis and Wound Healing. Carbohydr. Polym. 2022, 294, 119767. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, I.; Sandri, G.; Aguzzi, C.; Bonferoni, C.; Cerezo, P.; Sánchez-Espejo, R.; Viseras, C. Intestinal Permeability of Oxytetracycline from Chitosan-Montmorillonite Nanocomposites. Colloids Surf. B Biointerfaces 2014, 117, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, K.; Ding, C.; Sun, S.; Zheng, Y.; Ding, Q.; Hong, B.; Liu, W. Fabrication of Chitosan/PVP/Dihydroquercetin Nanocomposite Film for in vitro and in vivo Evaluation of Wound Healing. Int. J. Biol. Macromol. 2022, 206, 591–604. [Google Scholar] [CrossRef]
- D’souza, O.J.; Gasti, T.; Hiremani, V.D.; Pinto, J.P.; Contractor, S.S.; Shettar, A.K.; Olivia, D.; Arakera, S.B.; Masti, S.P.; Chougale, R.B. Basella Alba Stem Extract Integrated Poly (Vinyl Alcohol)/Chitosan Composite Films: A Promising Bio-Material for Wound Healing. Int. J. Biol. Macromol. 2023, 225, 673–686. [Google Scholar] [CrossRef]
- Manisha, U.K.; Ghosh, T.; Apoorva, V.P.; Divya, B.; Swathy, S.P.; Paul, A.; Basavraj, B.V. Isolation, Phytochemical Elucidation, and Wound Healing Potential of Chitosan-Based Film Loaded with Tagetes Erecta. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Tran, N.T.K.; Le, T.Q.; Nguyen, T.T.A.; Nguyen, L.T.M.; van Tran, T. Passion Fruit Peel Pectin/Chitosan Based Antibacterial Films Incorporated with Biosynthesized Silver Nanoparticles for Wound Healing Application. Alex. Eng. J. 2023, 69, 419–430. [Google Scholar] [CrossRef]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A Special Report on the Chitosan-Based Hemostatic Dressing: Experience in Current Combat Operations. J. Trauma-Inj. Infect. Crit. Care 2006, 60, 655–658. [Google Scholar] [CrossRef] [Green Version]
- da Pedretti, S.L.C.; de Rena, C.L.; Orellano, L.A.A.; de Lazari, M.G.; Campos, P.P.; Nunes, T.A. Benefits of Pentoxifylline for Skin Flap Tissue Repair in Rats. Acta Cir. Bras. 2020, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Dorjay, K.; Anwar, P. Pentoxifylline and Its Applications in Dermatology. Indian Dermatol. Online J. 2014, 5, 510. [Google Scholar] [CrossRef] [PubMed]
- Babaei, S.; Bayat, M.; Nouruzian, M.; Bayat, M. Pentoxifylline Improves Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Eur. J. Pharmacol. 2013, 700, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, A.; Kazemi, T.; Enayatifard, R.; Amiri, F.T.; Narenji, M. Investigating the Skin Penetration and Wound Healing Properties of Niosomal Pentoxifylline Cream. Eur. J. Pharm. Sci. 2020, 151, 105434. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Noves, A.; Whitney, W.R. The rate of solution of solid substances in their onw solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar]
- Korsmqer, R.W.; Gumy, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Padmaa Paarakh, M.; Ani Jose, P.; Setty, C.M.; Christoper, G.V.P. Release kinetics-concepts and applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Lowe, A.S.; Walker, M.D.; Cowan, R.; Baxter, G.D. Therapeutic Ultrasound and Wound Closure: Lack of Healing Effect on X-ray Irradiated Wounds in Murine Skin. Arch. Phys. Med. Rehabil. 2001, 82, 1507–1511. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wu, Y.; Guo, R.; Huang, Y.; Wen, S.; Shen, M.; Wang, J.; Shi, X. Laponite Nanodisks as an Efficient Platform for Doxorubicin Delivery to Cancer Cells. Langmuir 2013, 29, 5030–5036. [Google Scholar] [CrossRef]
