Kinin B2 and B1 Receptors Activation Sensitize the TRPA1 Channel Contributing to Anastrozole-Induced Pain Symptoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Animals
2.3. Anastrozole-Induced Pain Model
2.4. Study Design for Behavioral Assessment
2.4.1. Pain Induction by Anastrozole
2.4.2. Assessment of B2R, B1R, and TRPA1 Involvement in Anastrozole-Induced Pain
2.4.3. Interaction Assessment of the B2R, B1R, and TRPA1 in Anastrozole-Induced Painful Behaviors
2.4.4. Intracellular Pathways Dependent on Kinin Receptor Activation and TRPA1 Sensitization
2.5. Behavioral Experiments
2.5.1. Overt Nociceptive Behavior
2.5.2. Mechanical Allodynia
2.5.3. Muscle Strength
2.5.4. Desensitization Protocol
2.6. Statistical Analysis
3. Results
3.1. Systemic Anastrozole Induces Prolonged Pain Symptoms in Mice

3.2. Kinin B2 and B1 Receptors and the TRPA1 Channel Contribute to Pain Induced by Anastrozole
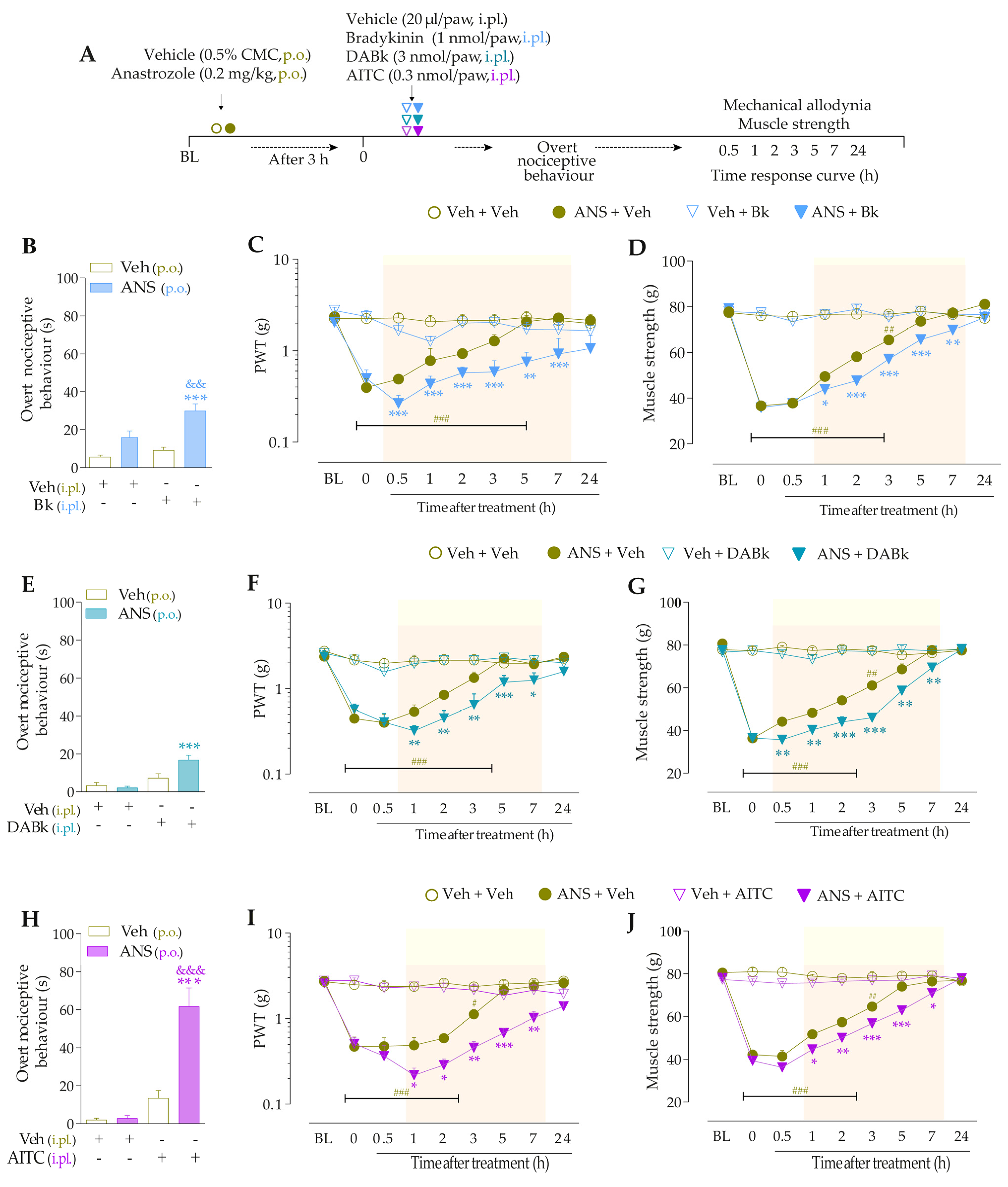
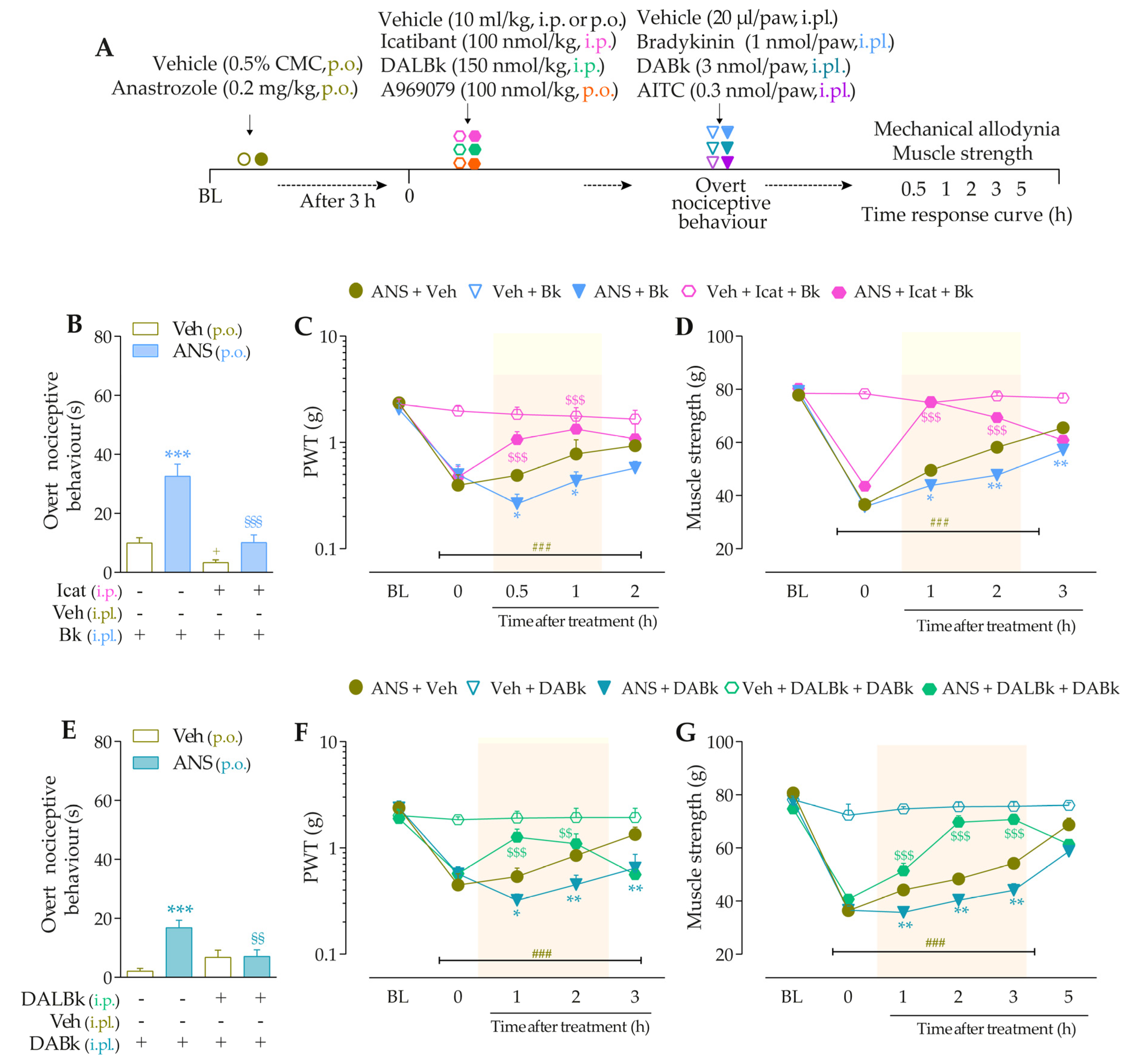
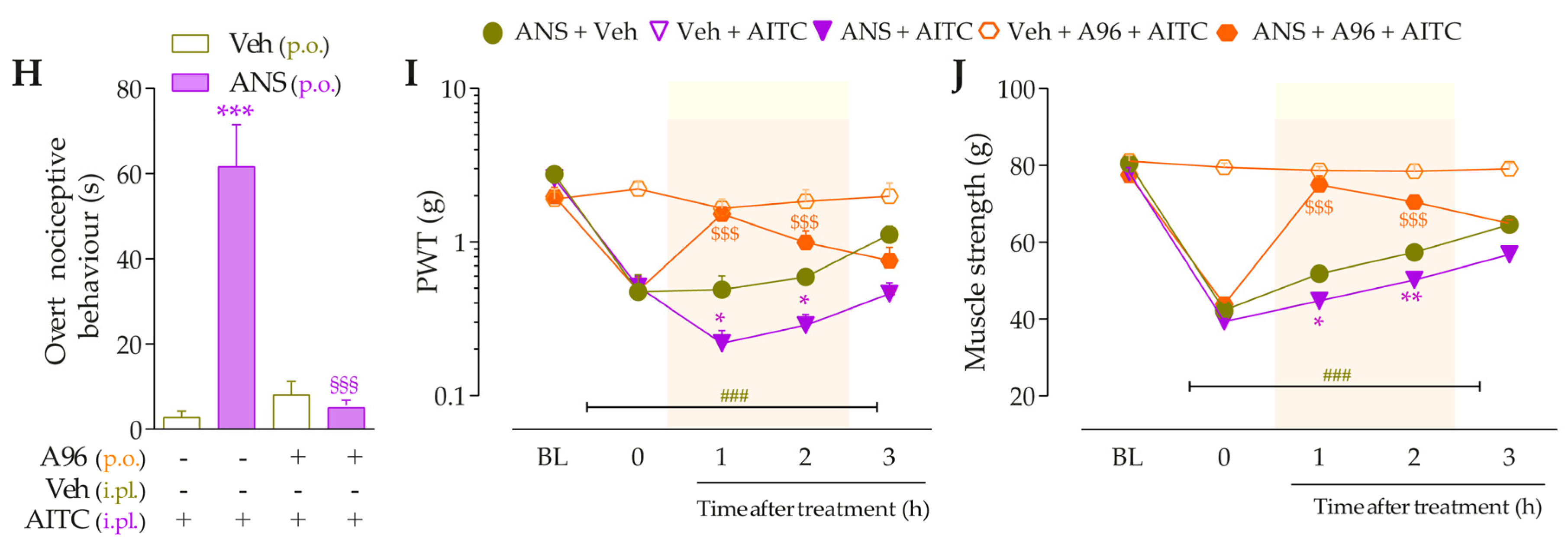
3.3. Interaction of Kinin B2 and B1 Receptors and the TRPA1 Channel Sustain the Anastrozole-Induced Pain


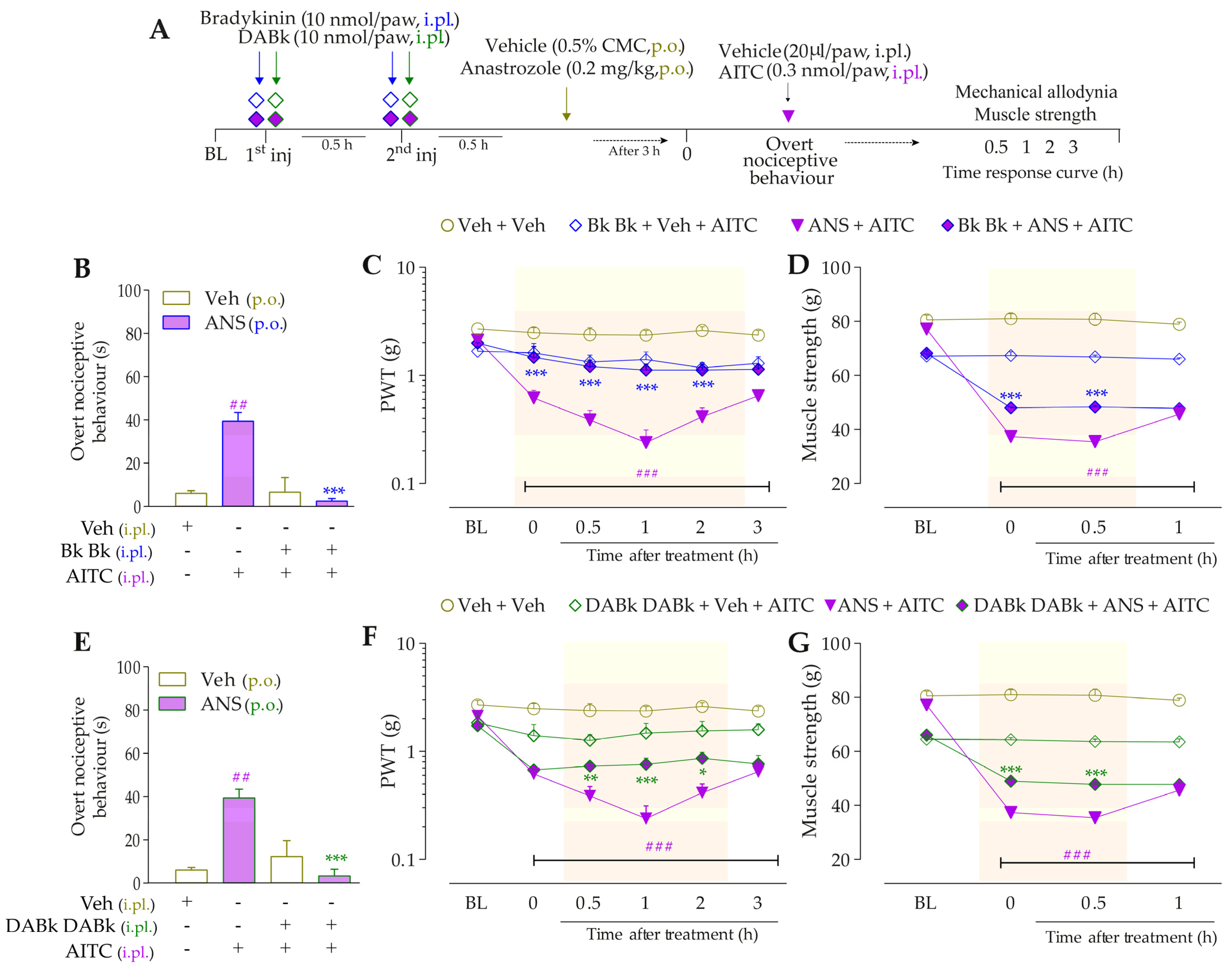
3.4. Intracellular Pathways Dependent on Kinin B2 and B1 Receptor Activation Cooperate to Sensitize TRPA1 in Anastrozole-Treated Mice
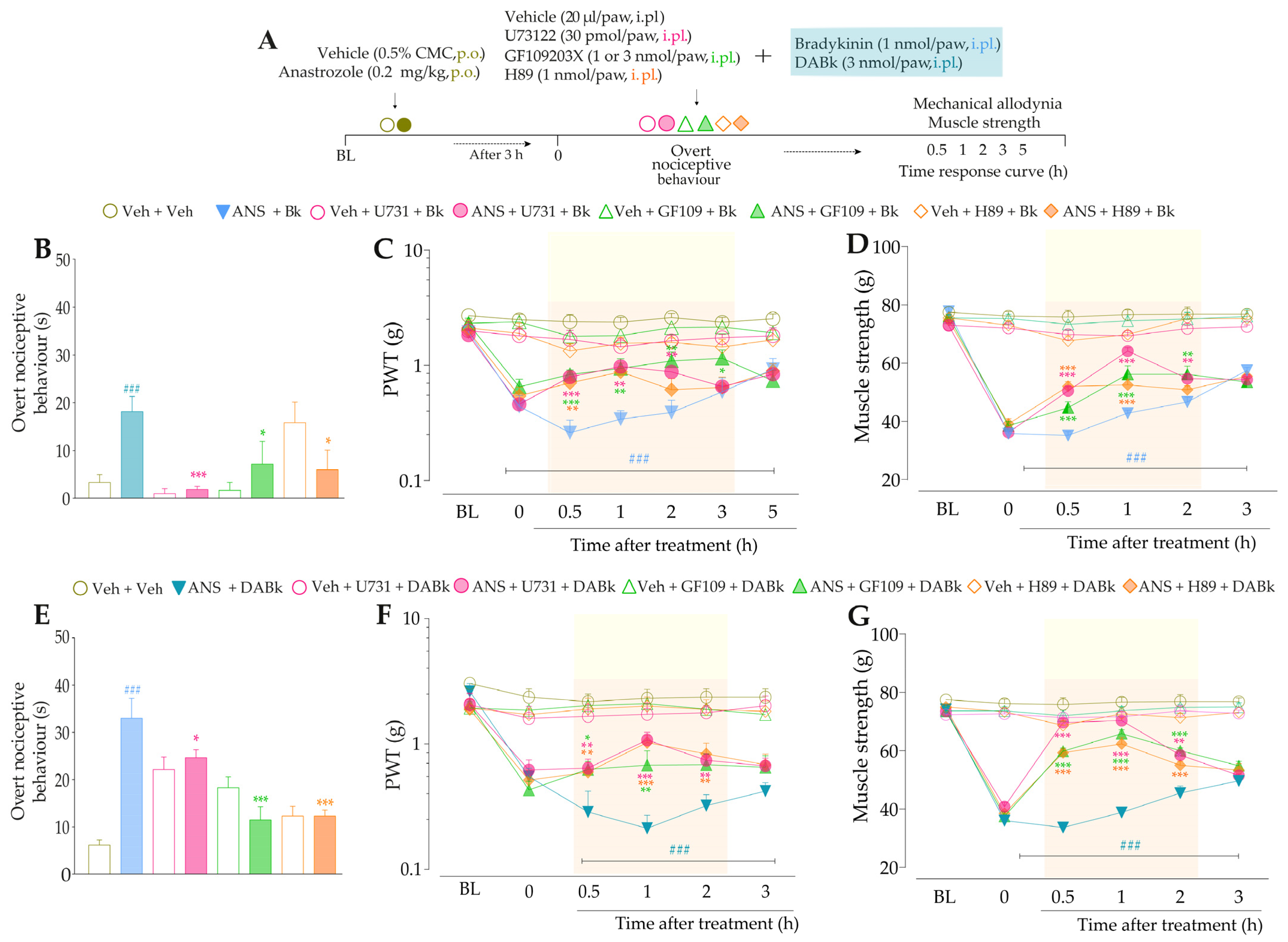
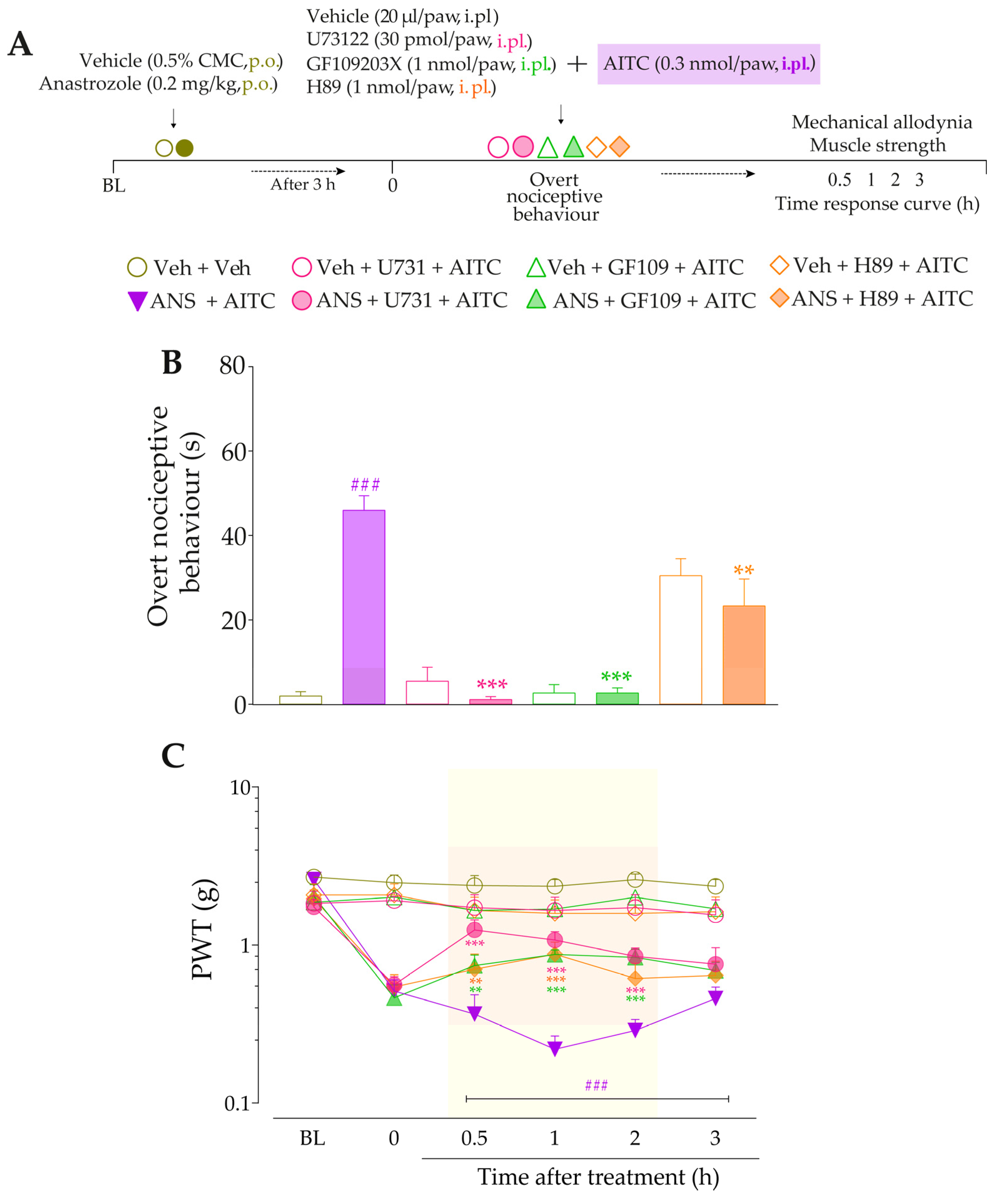
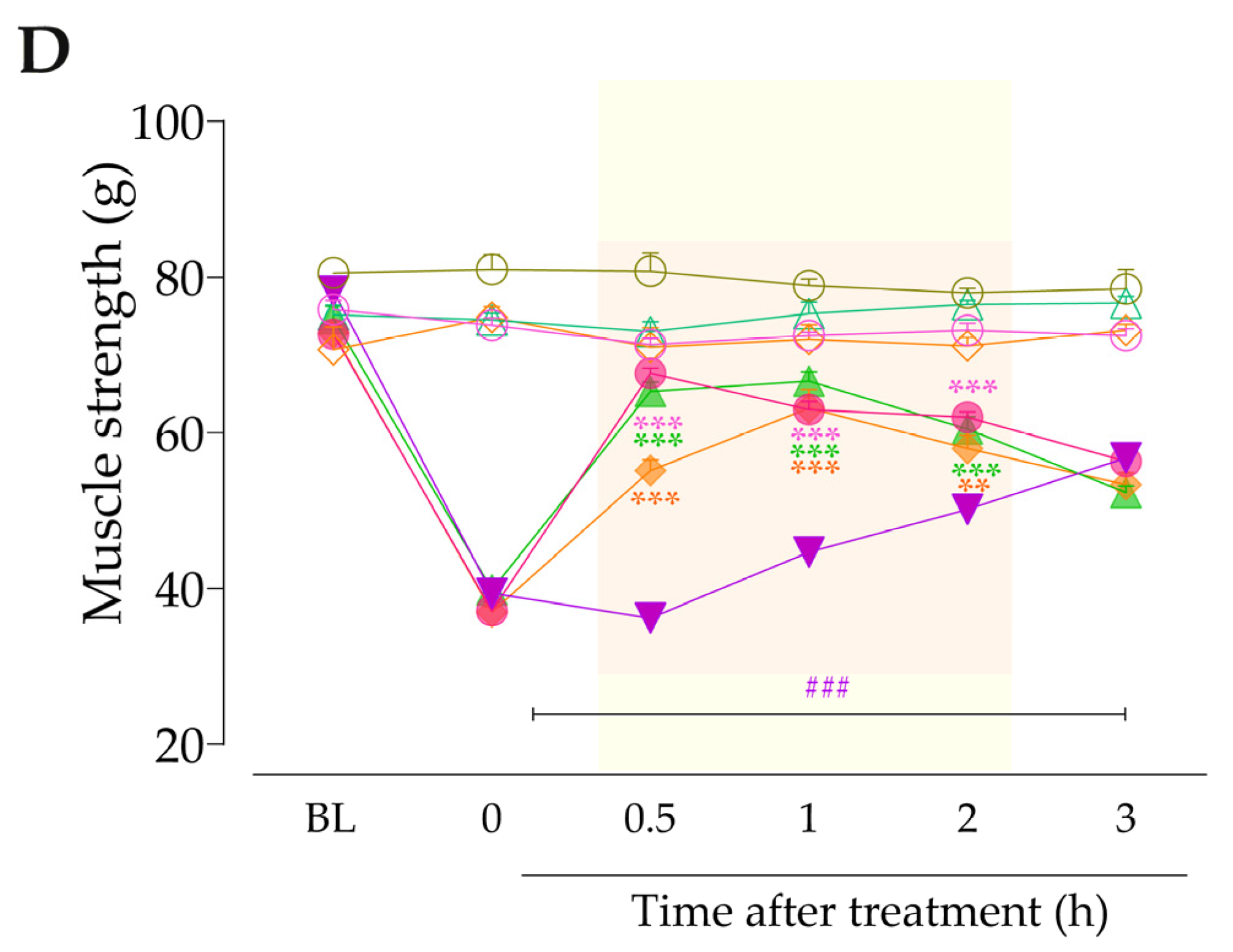
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bennett, M.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Tenti, S.; Correale, P.; Cheleschi, S.; Fioravanti, A.; Pirtoli, L. Aromatase Inhibitors—Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. Int. J. Mol. Sci. 2020, 21, 5625. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.; Lawrence, D.; Dawson, C.; Bliss, J. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst. Rev. 2009, 2021, CD003370. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Callahan, L.F.; Rini, C.; Altpeter, M.; Hackney, B.; DePue, A.; Wilson, A.; Schechter, A.; Muss, H.B. Aromatase inhibitor associated arthralgia: The importance of oncology provider-patient communication about side effects and potential management through physical activity. Support. Care Cancer 2016, 24, 2643–2650. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 288. [Google Scholar] [CrossRef]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor—Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef]
