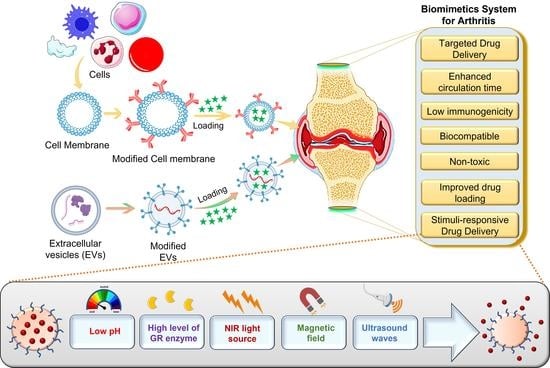
A Bird’s Eye View of Various Cell-Based Biomimetic Nanomedicines for the Treatment of Arthritis
Abstract
:1. Introduction
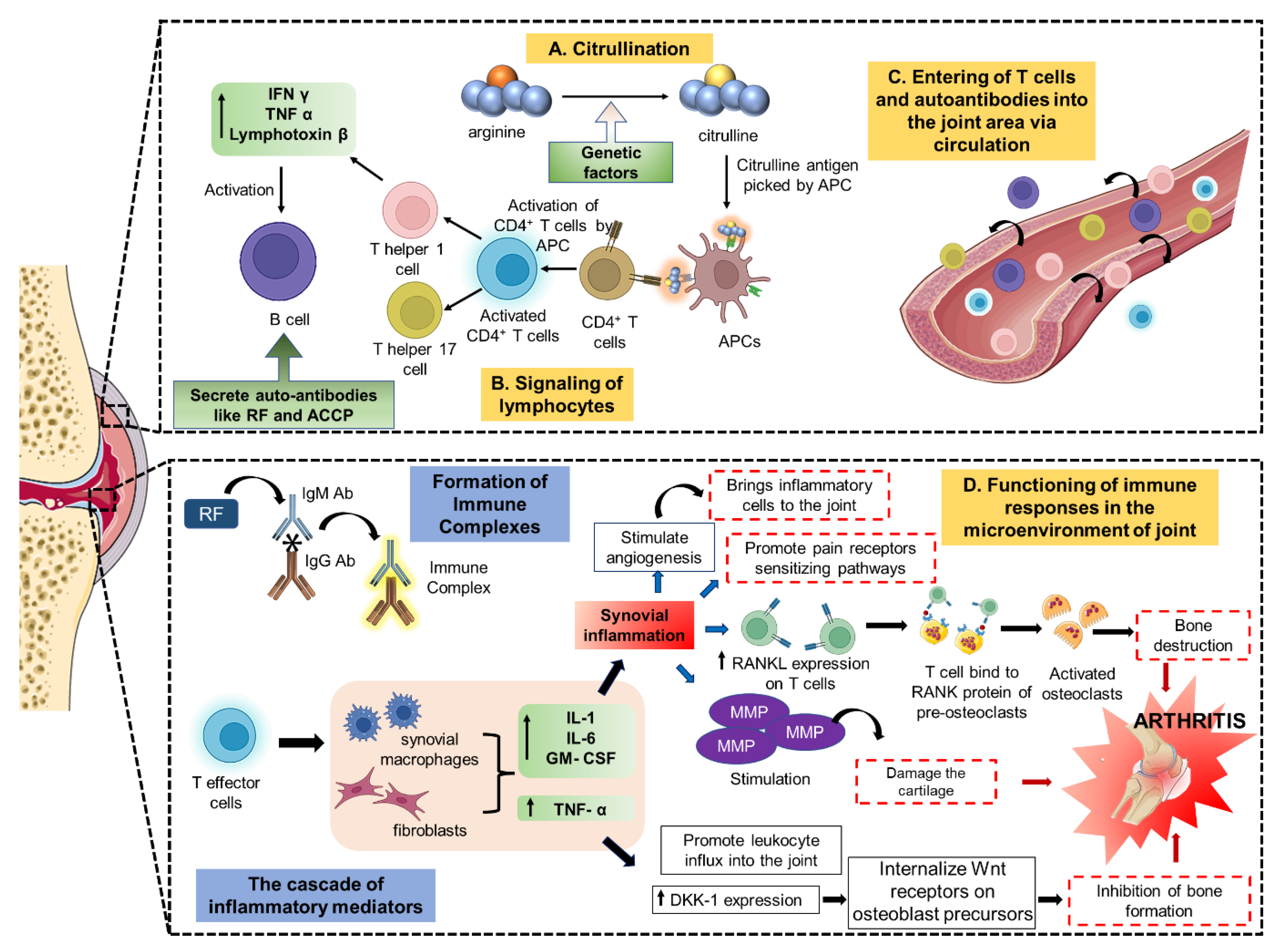
2. Pathophysiology and Therapeutic Targets of Arthritis
2.1. Intracellular Targets
2.2. Extracellular Targets
3. Biomechanics for Application of Biomimetic Systems in Arthritis
3.1. Biomimetic Surface
3.2. Biomimetic Movement
3.3. Shape and Surface
4. Cell-Membrane-Coated Biomimetic Nanomedicine for Arthritis
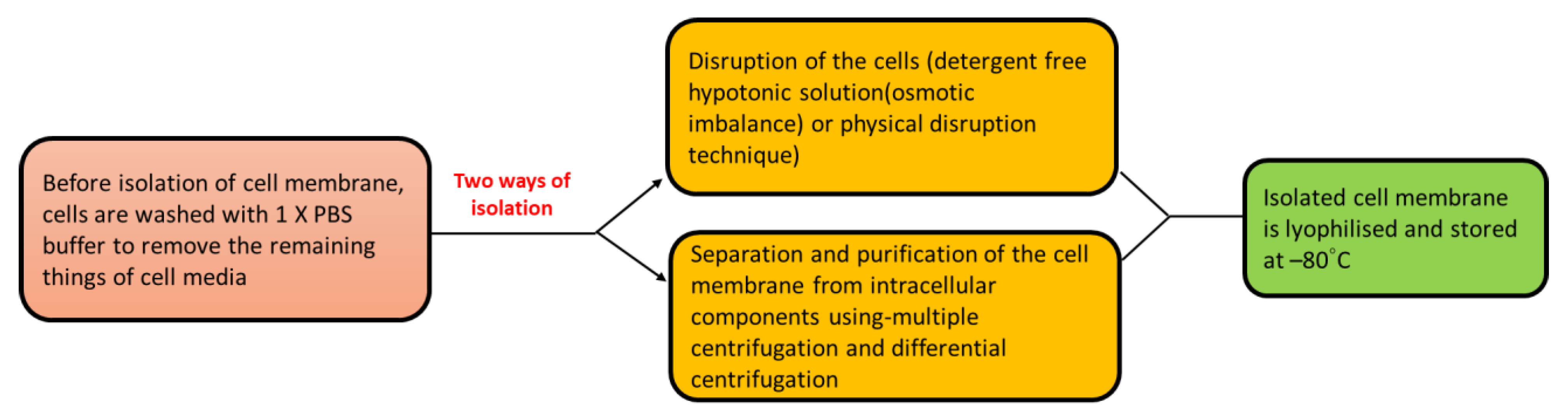
4.1. Isolation of Cell Membrane
4.2. Formulation of Cell-Membrane-Coated Nanomedicines
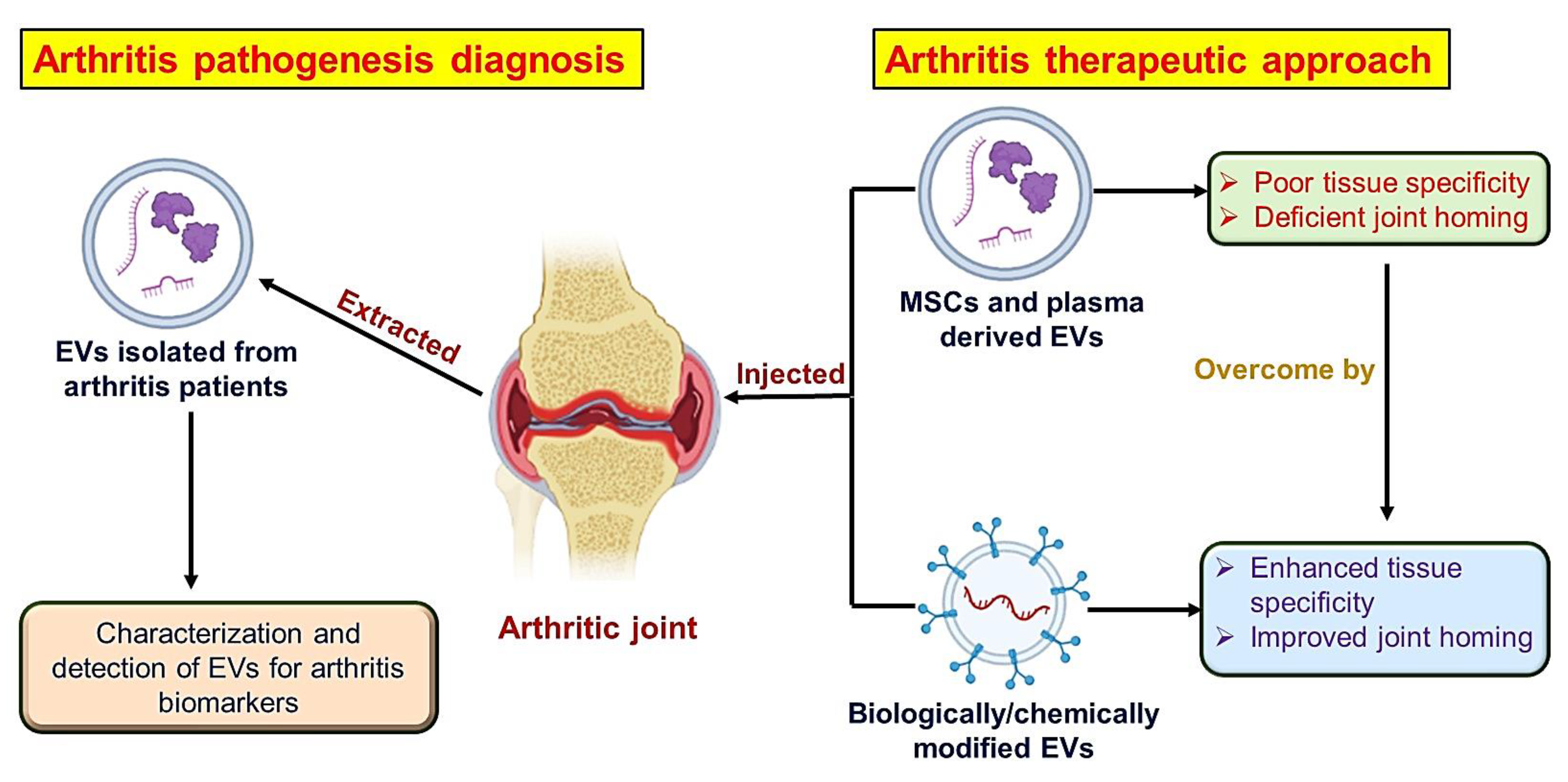
5. Extracellular Vesicles-Based Biomimetic Nanomedicine for Arthritis
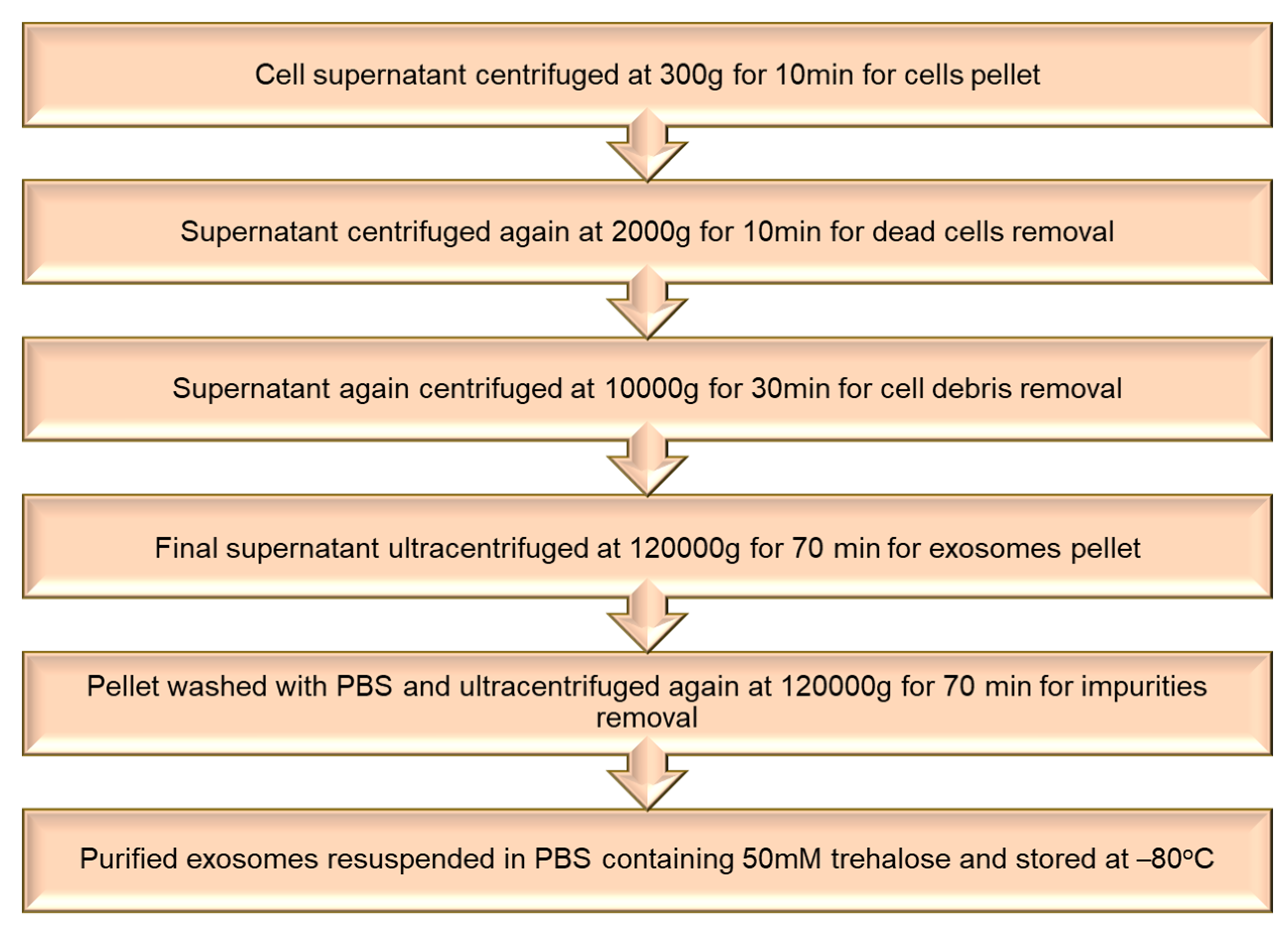
5.1. Isolation of Extracellular Vesicles
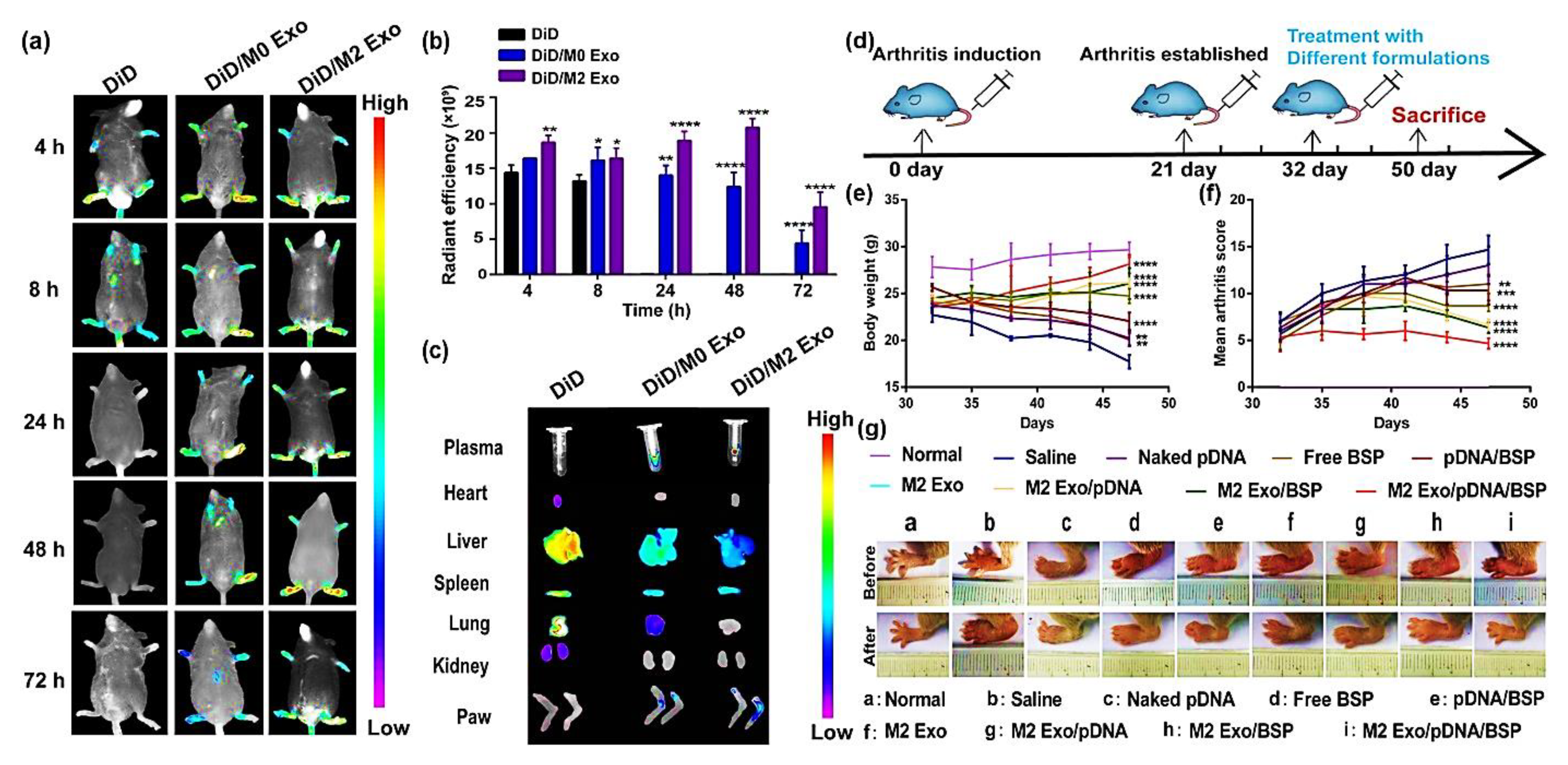
5.2. Loading of Therapeutic Agents in Extracellular Vesicles
6. Platelets Biomimetics System for Arthritis
Isolation of Platelet and Membrane Derivation
7. Functionalization of Biomimetic Nanomedicines
7.1. Biomimetic Nanomedicines with Targeting Ligand for Arthritis
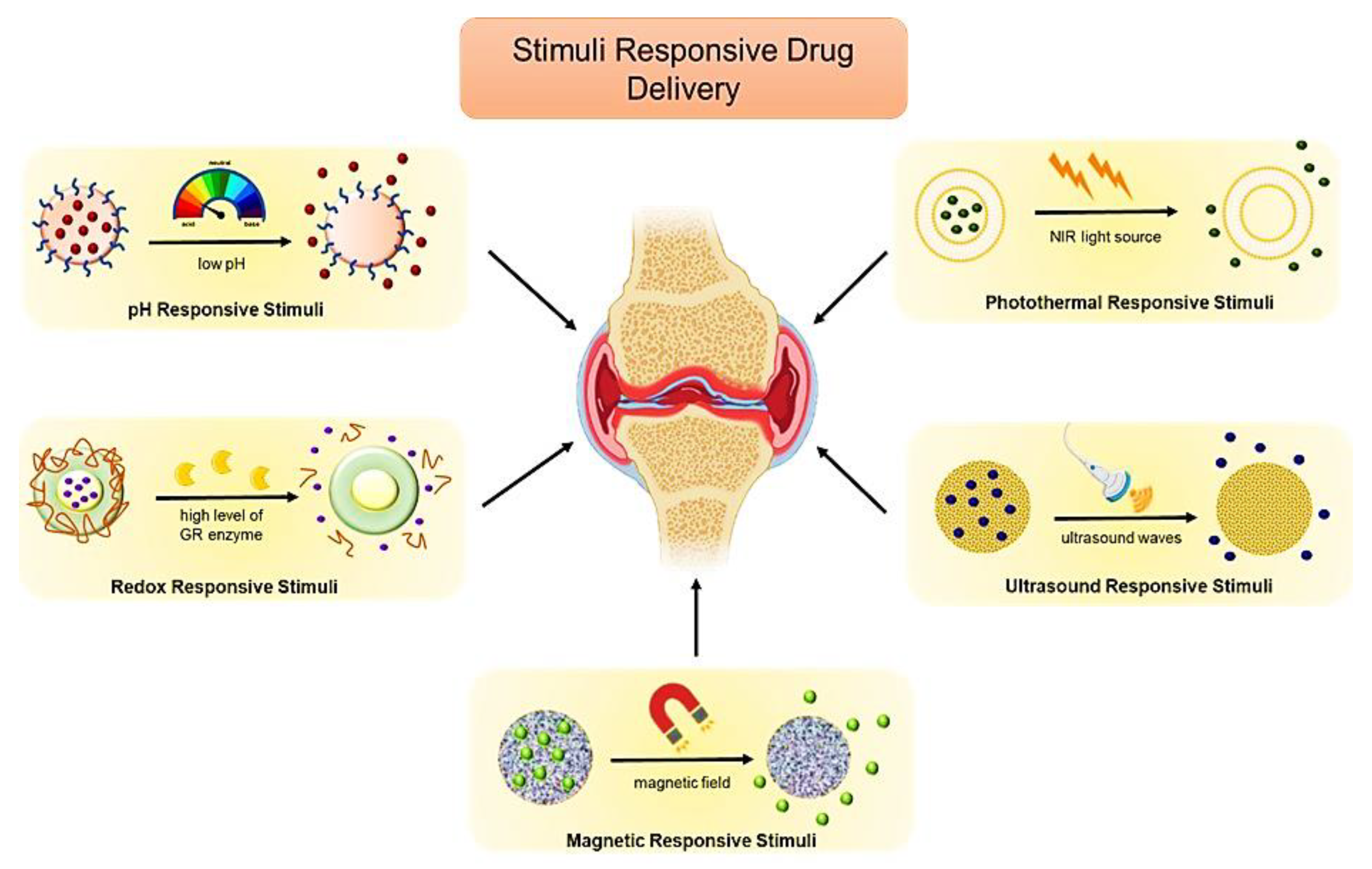
7.2. Stimuli-Responsive Biomimetic Nanomedicine
8. Challenges for Biomimetic Nanomedicines
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB | Apoptotic bodies |
| BSP | Betamethasone sodium phosphate |
| COX | Cyclooxygenase |
| DC | Dendritic cell |
| Dex | Dexamethasone sodium phosphate |
| EDTA | Ethylenediaminetetraacetic acid |
| EVs | Extracellular vesicles |
| FA | Folic acid |
| HSA | Human serum albumin |
| ICAM | Intercellular adhesion molecule |
| MCP-1 | Monocyte chemoattractant protein |
| MTX | Methotrexate |
| MVs | Microvesicles |
| OA | Osteoarthritis |
| PB | Prussian blue |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PLs | Platelets |
| POX | Poly-2-oxazolines |
| PTT | Photothermal therapy |
| QbD | Quality-by-design |
| RA | Rheumatoid arthritis |
| RES | Reticuloendothelial system |
| SEC | Size-exclusion chromatography |
| SF | Synovial fluid |
| SPARC | Secreted protein acidic and rich in cysteine |
| TMJ-OA | Temporomandibular joint osteoarthritis |
| TNF | Tumour necrosis factor |
| TRAIL | Tumour necrosis factor-related apoptosis-inducing ligand |
| VEGF | Vascular endothelial growth factor |
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Ledingham, J.; Snowden, N.; Ide, Z. Diagnosis and early management of inflammatory arthritis. BMJ 2017, 358, j3248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetland, M.L. Psoriatic arthritis: Still room for improvement. Lancet 2020, 395, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Pang, X.; Pi, G. Biomimetic and Bioinspired Intervention Strategies for the Treatment of Rheumatoid Arthritis. Adv. Funct. Mater. 2021, 31, 2104640. [Google Scholar] [CrossRef]
- Tang, C.-H. Research of Pathogenesis and Novel Therapeutics in Arthritis. Int. J. Mol. Sci. 2019, 20, 1646. [Google Scholar] [CrossRef] [Green Version]
- Gadeval, A.; Chaudhari, S.; Bollampally, S.P.; Polaka, S.; Kalyane, D.; Sengupta, P.; Kalia, K.; Tekade, R.K. Integrated nanomaterials for non-invasive photothermal therapy of rheumatoid arthritis. Drug Discov. Today 2021, 26, 2315–2328. [Google Scholar] [CrossRef]
- Pandey, P.K.; Maheshwari, R.; Raval, N.; Gondaliya, P.; Kalia, K.; Tekade, R.K. Nanogold-core multifunctional dendrimer for pulsatile chemo-, photothermal- and photodynamic- therapy of rheumatoid arthritis. J. Colloid Interface Sci. 2019, 544, 61–77. [Google Scholar] [CrossRef]