- Weinheimer-Haus, E.M.; Mirza, R.E.; Koh, T.J. Nod-like Receptor Protein-3 Inflammasome Plays an Important Role during Early Stages of Wound Healing. PLoS ONE 2015, 10, e0119106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciarlillo, D.; Celeste, C.; Carmeliet, P.; Boerboom, D.; Theoret, C. A Hypoxia Response Element in the Vegfa Promoter Is Required for Basal Vegfa Expression in Skin and Optimal Granulation Tissue Formation during Wound Healing in Mice. PLoS ONE 2017, 12, e0180586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, T.; Gupta, S.S.; Meena, A.; Serajuddin, A.T.M. Investigation of Thermal and Viscoelastic Properties of Polymers Relevant to Hot Melt Extrusion—III: Polymethacrylates and Polymethacrylic Acid Based Polymers. J. Excip. Food Chem. 2014, 5, 56–64. [Google Scholar] [CrossRef]
- Tang, J.; Liu, H.; Gao, C.; Mu, L.; Yang, S.; Rong, M.; Zhang, Z.; Liu, J.; Ding, Q.; Lai, R. A Small Peptide with Potential Ability to Promote Wound Healing. PLoS ONE 2014, 9, e92082. [Google Scholar] [CrossRef] [PubMed]
- Kolumam, G.; Wu, X.; Lee, W.P.; Hackney, J.A.; Zavala-Solorio, J.; Gandham, V.; Danilenko, D.M.; Arora, P.; Wang, X.; Ouyang, W. IL-22R Ligands IL-20, IL-22, and IL-24 Promote Wound Healing in Diabetic Db/Db Mice. PLoS ONE 2017, 12, e0170639. [Google Scholar] [CrossRef]
- Immonen, J.A.; Zagon, I.S.; McLaughlin, P.J. Topical Naltrexone as Treatment for Type 2 Diabetic Cutaneous Wounds. Adv. Wound Care (New Rochelle) 2014, 3, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/Clay Films’ Properties as Affected by Biopolymer and Clay Micro/Nanoparticles’ Concentrations. Food Hydrocoll. 2009, 23, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-Gelatin Composites and Bi-Layer Films with Potential Antimicrobial Activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Wanderley, D.M.S.; Melo, D.F.; Silva, L.M.; Silva, W.C.; Correia, L.P.; Oshiro-Junior, J.A.; Fook, M.V.L.; Moura, R.O.; Lima, R.S.C.; Damasceno, B.P.G.L. Physical–Chemical Characterization of N-Acylhydrazone Derivative Chitosan Films Using Spectroscopic and Thermoanalytical Techniques. J. Therm. Anal. Calorim. 2019, 138, 3789–3796. [Google Scholar] [CrossRef]
- Koc, B.; Akyuz, L.; Cakmak, Y.S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Ilk, S.; Cekic, F.O.; Akata, I.; Kaya, M. Production and Characterization of Chitosan-Fungal Extract Films. Food Biosci. 2020, 35, 100545. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Moorthi, A.; Prabaharan, M. Preparation and Characterization of Chitosan/Pectin/ZnO Porous Films for Wound Healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and Properties of Chitosan Films: Effect of the Type of Solvent Acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, M.; Jiang, Q.; Hu, K.; Ouyang, M.; Zhong, F.; Qin, M.; Zhuang, L.; Wang, G. Chitosan Membranes from Acetic Acid and Imidazolium Ionic Liquids: Effect of Imidazolium Structure on Membrane Properties. J. Mol. Liq. 2021, 340, 117209. [Google Scholar] [CrossRef]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR Spectroscopy Studies on the Spontaneous Neutralization of Chitosan Acetate Films by Moisture Conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A New Look towards the Thermal Decomposition of Chitins and Chitosans with Different Degrees of Deacetylation by Coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef]