- Henry, N.L.; Giles, J.T.; Ang, D.; Mohan, M.; Dadabhoy, D.; Robarge, J.; Hayden, J.; Lemler, S.; Shahverdi, K.; Powers, P.; et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res. Treat. 2008, 111, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Laroche, F.; Coste, J.; Medkour, T.; Cottu, P.H.; Pierga, J.-Y.; Lotz, J.-P.; Beerblock, K.; Tournigand, C.; Declèves, X.; de Cremoux, P.; et al. Classification of and Risk Factors for Estrogen Deprivation Pain Syndromes Related to Aromatase Inhibitor Treatments in Women With Breast Cancer: A Prospective Multicenter Cohort Study. J. Pain 2014, 15, 293–303. [Google Scholar] [CrossRef]
- Crew, K.D.; Greenlee, H.; Capodice, J.; Raptis, G.; Brafman, L.; Fuentes, D.; Sierra, A.; Hershman, D.L. Prevalence of Joint Symptoms in Postmenopausal Women Taking Aromatase Inhibitors for Early-Stage Breast Cancer. J. Clin. Oncol. 2007, 25, 3877–3883. [Google Scholar] [CrossRef]
- Gaillard, S.; Stearns, V. Aromatase inhibitor-associated bone and musculoskeletal effects: New evidence defining etiology and strategies for management. Breast Cancer Res. 2011, 13, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schug, S.A.; Lavand’Homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fusi, C.; Materazzi, S.; Benemei, S.; Coppi, E.; Trevisan, G.; Marone, I.M.; Minocci, D.; De Logu, F.; Tuccinardi, T.; Di Tommaso, M.R.; et al. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat. Commun. 2014, 5, 5736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassini, R.; Gees, M.; Harrison, S.; De Siena, G.; Materazzi, S.; Moretto, N.; Failli, P.; Preti, D.; Marchetti, N.; Cavazzini, A.; et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 2011, 152, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, S.; Fusi, C.; Benemei, S.; Pedretti, P.; Patacchini, R.; Nilius, B.; Prenen, J.; Creminon, C.; Geppetti, P.; Nassini, R. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflügers Arch.-Eur. J. Physiol. 2012, 463, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Materazzi, S.; Fusi, C.; Altomare, A.; Aldini, G.; Lodovici, M.; Patacchini, R.; Geppetti, P. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013, 73, 3120–3131. [Google Scholar] [CrossRef] [Green Version]
- Antoniazzi, C.; Nassini, R.; Rigo, F.K.; Milioli, A.M.; Bellinaso, F.; Camponogara, C.; Silva, C.R.; De Almeida, A.S.; Rossato, M.F.; De Logu, F.; et al. Transient receptor potential ankyrin 1 (TRPA1) plays a critical role in a mouse model of cancer pain. Int. J. Cancer 2019, 144, 355–365. [Google Scholar] [CrossRef]