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.; Fang, J.; Sun, X. Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies. Acta Pharm. Sin. B 2021, 11, 1158–1174. [Google Scholar] [CrossRef]
- Chen, M.; Kambere Amerigos Daddy, J.C.; Xiao, Y.; Ping, Q.; Zong, L. Advanced nanomedicine for rheumatoid arthritis treatment: Focus on active targeting. Expert Opin. Drug Deliv. 2017, 14, 1141–1144. [Google Scholar] [CrossRef] [Green Version]
- Qamar, N.; Arif, A.; Bhatti, A.; John, P. Nanomedicine: An emerging era of theranostics and therapeutics for rheumatoid arthritis. Rheumatology 2019, 58, 1715–1721. [Google Scholar] [CrossRef]
- Lin, H.; Yang, C.; Luo, Y.; Ge, M.; Shen, H.; Zhang, X.; Shi, J. Biomimetic Nanomedicine-Triggered in Situ Vaccination for Innate and Adaptive Immunity Activations for Bacterial Osteomyelitis Treatment. ACS Nano 2022, 16, 5943–5960. [Google Scholar] [CrossRef]
- Singh, E.; Osmani, R.A.M.; Banerjee, R.; Abu Lila, A.S.; Moin, A.; Almansour, K.; Arab, H.H.; Alotaibi, H.F.; Khafagy, E.-S. Poly ε-Caprolactone Nanoparticles for Sustained Intra-Articular Immune Modulation in Adjuvant-Induced Arthritis Rodent Model. Pharmaceutics 2022, 14, 519. [Google Scholar] [CrossRef]
- Shinde, C.G.; Pramod Kumar, T.M.; Venkatesh, M.P.; Rajesh, K.S.; Srivastava, A.; Osmani, R.A.M.; Sonawane, Y.H. Intra-articular delivery of a methotrexate loaded nanostructured lipid carrier based smart gel for effective treatment of rheumatic diseases. RSC Adv. 2016, 6, 12913–12924. [Google Scholar] [CrossRef]
- Osmani, R.A.M.; Aloorkar, N.H.; Ingale, D.J.; Kulkarni, P.K.; Hani, U.; Bhosale, R.R.; Jayachandra Dev, D. Microsponges based novel drug delivery system for augmented arthritis therapy. Saudi Pharm. J. 2015, 23, 562–572. [Google Scholar] [CrossRef] [Green Version]
- Sabu, C.; Rejo, C.; Kotta, S.; Pramod, K. Bioinspired and biomimetic systems for advanced drug and gene delivery. J. Control. Release 2018, 287, 142–155. [Google Scholar] [CrossRef]
- Li, A.; Zhao, Y.; Li, Y.; Jiang, L.; Gu, Y.; Liu, J. Cell-derived biomimetic nanocarriers for targeted cancer therapy: Cell membranes and extracellular vesicles. Drug Deliv. 2021, 28, 1237–1255. [Google Scholar] [CrossRef]
- Jin, J.; Luo, Z.; Zhang, B.; Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B 2017, 8, 23–33. [Google Scholar] [CrossRef]
- Li, C.; Zheng, X.; Hu, M.; Jia, M.; Jin, R.; Nie, Y. Recent progress in therapeutic strategies and biomimetic nanomedicines for rheumatoid arthritis treatment. Expert Opin. Drug Deliv. 2022, 19, 883–898. [Google Scholar] [CrossRef]
- Koenders, M.I.; van den Berg, W.B. Novel therapeutic targets in rheumatoid arthritis. Trends Pharmacol. Sci. 2015, 36, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fu, X.; Chen, X.; Li, Z.; Huang, Y.; Liang, C. Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 686155. [Google Scholar] [CrossRef] [PubMed]
- Cooles, F.; Isaacs, J. Pathophysiology of rheumatoid arthritis. Curr. Opin. Rheumatol. 2011, 23, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, T.; Liu, M.; Wang, S.; Liu, S.; Yang, Y.; Yang, Y.; Nan, Y.; Huang, Q.; Ai, K. Rheumatoid arthritis microenvironment insights into treatment effect of nanomaterials. Nano Today 2022, 42, 101358. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, W.; Sui, L.; Huang, Q.; Nan, Y.; Liu, J.; Ai, K. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact. Mater. 2022, 7, 47–72. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Ming Di, Y.; Zhou, Z.-W.; Guang Li, C.; Zhou, S.-F. Current and Future Therapeutic Targets of Rheumatoid Arthritis. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2011, 10, 92–120. [Google Scholar] [CrossRef]