- al Shuwaili, A.H.; Rasool, B.K.A.; Abdulrasool, A.A. Optimization of Elastic Transfersomes Formulations for Transdermal Delivery of Pentoxifylline. Eur. J. Pharm. Biopharm. 2016, 102, 101–114. [Google Scholar] [CrossRef]
- Karava, A.; Lazaridou, M.; Nanaki, S.; Michailidou, G.; Christodoulou, E.; Kostoglou, M.; Iatrou, H.; Bikiaris, D.N. Chitosan Derivatives with Mucoadhesive and Antimicrobial Properties for Simultaneous Nanoencapsulation and Extended Ocular Release Formulations of Dexamethasone and Chloramphenicol Drugs. Pharmaceutics 2020, 12, 594. [Google Scholar] [CrossRef]
- Cervera, M.F.; Heinämäki, J.; de la Paz, N.; López, O.; Maunu, S.L.; Virtanen, T.; Hatanpää, T.; Antikainen, O.; Nogueira, A.; Fundora, J.; et al. Effects of Spray Drying on Physicochemical Properties of Chitosan Acid Salts. AAPS PharmSciTech 2011, 12, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Qin, J.; Zhang, P.; Chen, X.; You, X.; Zhang, F.; Zuo, B.; Yao, M. Facile Preparation of a Strong Chitosan-Silk Biocomposite Film. Carbohydr. Polym. 2020, 229, 115515. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Hussain, I.; Murtaza, G. Chemical Synthesis and Characterization of Chitosan/Silver Nanocomposites Films and Their Potential Antibacterial Activity. Int. J. Biol. Macromol. 2018, 116, 520–529. [Google Scholar] [CrossRef]
- Mazloom-Jalali, A.; Shariatinia, Z.; Tamai, I.A.; Pakzad, S.R.; Malakootikhah, J. Fabrication of Chitosan–Polyethylene Glycol Nanocomposite Films Containing ZIF-8 Nanoparticles for Application as Wound Dressing Materials. Int. J. Biol. Macromol. 2020, 153, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, J.M.F.; dos Santos, N.Z.; May, I.C.; Pollo, L.D.; Tessaro, I.C. Impact of Acid Type and Glutaraldehyde Crosslinking in the Physicochemical and Mechanical Properties and Biodegradability of Chitosan Films. Polymer. Bulletin. 2021, 78, 981–1000. [Google Scholar] [CrossRef]
- Cavalcanti, A.L.M.; Reis, M.Y.F.A.; Silva, G.C.L.; Ramalho, Í.M.M.; Guimarães, G.P.; Silva, J.A.; Saraiva, K.L.A.; Damasceno, B.P.G.L. Microemulsion for Topical Application of Pentoxifylline: In vitro Release and in vivo Evaluation. Int. J. Pharm. 2016, 506, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Li, F.; Mei, X. Structure, Mechanical and Physical Properties of Hordein/Chitosan Composite Films. LWT 2022, 163, 113596. [Google Scholar] [CrossRef]
- Sole, R.; Buranello, C.; di Michele, A.; Beghetto, V. Boosting Physical-Mechanical Properties of Adipic Acid/Chitosan Films by DMTMM cross-Linking. Int. J. Biol. Macromol. 2022, 209, 2009–2019. [Google Scholar] [CrossRef]
- Rahim, S.A.; Carter, P.A.; Elkordy, A.A. Design and Evaluation of Effervescent Floating Tablets Based on Hydroxyethyl Cellulose and Sodium Alginate Using Pentoxifylline as a Model Drug. Drug Des. Devel. Ther. 2015, 9, 1843–1857. [Google Scholar] [CrossRef] [Green Version]