- de Almeida, A.S.; Rigo, F.K.; De Prá, S.D.-T.; Milioli, A.M.; Pereira, G.C.; Lückemeyer, D.D.; Antoniazzi, C.T.; Kudsi, S.Q.; Araújo, D.M.P.A.; Oliveira, S.M.; et al. Role of transient receptor potential ankyrin 1 (TRPA1) on nociception caused by a murine model of breast carcinoma. Pharmacol. Res. 2020, 152, 104576. [Google Scholar] [CrossRef]
- Brusco, I.; Puma, S.L.; Chiepe, K.B.; Brum, E.S.; Antoniazzi, C.T.D.; Almeida, A.S.; Camponogara, C.; Silva, C.R.; De Logu, F.; Andrade, V.M.; et al. Dacarbazine alone or associated with melanoma-bearing cancer pain model induces painful hypersensitivity by TRPA1 activation in mice. Int. J. Cancer 2020, 146, 2797–2809. [Google Scholar] [CrossRef] [Green Version]
- De Logu, F.; Marini, M.; Landini, L.; Souza Monteiro de Araujo, D.; Bartalucci, N.; Trevisan, G.; Gennaro Bruno, G.; Marangoni, M.; Schmidt, B.L.; Nigel, W.; et al. Peripheral Nerve Resident Macrophages and Schwann Cells Mediate Cancer-induced Pain. Cancer Res. 2021, 81, 3387–3401. [Google Scholar] [CrossRef]
- Bujalska, M.; Makulska-Nowak, H. Bradykinin receptor antagonists and cyclooxygenase inhibitors in vincristine-and streptozotocin-induced hyperalgesia. Pharmacol. Rep. 2009, 61, 631–640. [Google Scholar] [CrossRef]
- Bujalska, M.; Tatarkiewicz, J.; Gumułka, S.W. Effect of Bradykinin Receptor Antagonists on Vincristine- and Streptozotocin-Induced Hyperalgesia in a Rat Model of Chemotherapy-Induced and Diabetic Neuropathy. Pharmacology 2008, 81, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Motta, E.M.; Dutra, R.C.; Manjavachi, M.N.; Bento, A.F.; Malinsky, F.R.; Pesquero, J.B.; Calixto, J.B. Anti-nociceptive effect of kinin B 1 and B 2 receptor antagonists on peripheral neuropathy induced by paclitaxel in mice. Br. J. Pharmacol. 2011, 164, 681–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusco, I.; Silva, C.R.; Trevisan, G.; Gewehr, C.D.C.V.; Rigo, F.K.; Tamiozzo, L.L.R.; Rossato, M.F.; Tonello, R.; Dalmolin, G.D.; Cabrini, D.D.A.; et al. Potentiation of Paclitaxel-Induced Pain Syndrome in Mice by Angiotensin I Converting Enzyme Inhibition and Involvement of Kinins. Mol. Neurobiol. 2017, 54, 7824–7837. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, G.; Winter, J. Bradykinin B1 receptor is constitutively expressed in the rat sensory nervous system. Neurosci. Lett. 2000, 294, 175–178. [Google Scholar] [CrossRef]