- Rasheed, Z.; Haqqi, T.M. Update on Targets of Biologic Therapies for Rheumatoid Arthritis. Curr. Rheumatol. Rev. 2008, 4, 246. [Google Scholar] [CrossRef] [Green Version]
- Schindler, C.; Levy, D.E.; Decker, T. JAK-STAT Signaling: From Interferons to Cytokines. J. Biol. Chem. 2007, 282, 20059–20063. [Google Scholar] [CrossRef] [Green Version]
- Shuai, K.; Liu, B. Regulation of JAK–STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Yamaoka, K.; Saharinen, P.; Pesu, M.; Holt, V.E.T., 3rd; Silvennoinen, O.; O’Shea, J.J. The Janus kinases (Jaks). Genome Biol. 2004, 5, 253. [Google Scholar] [CrossRef] [Green Version]
- Cheung, T.T.; McInnes, I.B. Future therapeutic targets in rheumatoid arthritis? Semin. Immunopathol. 2017, 39, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Banham-Hall, E.; Clatworthy, M.R.; Okkenhaug, K. The Therapeutic Potential for PI3K Inhibitors in Autoimmune Rheumatic Diseases. Open Rheumatol. J. 2012, 6, 245–258. [Google Scholar] [CrossRef]
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Liu, S.; Ma, H.; Zhang, H.; Deng, C.; Xin, P. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 2021, 230, 108793. [Google Scholar] [CrossRef]
- Rabelo, F.d.S.; da Mota, L.M.H.; Lima, R.A.C.; Lima, F.A.C.; Barra, G.B.; de Carvalho, J.F.; Amato, A.A. The Wnt signaling pathway and rheumatoid arthritis. Autoimmun. Rev. 2010, 9, 207–210. [Google Scholar] [CrossRef]
- Nejatbakhsh Samimi, L.; Farhadi, E.; Tahmasebi, M.N.; Jamshidi, A.; Sharafat Vaziri, A.; Mahmoudi, M. NF-κB signaling in rheumatoid arthritis with focus on fibroblast-like synoviocytes. Autoimmun. Highlights 2020, 11, 11. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling pathways in rheumatoid arthritis: Implications for targeted therapy. Signal Transduct. Target. 2023, 8, 68. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Lawrence, T.; Hamilton, J.A.; Cook, A.D. Granulocyte-Macrophage Colony-Stimulating Factor (CSF) and Macrophage CSF-Dependent Macrophage Phenotypes Display Differences in Cytokine Profiles and Transcription Factor Activities: Implications for CSF Blockade in Inflammation1. J. Immunol. 2007, 178, 5245–5252. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Lim, D.-S.; Choi, Y.-E.; Jeong, Y.; Yoo, S.-A.; Kim, W.-U.; Bae, Y.-S. MLN51 and GM-CSF involvement in the proliferation of fibroblast-like synoviocytes in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R170. [Google Scholar] [CrossRef] [Green Version]
- Torchinsky, M.B.; Garaude, J.; Martin, A.P.; Blander, J.M. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature 2009, 458, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Nabeel, F.; Raza, A.; Bilal, M.; Iqbal, H. Biomimetic nanostructures/cues as drug delivery systems: A review. Mater. Today Chem. 2019, 13, 147–157. [Google Scholar] [CrossRef]
- Dash, P.; Piras, A.M.; Dash, M. Cell membrane coated nanocarriers-an efficient biomimetic platform for targeted therapy. J. Control. Release 2020, 327, 546–570. [Google Scholar] [CrossRef] [PubMed]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wang, Y.; Jiang, T.; Xia, H.; Gu, X.; Chen, H. Recent progress of biomimetic motions—From microscopic micro/nanomotors to macroscopic actuators and soft robotics. RSC Adv. 2021, 11, 27406–27419. [Google Scholar] [CrossRef]
- Shi, Q.; Gao, Z.; Jia, G.; Li, C.; Huang, Q.; Ishii, H.; Takanishi, A.; Fukuda, T. Implementing rat-like motion for a small-sized biomimetic robot based on extraction of key movement joints. IEEE Trans. Robot. 2020, 37, 747–762. [Google Scholar] [CrossRef]