- Ghate, V.M.; Kodoth, A.K.; Shah, A.; Vishalakshi, B.; Lewis, S.A. Colloidal Nanostructured Lipid Carriers of Pentoxifylline Produced by Microwave Irradiation Ameliorates Imiquimod-Induced Psoriasis in Mice. Colloids Surf. B Biointerfaces 2019, 181, 389–399. [Google Scholar] [CrossRef]
- Tita, B.; Jurca, T.; Tita, D. Thermal Stability of Pentoxifylline: Active Substance and Tablets: Part 1. Kinetic Study of the Active Substance under Non-Isothermal Conditions. J. Therm. Anal. Calorim. 2013, 113, 291–299. [Google Scholar] [CrossRef]
- Kim, J.O.; Noh, J.K.; Thapa, R.K.; Hasan, N.; Choi, M.; Kim, J.H.; Lee, J.H.; Ku, S.K.; Yoo, J.W. Nitric Oxide-Releasing Chitosan Film for Enhanced Antibacterial and in vivo Wound-Healing Efficacy. Int. J. Biol. Macromol. 2015, 79, 217–225. [Google Scholar] [CrossRef]
- Ashri, L.Y.; Abou El Ela, A.E.S.F.; Ibrahim, M.A.; Alshora, D.H.; Naguib, M.J. Optimization and Evaluation of Chitosan Buccal Films Containing Tenoxicam for Treating Chronic Periodontitis: In vitro and in vivo Studies. J. Drug Deliv. Sci. Technol. 2020, 57, 101720. [Google Scholar] [CrossRef]
- Arantes, V.T.; Faraco, A.A.G.; Ferreira, F.B.; Oliveira, C.A.; Martins-Santos, E.; Cassini-Vieira, P.; Barcelos, L.S.; Ferreira, L.A.M.; Goulart, G.A.C. Retinoic Acid-Loaded Solid Lipid Nanoparticles Surrounded by Chitosan Film Support Diabetic Wound Healing in in vivo Study. Colloids Surf. B Biointerfaces 2020, 188, 110749. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Feki, A.; Bardaa, S.; Li, S.; Nagarajan, S.; Mellouli, M.; Boudawara, T.; Sahnoun, Z.; Nasri, M.; Nasri, R. A Novel Blue Crab Chitosan/Protein Composite Hydrogel Enriched with Carotenoids Endowed with Distinguished Wound Healing Capability: In vitro Characterization and in vivo Assessment. Mater. Sci. Eng. C 2020, 113, 110978. [Google Scholar] [CrossRef]
- Bardaa, S.; Chabchoub, N.; Jridi, M.; Moalla, D.; Mseddi, M.; Rebai, T.; Sahnoun, Z. The Effect of Natural Extracts on Laser Burn Wound Healing. J. Surg. Res. 2016, 201, 464–472. [Google Scholar] [CrossRef]
- Tamer, T.M.; Hassan, M.A.; Valachová, K.; Omer, A.M.; El-Shafeey, M.E.A.; Mohy Eldin, M.S.; Šoltés, L. Enhancement of Wound Healing by Chitosan/Hyaluronan Polyelectrolyte Membrane Loaded with Glutathione: In vitro and in vivo Evaluations. J. Biotechnol. 2020, 310, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.E.; Machado, B.E.K.; da Silva, M.G.C.; da Silva, M.M.A.; Bosco, L.D.; Marques, M.S.; Horn, A.P.; Persich, L.; Geller, F.C.; Argenta, D.; et al. Coumestrol/Hydroxypropyl-β-Cyclodextrin Association Incorporated in Hydroxypropyl Methylcellulose Hydrogel Exhibits Wound Healing Effect: In vitro and in vivo Study. Eur. J. Pharm. Sci. 2018, 119, 179–188. [Google Scholar] [CrossRef]
- Hajji, S.; ben Khedir, S.; Hamza-Mnif, I.; Hamdi, M.; Jedidi, I.; Kallel, R.; Boufi, S.; Nasri, M. Biomedical Potential of Chitosan-Silver Nanoparticles with Special Reference to Antioxidant, Antibacterial, Hemolytic and in vivo Cutaneous Wound Healing Effects. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 241–254. [Google Scholar] [CrossRef]