- Ca, Q.M.; Heavens, R. Basal expression of bradykinin B 1 receptor in the spinal cord in humans and rats. Neuroreport 2001, 12, 2311–2314. [Google Scholar]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Jordt, S.-E.; Bautista, D.M.; Chuang, H.-H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Moreau, M.E.; Garbacki, N.; Molinaro, G.; Brown, N.J.; Marceau, F.; Adam, A. The kallikrein-kinin system: Current and future pharmacological targets. J. Pharmacol. Sci. 2005, 99, 6–38. [Google Scholar] [CrossRef] [Green Version]
- Bautista, D.M.; Jordt, S.-E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Dai, Y.; Fukuoka, T.; Yamanaka, H.; Kobayashi, K.; Obata, K.; Cui, X.; Tominaga, M.; Noguchi, K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: A molecular mechanism of inflammatory pain. Brain 2008, 131, 1241–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Dubin, A.E.; Petrus, M.J.; Earley, T.J. Nociceptive Signals Induce Trafficking of TRPA1 to the Plasma Membrane. Neuron 2009, 64, 498–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meotti, F.C.; Figueiredo, C.P.; Manjavachi, M.; Calixto, J.B. The transient receptor potential ankyrin-1 mediates mechanical hyperalgesia induced by the activation of B1 receptor in mice. Biochem. Pharmacol. 2017, 125, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.; Meotti, F.; Calixto, J. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012, 133, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Da Silva, G.L.; Calixto, J.B. Contribution of vanilloid receptors to the overt nociception induced by B 2 kinin receptor activation in mice. Br. J. Pharmacol. 2004, 141, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Andrade, E.; Luiz, A.; Ferreira, J.; Calixto, J. Pronociceptive response elicited by TRPA1 receptor activation in mice. Neuroscience 2008, 152, 511–520. [Google Scholar] [CrossRef]
- Costa, R.; Bicca, M.A.; Manjavachi, M.N.; Segat, G.C.; Dias, F.C.; Fernandes, E.S.; Calixto, J.B. Kinin Receptors Sensitize TRPV4 Channel and Induce Mechanical Hyperalgesia: Relevance to Paclitaxel-Induced Peripheral Neuropathy in Mice. Mol. Neurobiol. 2018, 55, 2150–2161. [Google Scholar] [CrossRef]
- Brusco, I.; Justino, A.B.; Silva, C.R.; Fischer, S.; Cunha, T.M.; Scussel, R.; Machado-De-Ávila, R.A.; Ferreira, J.; Oliveira, S.M. Kinins and their B1 and B2 receptors are involved in fibromyalgia-like pain symptoms in mice. Biochem. Pharmacol. 2019, 168, 119–132. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- De Logu, F.; Tonello, R.; Materazzi, S.; Nassini, R.; Fusi, C.; Coppi, E.; Puma, S.L.; Marone, I.M.; Sadofsky, L.R.; Morice, A.H.; et al. TRPA1 Mediates Aromatase Inhibitor–Evoked Pain by the Aromatase Substrate Androstenedione. Cancer Res. 2016, 76, 7024–7035. [Google Scholar] [CrossRef] [Green Version]