- Akin, D.; Sturgis, J.; Ragheb, K.; Sherman, D.; Burkholder, K.; Robinson, J.P.; Bhunia, A.K.; Mohammed, S.; Bashir, R. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat. Nanotechnol. 2007, 2, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Bian, Q.; Wang, R.; Gao, J. Micro/nanorobots for precise drug delivery via targeted transport and triggered release: A review. Int. J. Pharm. 2022, 616, 121551. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Woldstad, C.; Ottemann, B.M.; Dash, P.; Sajja, B.R.; Lamberty, B.; Morsey, B.; Kocher, T.; Dutta, R.; Bade, A.N. Multimodal theranostic nanoformulations permit magnetic resonance bioimaging of antiretroviral drug particle tissue-cell biodistribution. Theranostics 2018, 8, 256. [Google Scholar] [CrossRef]
- Rampersaud, S.; Fang, J.; Wei, Z.; Fabijanic, K.; Silver, S.; Jaikaran, T.; Ruiz, Y.; Houssou, M.; Yin, Z.; Zheng, S. The effect of cage shape on nanoparticle-based drug carriers: Anticancer drug release and efficacy via receptor blockade using dextran-coated iron oxide nanocages. Nano Lett. 2016, 16, 7357–7363. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, P.; Wu, M.; Meng, Q.; Chen, H.; Shu, Z.; Wang, J.; Zhang, L.; Li, Y.; Shi, J. Colloidal RBC-Shaped, Hydrophilic, and Hollow Mesoporous Carbon Nanocapsules for Highly Efficient Biomedical Engineering. Adv. Mater. 2014, 26, 4294–4301. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Little, N.; Lu, J. Self-assembling prodrug nanotherapeutics for synergistic tumor targeted drug delivery. Acta Biomater. 2020, 111, 20–28. [Google Scholar] [CrossRef]
- Darmawan, B.A.; Lee, S.B.; Go, G.; Nguyen, K.T.; Lee, H.-S.; Nan, M.; Hong, A.; Kim, C.-S.; Li, H.; Bang, D. Self-folded microrobot for active drug delivery and rapid ultrasound-triggered drug release. Sens. Actuators B Chem. 2020, 324, 128752. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, T.P.; Pandey, B.; Gupta, V.; Singh, S.P. Engineering nanomaterials for smart drug release: Recent advances and challenges. Appl. Target. Nano Drugs Deliv. Syst. 2019, 411–449. [Google Scholar] [CrossRef]
- Sarode, A.; Annapragada, A.; Guo, J.; Mitragotri, S. Layered self-assemblies for controlled drug delivery: A translational overview. Biomaterials 2020, 242, 119929. [Google Scholar] [CrossRef]
- Lechanteur, A.; das Neves, J.; Sarmento, B. The role of mucus in cell-based models used to screen mucosal drug delivery. Adv. Drug Deliv. Rev. 2018, 124, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Topuz, F.; Uyar, T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef]
- Chugh, V.; Vijaya Krishna, K.; Pandit, A. Cell membrane-coated mimics: A methodological approach for fabrication, characterization for therapeutic applications, and challenges for clinical translation. ACS Nano 2021, 15, 17080–17123. [Google Scholar] [CrossRef]
- Goñi, F.M. The basic structure and dynamics of cell membranes: An update of the Singer–Nicolson model. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1467–1476. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.; Zhu, Q.; Wei, D.; Feng, J.; Yao, J.; Jiang, T.; Song, Q.; Wei, X.; Chen, H.; Gao, X. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano 2017, 11, 1397–1411. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, C.; Jin, Y.; Li, Y.; Zhong, C.; Ma, J.; Yang, J.; Zhang, N.; Li, Y.; Wang, C. Artificial mini dendritic cells boost T cell–based immunotherapy for ovarian cancer. Adv. Sci. 2020, 7, 1903301. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomater. Sci. 2020, 8, 2939–2954. [Google Scholar] [CrossRef]