- Moangella Andrade de Assis, K.; Maísa da Silva Leite, J.; Ferreira de Melo, D.; Cordeiro Borges, J.; Matheus Barreto Santana, L.; Maria Lucas dos Reis, M.; Martins Moreira, V.; Rainny Vieira da Rocha, W.; Mayer Ramalho Catão, R.; Golzio dos Santos, S.; et al. Bicontinuous Microemulsions Containing Melaleuca Alternifolia Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing. Drug Deliv. Transl. Res. 2020, 10, 1748–1763. [Google Scholar] [CrossRef]
- Babaei, S.; Bayat, M. Pentoxifylline Accelerates Wound Healing Process by Modulating Gene Expression of MMP-1, MMP-3, and TIMP-1 in Normoglycemic Rats. J. Investig. Surg. 2015, 28, 196–201. [Google Scholar] [CrossRef]
- Eğin, S.; Açıksarı, K.; Ercan, G.; Aydın, A.F.; Üstyol, E.A.; Eser, M.; Tanrıverdi, G.; Yanar, H.T. Effects of Pentoxifylline on Oxidative Stress in Rats with Abdominal Compartment Syndrome Model. Int. J. Surg. Open 2016, 5, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Sunil, V.R.; Vayas, K.N.; Cervelli, J.A.; Malaviya, R.; Hall, L.R.; Massa, C.B.; Gow, A.J.; Laskin, J.D.; Laskin, D.L. Pentoxifylline Attenuates Nitrogen Mustard-Induced Acute Lung Injury, Oxidative Stress, and Inflammation. Exp. Mol. Pathol. 2014, 97, 89. [Google Scholar] [CrossRef] [Green Version]
- Dhulqarnain, A.O.; Takzaree, N.; Hassanzadeh, G.; Tooli, H.; Malekzadeh, M.; Khanmohammadi, N.; Yaghobinejad, M.; Solhjoo, S.; Rastegar, T. Pentoxifylline Improves the Survival of Spermatogenic Cells via Oxidative Stress Suppression and Upregulation of PI3K/AKT Pathway in Mouse Model of Testicular Torsion-Detorsion. Heliyon 2021, 7, e06868. [Google Scholar] [CrossRef]
- Radfar, M.; Larijani, B.; Hadjibabaie, M.; Rajabipour, B.; Mojtahedi, A.; Abdollahi, M. Effects of Pentoxifylline on Oxidative Stress and Levels of EGF and NO in Blood of Diabetic Type-2 Patients; a Randomized, Double-Blind Placebo-Controlled Clinical Trial. Biomed. Pharmacother. 2005, 59, 302–306. [Google Scholar] [CrossRef]
- Dinckan, A.; Sahin, E.; Ogus, M.; Emek, K.; Gumuslu, S. The Effect of Pentoxifylline on Oxidative Stress in CO2 Pneumoperitoneum. Surg. Endosc. Other Interv. Tech. 2009, 23, 534–538. [Google Scholar] [CrossRef]
- Nesek-Adam, V.; Vnuk, D.; Rašić, Ž.; Rumenjak, V.; Kos, J.; Krstonijević, Z. Comparison of the Effects of Low Intra-Abdominal Pressure and Pentoxifylline on Oxidative Stress during CO2 Pneumoperitoneum in Rabbits. Eur. Surg. Res. 2009, 43, 330–337. [Google Scholar] [CrossRef]
- Ahmad, M.; Abu-Taweel, G.M.; Aboshaiqah, A.E.; Ajarem, J.S. The Effects of Quinacrine, Proglumide, and Pentoxifylline on Seizure Activity, Cognitive Deficit, and Oxidative Stress in Rat Lithium-Pilocarpine Model of Status Epilepticus. Oxid. Med. Cell. Longev. 2014, 2014, 630509. [Google Scholar] [CrossRef] [Green Version]
- Chavarría, A.P.; Vázquez, R.R.V.; Cherit, J.G.D.; Bello, H.H.; Suastegui, H.C.; Moreno-Castañeda, L.; Alanís Estrada, G.; Hernández, F.; González-Marcos, O.; Saucedo-Orozco, H.; et al. Antioxidants and Pentoxifylline as Coadjuvant Measures to Standard Therapy to Improve Prognosis of Patients with Pneumonia by COVID-19. Comput. Struct. Biotechnol. J. 2021, 19, 1379. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and Angiogenic Chitosan Microneedle Array Patch for Promoting Wound Healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Haktaniyan, M.; Bradley, M. Polymers Showing Intrinsic Antimicrobial Activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef]