- Calixto, J.B.; Medeiros, R.; Fernandes, E.S.; Ferreira, J.; A Cabrini, D.; Campos, M.M. Kinin B 1 receptors: Key G-protein-coupled receptors and their role in inflammatory and painful processes. Br. J. Pharmacol. 2004, 143, 803–818. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Zameer, S.; Vohora, D. Effect of aromatase inhibitors on learning and memory and modulation of hippocampal dickkopf-1 and sclerostin in female mice. Pharmacol. Rep. 2017, 69, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Brusco, I.; Becker, G.; Palma, T.V.; Pillat, M.M.; Scussel, R.; Steiner, B.T.; Sampaio, T.B.; Ardisson-Araújo, D.M.P.; de Andrade, C.M.; Oliveira, M.S.; et al. Kinin B1 and B2 receptors mediate breast cancer pain associated with both the tumor and oncology therapy using aromatase inhibitors. Sci. Rep. 2023, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Oliveira, S.; Silva, C.R.; Ferreira, J. Critical role of protease-activated receptor 2 activation by mast cell tryptase in the development of postoperative pain. Anesthesiology 2013, 118, 679–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostek, M.; Polaski, A.; Kolber, B.; Ramsey, A.; Kranjec, A.; Szucs, K. A Protocol of Manual Tests to Measure Sensation and Pain in Humans. J. Vis. Exp. 2016, 118, e54130. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient Analysis of Experimental Observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Brum, E.D.S.; Fialho, M.F.P.; Fischer, S.P.M.; Hartmann, D.D.; Gonçalves, D.F.; Scussel, R.; Machado-De-Ávila, R.A.; Corte, C.D.; Soares, F.A.A.; Oliveira, S.M. Relevance of Mitochondrial Dysfunction in the Reserpine-Induced Experimental Fibromyalgia Model. Mol. Neurobiol. 2020, 57, 4202–4217. [Google Scholar] [CrossRef]
- Montilla-García, Á.; Tejada, M.Á.; Perazzoli, G.; Entrena, J.M.; Portillo-Salido, E.; Fernández-Segura, E.; Cañizares, F.J.; Cobos, E.J. Grip strength in mice with joint inflammation: A rheumatology function test sensitive to pain and analgesia. Neuropharmacology 2017, 125, 231–242. [Google Scholar] [CrossRef]