- Yu, H.; Fan, J.; Shehla, N.; Qiu, Y.; Lin, Y.; Wang, Z.; Cao, L.; Li, B.; Daniyal, M.; Qin, Y.; et al. Biomimetic Hybrid Membrane-Coated Xuetongsu Assisted with Laser Irradiation for Efficient Rheumatoid Arthritis Therapy. ACS Nano 2022, 16, 502–521. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, C.; Li, H.; Liu, C.; Zhang, P.; Gong, P.; Cai, L. T cell/Macrophage Dual-Targeting Biomimetic Triptolide Self-Assembly Nanodrugs for Rheumatoid Arthritis Therapy by Inflammatory Microenvironment Remodeling. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Shi, Y.; Xie, F.; Rao, P.; Qian, H.; Chen, R.; Chen, H.; Li, D.; Mu, D.; Zhang, L.; Lv, P.; et al. TRAIL-expressing cell membrane nanovesicles as an anti-inflammatory platform for rheumatoid arthritis therapy. J. Control. Release 2020, 320, 304–313. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhu, Y.; Jiang, L.; Zhang, S.; Qin, J.; Wu, Q.; Dai, W.; Shen, S.; Pang, Z. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2018, 19, 124–134. [Google Scholar] [CrossRef]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Zhou, Z.; Bai, D.; Zhang, Q.; Ai, X.; Gao, W.; Zhang, L. White blood cell membrane-coated nanoparticles: Recent development and medical applications. Adv. Healthc. Mater. 2022, 11, 2101349. [Google Scholar] [CrossRef]
- Xue, X.; Liu, H.; Wang, S.; Hu, Y.; Huang, B.; Li, M.; Gao, J.; Wang, X.; Su, J. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/anti-inflammatory therapy of osteoarthritis. Compos. Part B Eng. 2022, 237, 109855. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Cosenza, S.; Ruiz, M.; Maumus, M.; Jorgensen, C.; Noël, D. Pathogenic or Therapeutic Extracellular Vesicles in Rheumatic Diseases: Role of Mesenchymal Stem Cell-Derived Vesicles. Int. J. Mol. Sci. 2017, 18, 889. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Ho, L.W.C.; Yin, B.; Dai, G.; Choi, C.H.J. Effect of Surface Modification with Hydrocarbyl Groups on the Exocytosis of Nanoparticles. Biochemistry 2021, 60, 1019–1030. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Riekkola, M.L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Yin, B.; Ni, J.; Witherel, C.E.; Yang, M.; Burdick, J.A.; Wen, C.; Wong, S.H.D. Harnessing Tissue-derived Extracellular Vesicles for Osteoarthritis Theranostics. Theranostics 2022, 12, 207–231. [Google Scholar] [CrossRef]
- Gao, T.; Guo, W.; Chen, M.; Huang, J.; Yuan, Z.; Zhang, Y.; Wang, M.; Li, P.; Peng, J.; Wang, A.; et al. Extracellular Vesicles and Autophagy in Osteoarthritis. BioMed Res. Int. 2016, 2016, 2428915. [Google Scholar] [CrossRef] [Green Version]
- Rong, J.; Pool, B.; Zhu, M.; Munro, J.; Cornish, J.; McCarthy, G.M.; Dalbeth, N.; Poulsen, R. Basic Calcium Phosphate Crystals Induce Osteoarthritis-Associated Changes in Phenotype Markers in Primary Human Chondrocytes by a Calcium/Calmodulin Kinase 2-Dependent Mechanism. Calcif. Tissue Int. 2019, 104, 331–343. [Google Scholar] [CrossRef]
- Withrow, J.; Murphy, C.; Liu, Y.; Hunter, M.; Fulzele, S.; Hamrick, M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res. 2016, 18, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berckmans, R.J.; Nieuwland, R.; Tak, P.P.; Böing, A.N.; Romijn, F.P.; Kraan, M.C.; Breedveld, F.C.; Hack, C.E.; Sturk, A. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002, 46, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Berckmans, R.J.; Nieuwland, R.; Kraan, M.C.; Schaap, M.C.; Pots, D.; Smeets, T.J.; Sturk, A.; Tak, P.P. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res. 2005, 7, R536-544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skriner, K.; Adolph, K.; Jungblut, P.R.; Burmester, G.R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006, 54, 3809–3814. [Google Scholar] [CrossRef]