- Baran, A.A.; Keskin, C.; Fırat Baran, M.; Huseynova, I.; Khalilov, R.; Eftekhari, A.; Irtegun-Kandemir, S.; Kavak, D.E. Ecofriendly Synthesis of Silver Nanoparticles Using Ananas Comosus Fruit Peels: Anticancer and Antimicrobial Activities. Bioinorg. Chem. Appl. 2021, 2021, 2058149. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Gholipour, B.; Rostamnia, S.; Eftekhari, A.; Tanomand, A.; Valizadeh, K.A.; Khaksar, S.; Khalilov, R. Biosynthesis of AgNPs onto the Urea-Based Periodic Mesoporous Organosilica (AgxNPs/Ur-PMO) for Antibacterial and Cell Viability Assay. J. Colloid Interface Sci. 2021, 585, 676–683. [Google Scholar] [CrossRef]



| Group | Treatment | Number of Animals | Duration of the Treatment |
|---|---|---|---|
| G1 | Sterile saline solution 0.9% (200 µL) (SS) | 7 | 9 days |
| G2 | CSF | 7 | |
| G3 | F1 (1370.72 µg PTX) | 7 | |
| G4 | F2 (2741.45 µg PTX) | 7 | |
| G5 | Betamethasone Dipropionate Ointment with Gentamicin Sulfate (BD + GS O) | 7 | |
| The total amount of animals | 35 | ||
| Formulations | |||||
|---|---|---|---|---|---|
| Models | F1 | F2 | |||
| k0 (µg.min−1) | r2 | k (µg.min−1) | r2 | ||
| Zero-order | t ≤ 2 h | 30.54 | 0.9519 | 32.21 | 0.9585 |
| t ≥ 2 h | 1.75 | 0.9453 | 1.86 | 0.9570 | |
| First-order | k1 (min−1) × 10−3 | k1 (min−1) × 10−3 | |||
| t ≤ 2 h | 0.40 | 0.9735 | 0.43 | 0.9790 | |
| t ≥ 2 h | 0.04 | 0.9806 | 0.05 | 0.9830 | |
| Higuchi | kH (µg.min−1/2) | kH (µg.min−1/2) | |||
| t ≤ 2 h | 39.86 | 0.9821 | 42.03 | 0.9855 | |
| t ≥ 2 h | 12.48 | 0.9857 | 13.25 | 0.9832 | |
| Korsmeyer–Peppas | kkp(min−n) × 10−3 | kH (µg.min−1/2) | |||
| t ≤ 2 h * | 42.81 | 0.9958 | 45.13 | 0.9965 | |
| n * | 0.2060 | 0.1081 | |||
| t ≥ 2 h ** | 34.98 | 0.9906 | 39.07 | 0.9974 | |
| n ** | 0.1074 | 0.1914 | |||
| Hixson–Crowell | kHC (µg1/3.min−1) × 10−3 | kHC (µg1/3.min−1) × 10−3 | |||
| t ≥ 2 h | 0.12 | 0.9678 | 0.13 | 0.9737 | |
| t ≤ 2 h | 0.01 | 0.9854 | 0.01 | 0.9852 | |
| Best Fit | Korsmeyer–Peppas | Korsmeyer–Peppas | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, V.M.; Leite, J.M.d.S.; Medeiros, K.d.A.; Assis, K.M.A.d.; Borges, J.C.; Santana, L.M.B.; Moreira, L.M.C.d.C.; Alves, L.P.; Oliveira, T.K.B.d.; Silveira, J.W.d.S.d.; et al. Pentoxifylline/Chitosan Films on Wound Healing: In Vitro/In Vivo Evaluation. Pharmaceutics 2023, 15, 1122. https://doi.org/10.3390/pharmaceutics15041122
Moreira VM, Leite JMdS, Medeiros KdA, Assis KMAd, Borges JC, Santana LMB, Moreira LMCdC, Alves LP, Oliveira TKBd, Silveira JWdSd, et al. Pentoxifylline/Chitosan Films on Wound Healing: In Vitro/In Vivo Evaluation. Pharmaceutics. 2023; 15(4):1122. https://doi.org/10.3390/pharmaceutics15041122
Chicago/Turabian StyleMoreira, Vandiara Martins, Joandra Maísa da Silva Leite, Kaline de Araújo Medeiros, Karoll Moangella Andrade de Assis, Joyce Cordeiro Borges, Lucas Matheus Barreto Santana, Lívia Maria Coelho de Carvalho Moreira, Larissa Pereira Alves, Tharcia Kiara Beserra de Oliveira, João Walter de Souza da Silveira, and et al. 2023. "Pentoxifylline/Chitosan Films on Wound Healing: In Vitro/In Vivo Evaluation" Pharmaceutics 15, no. 4: 1122. https://doi.org/10.3390/pharmaceutics15041122