- Lintermans, A.; Van Calster, B.; Van Hoydonck, M.; Pans, S.; Verhaeghe, J.; Westhovens, R.; Henry, N.L.; Wildiers, H.; Paridaens, R.; Dieudonné, A.S.; et al. Aromatase inhibitor-induced loss of grip strength is body mass index dependent: Hypothesis-generating findings for its pathogenesis. Ann. Oncol. 2011, 22, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; He, D.; Ru, X.; Chen, Y.; Cai, Y.; Bruce, I.C.; Xia, Q.; Yao, X.; Jin, J. Apigenin, a plant-derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br. J. Pharmacol. 2012, 166, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.R.; Oliveira, S.M.; Hoffmeister, C.; Funck, V.; Guerra, G.P.; Trevisan, G.; Tonello, R.; Rossato, M.F.; Pesquero, J.B.; Bader, M.; et al. The role of kinin B 1 receptor and the effect of angiotensin I-converting enzyme inhibition on acute gout attacks in rodents. Ann. Rheum. Dis. 2016, 75, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, R.; Dowsett, M.; Fisher, G.; Selen, A.; Wyld, P. Arimidex (ZD1033): A selective, potent inhibitor of aromatase in post-menopausal female volunteers. Br. J. Cancer 1996, 73, 543–548. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, G.G.; Li, R.; Ng, M.P.E.; Lemes, J.B.P.; Vieira, W.F.; Nagy, I.; Tambeli, C.H.; Parada, C.A. CB 1 receptor-dependent desensitisation of TRPV1 channels contributes to the analgesic effect of dipyrone in sensitised primary sensory neurons. Br. J. Pharmacol. 2020, 177, 4615–4626. [Google Scholar] [CrossRef] [PubMed]
- Paterson, K.J.; Zambreanu, L.; Bennett, D.L.; McMahon, S.B. Characterisation and mechanisms of bradykinin-evoked pain in man using iontophoresis. Pain 2013, 154, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Perin-Martins, A.; Teixeira, J.M.; Tambeli, C.H.; Parada, C.A.; Fischer, L. Mechanisms underlying transient receptor potential ankyrin 1 (TRPA1)-mediated hyperalgesia and edema. J. Peripher. Nerv. Syst. 2013, 18, 62–74. [Google Scholar] [CrossRef]
- Ferreira, J.; Trichês, K.M.; Medeiros, R.; Cabrini, D.A.; Mori, M.A.; Pesquero, J.B.; Bader, M.; Calixto, J.B. The role of kinin B1 receptors in the nociception produced by peripheral protein kinase C activation in mice. Neuropharmacology 2008, 54, 597–604. [Google Scholar] [CrossRef]