- Zhang, H.G.; Liu, C.; Su, K.; Yu, S.; Zhang, L.; Zhang, S.; Wang, J.; Cao, X.; Grizzle, W.; Kimberly, R.P. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J. Immunol. 2006, 176, 7385–7393. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Zhong, Z.; Wang, Y.; Feng, Y.; Mei, Z.; Li, H.; Chen, X.; Cai, L.; Li, C. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J. Nanobiotechnol. 2020, 18, 115. [Google Scholar] [CrossRef]
- Kim, S.H.; Lechman, E.R.; Bianco, N.; Menon, R.; Keravala, A.; Nash, J.; Mi, Z.; Watkins, S.C.; Gambotto, A.; Robbins, P.D. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 2005, 174, 6440–6448. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, S.; Xue, Q.; Hong, Y.; Liu, L.; Song, L.; Fang, C.; Zhang, H.; Wang, B.; Sedgwick, A.C.; et al. Photoacoustic image-guided biomimetic nanoparticles targeting rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2022, 119, e2213373119. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, M.; Zheng, X.; Wang, C.; Zhou, Y.; Pan, H.; Liu, Y.; Lu, J.; Mei, Z.; Li, C. Microvesicle-camouflaged biomimetic nanoparticles encapsulating a metal-organic framework for targeted rheumatoid arthritis therapy. J. Nanobiotechnol. 2022, 20, 253. [Google Scholar] [CrossRef]
- Yang, N.; Li, M.; Wu, L.; Song, Y.; Yu, S.; Wan, Y.; Cheng, W.; Yang, B.; Mou, X.; Yu, H.; et al. Peptide-anchored neutrophil membrane-coated biomimetic nanodrug for targeted treatment of rheumatoid arthritis. J. Nanobiotechnol. 2023, 21, 13. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Zheng, X.; Jia, M.; Mei, Z.; Wang, Y.; Zhang, Z.; Zhou, M.; Li, C. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J. Control. Release Off. J. Control. Release Soc. 2022, 341, 16–30. [Google Scholar] [CrossRef]
- Liu, L.; Hu, F.; Wang, H.; Wu, X.; Eltahan, A.S.; Stanford, S.; Bottini, N.; Xiao, H.; Bottini, M.; Guo, W.; et al. Secreted Protein Acidic and Rich in Cysteine Mediated Biomimetic Delivery of Methotrexate by Albumin-Based Nanomedicines for Rheumatoid Arthritis Therapy. ACS Nano 2019, 13, 5036–5048. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- You, D.G.; Lim, G.T.; Kwon, S.; Um, W.; Oh, B.H.; Song, S.H.; Lee, J.; Jo, D.G.; Cho, Y.W.; Park, J.H. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci. Adv. 2021, 7, eabe0083. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Jia, M.; Zheng, X.; Liu, Y.; Wang, C.; Lei, F.; Niu, H.; Li, C. ZIF-8 nanoparticles coated with macrophage-derived microvesicles for sustained, targeted delivery of dexamethasone to arthritic joints. J. Drug Target. 2022, 30, 1006–1016. [Google Scholar] [CrossRef]
- Tramś, E.; Malesa, K.; Pomianowski, S.; Kamiński, R. Role of Platelets in Osteoarthritis-Updated Systematic Review and Meta-Analysis on the Role of Platelet-Rich Plasma in Osteoarthritis. Cells 2022, 11, 1080. [Google Scholar] [CrossRef]
- Olumuyiwa-Akeredolu, O.O.; Page, M.J.; Soma, P.; Pretorius, E. Platelets: Emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 237–248. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Gao, W.; Karshalev, E.; Zhang, L.; Wang, J. Cell-Like Micromotors. Acc. Chem. Res. 2018, 51, 1901–1910. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Q.; Jiang, C.; Gu, Z. Platelet for drug delivery. Curr. Opin. Biotechnol. 2019, 58, 81–91. [Google Scholar] [CrossRef]