- Ma, Q. The expression of bradykinin B 1 receptors on primary sensory neurones that give rise to small caliber sciatic nerve fibres in rats. Neuroscience 2001, 107, 665–673. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Ouyang, A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 296, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Shenoy, S.K. GPCR Desensitization: Acute and Prolonged Phases. Cell. Signal. 2019, 41, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Leeb-Lundberg, L.M.F.; Marceau, F.; Müller-Esterl, W.; Pettibone, D.J.; Zuraw, B. International Union of Pharmacology. XLV. Classification of the Kinin Receptor Family: From Molecular Mechanisms to Pathophysiological Consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef] [Green Version]
- Nezhady, M.A.M.; Rivera, J.C.; Chemtob, S. Location Bias as Emerging Paradigm in GPCR Biology and Drug Discovery. iScience 2020, 23, 101643. [Google Scholar] [CrossRef] [PubMed]
- Di Castro, A.; Drew, L.J.; Wood, J.N.; Cesare, P. Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 4699–4704. [Google Scholar] [CrossRef] [Green Version]
- Velazquez, K.; Mohammad, H.; Sweitzer, S. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol. Res. 2007, 55, 578–589. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-S.; Lei, J.; He, X.; Qu, F.; Wang, Y.; Wen, W.-W.; You, H.-J.; Arendt-Nielsen, L. Peripheral involvement of PKA and PKC in subcutaneous bee venom-induced persistent nociception, mechanical hyperalgesia, and inflammation in rats. Pain 2008, 135, 31–36. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Yu, X.; Zhang, Q.; Qin, B. Effect of PKC/NF- κ B on the Regulation of P2X 3 Receptor in Dorsal Root Ganglion in Rats with Sciatic Nerve Injury. Pain Res. Manag. 2020, 2020, 7104392. [Google Scholar] [CrossRef]
- Meotti, F.; Campos, R.; da Silva, K.; Paszcuk, A.; Costa, R.; Calixto, J. Inflammatory muscle pain is dependent on the activation of kinin B 1 and B 2 receptors and intracellular kinase pathways. Br. J. Pharmacol. 2012, 166, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-I.; Hwang, S.W. Depolarizing Effectors of Bradykinin Signaling in Nociceptor Excitation in Pain Perception. Biomol. Ther. 2018, 26, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Review Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutra, R.; Bicca, M.; Segat, G.; Silva, K.; Motta, E.; Pianowski, L.; Costa, R.; Calixto, J. The antinociceptive effects of the tetracyclic triterpene euphol in inflammatory and neuropathic pain models: The potential role of PKCε. Neuroscience 2015, 303, 126–137. [Google Scholar] [CrossRef]