- Han, H.; Bártolo, R.; Li, J.; Shahbazi, M.A.; Santos, H.A. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. 2022, 172, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Narain, A.; Asawa, S.; Chhabria, V.; Patil-Sen, Y. Cell membrane coated nanoparticles: Next-generation therapeutics. Nanomedicine 2017, 12, 2677–2692. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, R.; Liang, J.; Zhu, Y.; Zhang, S.; Zheng, Z.; Qin, J.; Pang, Z.; Wang, J. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Res. 2018, 11, 6086–6101. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.; Noh, I. Overviews of Biomimetic Medical Materials. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Springer: Singapore, 2018; pp. 3–24. [Google Scholar]
- Wu, M.; Le, W.; Mei, T.; Wang, Y.; Chen, B.; Liu, Z.; Xue, C. Cell membrane camouflaged nanoparticles: A new biomimetic platform for cancer photothermal therapy. Int. J. Nanomed. 2019, 14, 4431–4448. [Google Scholar] [CrossRef] [Green Version]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Toledano Furman, N.E.; Hartman, K.A.; Sherman, M.B.; De Rosa, E.; Kirui, D.K.; Salvatore, F.; Tasciotti, E. Unveiling the in Vivo Protein Corona of Circulating Leukocyte-like Carriers. ACS Nano 2017, 11, 3262–3273. [Google Scholar] [CrossRef]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or cell membrane-based drug delivery systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef] [Green Version]
- Bose, R.J.C.; Lee, S.-H.; Park, H. Biofunctionalized nanoparticles: An emerging drug delivery platform for various disease treatments. Drug Discov. Today 2016, 21, 1303–1312. [Google Scholar] [CrossRef]
- Oldenborg, P.-A. Role of CD47 in Erythroid Cells and in Autoimmunity. Leuk. Lymphoma 2004, 45, 1319–1327. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.-L.; Xu, J.-H.; Cai, B.; Yu, G.-T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.-S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.-M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Gnanasammandhan, M.; Xie, C.; Huang, K.; Cui, M.; Chan, J.J.N. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale 2016, 8, 6981–6985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Lv, Y.; Ni, D.; Wang, J.; Tian, Z.; Wei, W.; Ma, G. Erythrocyte membrane-coated NIR-triggered biomimetic nanovectors with programmed delivery for photodynamic therapy of cancer. Nanoscale 2015, 7, 9806–9815. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; Xu, J.-H.; Qi, G.-B.; Zhao, X.; Yu, F.; Wang, H. Core–Shell Supramolecular Gelatin Nanoparticles for Adaptive and “On-Demand” Antibiotic Delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.-M.J.; Chen, K.N.; Luk, B.T.; Carpenter, C.W.; Gao, W.; Li, S.; Zhang, D.-E.; Lu, W.; Zhang, L.J.N. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale 5 2013, 5, 8884–8888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayan, V.; Uthaman, S.; Park, I.-K. Cell Membrane Coated Nanoparticles: An Emerging Biomimetic Nanoplatform for Targeted Bioimaging and Therapy. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Springer: Singapore, 2018; pp. 45–59. [Google Scholar]
- De Ávila, B.E.-F.; Gao, W.; Karshalev, E.; Zhang, L.; Wang, J.J.A.C.R. Cell-like micromotors. Acc. Chem. Res. 2018, 51, 1901–1910. [Google Scholar] [CrossRef]
- Tavasolian, F.; Moghaddam, A.S.; Rohani, F.; Abdollahi, E.; Janzamin, E.; Momtazi-Borojeni, A.A.; Moallem, S.A.; Jamialahmadi, T.; Sahebkar, A. Exosomes: Effectual players in rheumatoid arthritis. Autoimmun. Rev. 2020, 19, 102511. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, L.; Bai, X.; Du, X.; Wei, J.; Wang, J.; Lin, Y.; Chen, Z.; Liu, Z.; Wu, J.; et al. Treatment of Rheumatoid Arthritis by Serum Albumin Nanoparticles Coated with Mannose to Target Neutrophils. ACS Appl. Mater. Interfaces 2021, 13, 266–276. [Google Scholar] [CrossRef]