- Fu, W.; Nelson, T.S.; Santos, D.F.; Doolen, S.; Gutierrez, J.J.; Na Ye, N.; Zhou, J.; Taylor, B.K. An NPY Y1 receptor antagonist unmasks latent sensitization and reveals the contribution of protein kinase A and Epac to chronic inflammatory pain. Pain 2019, 160, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, L.; Xie, Q.; Yang, F.; Li, G.; Zhang, G.; Li, S.; Wu, Z.; Wang, J.; Kang, X. Protein Kinase A Is Involved in Neuropathic Pain by Activating the p38MAPK Pathway to Mediate Spinal Cord Cell Apoptosis. Mediat. Inflamm. 2020, 2020, 6420425-17. [Google Scholar] [CrossRef] [PubMed]
- Aley, K.O.; Levine, J.D. Role of Protein Kinase A in the Maintenance of Inflammatory Pain. J. Neurosci. 1999, 19, 2181–2186. [Google Scholar] [CrossRef] [Green Version]
- Yajima, Y.; Narita, M.; Shimamura, M.; Narita, M.; Kubota, C.; Suzuki, T. Differential involvement of spinal protein kinase C and protein kinase A in neuropathic and inflammatory pain in mice. Brain Res. 2003, 992, 288–293. [Google Scholar] [CrossRef]
- Meents, J.E.; Fischer, M.J.M.; McNaughton, P.A. Sensitization of TRPA1 by Protein Kinase, A. PLoS ONE 2017, 12, e0170097. [Google Scholar] [CrossRef] [Green Version]
- Miyajima, M.; Kusuhara, H.; Takahashi, K.; Takashima, T.; Hosoya, T.; Watanabe, Y.; Sugiyama, Y. Investigation of the effect of active efflux at the blood–brain barrier on the distribution of nonsteroidal aromatase inhibitors in the central nervous system. J. Pharm. Sci. 2013, 102, 3309–3319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fialho, M.F.P.; Brum, E.S.; Becker, G.; Brusco, I.; Oliveira, S.M. Kinin B2 and B1 Receptors Activation Sensitize the TRPA1 Channel Contributing to Anastrozole-Induced Pain Symptoms. Pharmaceutics 2023, 15, 1136. https://doi.org/10.3390/pharmaceutics15041136
Fialho MFP, Brum ES, Becker G, Brusco I, Oliveira SM. Kinin B2 and B1 Receptors Activation Sensitize the TRPA1 Channel Contributing to Anastrozole-Induced Pain Symptoms. Pharmaceutics. 2023; 15(4):1136. https://doi.org/10.3390/pharmaceutics15041136
Chicago/Turabian StyleFialho, Maria Fernanda Pessano, Evelyne Silva Brum, Gabriela Becker, Indiara Brusco, and Sara Marchesan Oliveira. 2023. "Kinin B2 and B1 Receptors Activation Sensitize the TRPA1 Channel Contributing to Anastrozole-Induced Pain Symptoms" Pharmaceutics 15, no. 4: 1136. https://doi.org/10.3390/pharmaceutics15041136
APA StyleFialho, M. F. P., Brum, E. S., Becker, G., Brusco, I., & Oliveira, S. M. (2023). Kinin B2 and B1 Receptors Activation Sensitize the TRPA1 Channel Contributing to Anastrozole-Induced Pain Symptoms. Pharmaceutics, 15(4), 1136. https://doi.org/10.3390/pharmaceutics15041136