- Jones, M.R.; Seeman, N.C.; Mirkin, C.A.J.S.N.A. Programmable materials and the nature of the DNA bond. Spherical Nucleic Acids 2020, 347, 167–197. [Google Scholar]
- Nicolson, F.; Ali, A.; Kircher, M.F.; Pal, S. DNA Nanostructures and DNA-Functionalized Nanoparticles for Cancer Theranostics. Adv. Sci. 2020, 7, 2001669. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Wang, H.; Jreyssaty, C.; Benderdour, M.; Lavigne, P.; Qiu, X.; Winnik, F.M.; Zhang, X.; Dai, K.; Shi, Q. Bone-protective Effects of Nonviral Gene Therapy With Folate–Chitosan DNA Nanoparticle Containing Interleukin-1 Receptor Antagonist Gene in Rats With Adjuvant-induced Arthritis. Mol. Ther. 2008, 16, 1243–1251. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid–Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019, 31, 1803549. [Google Scholar] [CrossRef]
- Pirmardvand Chegini, S.; Varshosaz, J.; Taymouri, S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Eniola, A.O.; Rodgers, S.D.; Hammer, D.A. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials 2002, 23, 2167–2177. [Google Scholar] [CrossRef]
- Jeong, M.; Park, J.-H. Nanomedicine for the Treatment of Rheumatoid Arthritis. Mol. Pharm. 2021, 18, 539–549. [Google Scholar] [CrossRef]
- Gerlag, D.M.; Borges, E.; Tak, P.P.; Ellerby, H.M.; Bredesen, D.E.; Pasqualini, R.; Ruoslahti, E.; Firestein, G.S. Suppression of murine collagen-induced arthritis by targeted apoptosis of synovial neovasculature. Arthritis Res. Ther. 2001, 3, 357. [Google Scholar] [CrossRef] [Green Version]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Yang, Z.; Zhang, D.; Lu, Y.; Zheng, M.; Xue, X.; Geng, J.; Chung, R.; Shi, B. Effective and Targeted Human Orthotopic Glioblastoma Xenograft Therapy via a Multifunctional Biomimetic Nanomedicine. Adv. Mater. 2018, 30, 1803717. [Google Scholar] [CrossRef]
- Wang, S.; Lv, J.; Meng, S.; Tang, J.; Nie, L. Recent Advances in Nanotheranostics for Treat-to-Target of Rheumatoid Arthritis. Adv. Healthc. Mater. 2020, 9, 1901541. [Google Scholar] [CrossRef]
- Wang, X.; Cao, W.; Sun, C.; Wang, Y.; Wang, M.; Wu, J. Development of pH-sensitive dextran-based methotrexate nanodrug for rheumatoid arthritis therapy through inhibition of JAK-STAT pathways. Int. J. Pharm. 2022, 622, 121874. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wang, Z.; Shao, D.; Chang, Z.; Hu, R.; Li, L.; Luo, S.-z.; Dong, W.-f.J.R.a. Cancer cell membrane-modified biodegradable mesoporous silica nanocarriers for berberine therapy of liver cancer. RSC Adv. 2018, 8, 40288–40297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Tuguntaev, R.G.; Mao, C.; Chen, H.; Tao, Y.; Wang, S.; Yang, B.; Guo, W. Stimuli-responsive polymeric nanomaterials for rheumatoid arthritis therapy. Biophys. Rep. 2020, 6, 193–210. [Google Scholar] [CrossRef]
- Lima, A.C.; Reis, R.L.; Ferreira, H.; Neves, N.M. Glutathione Reductase-Sensitive Polymeric Micelles for Controlled Drug Delivery on Arthritic Diseases. ACS Biomater. Sci. Eng. 2021, 7, 3229–3241. [Google Scholar] [CrossRef]
- Mai, B.T.; Fernandes, S.; Balakrishnan, P.B.; Pellegrino, T. Nanosystems Based on Magnetic Nanoparticles and Thermo- or pH-Responsive Polymers: An Update and Future Perspectives. Acc. Chem. Res. 2018, 51, 999–1013. [Google Scholar] [CrossRef]
- Li, J.; Angsantikul, P.; Liu, W.; Esteban-Fernández de Ávila, B.; Chang, X.; Sandraz, E.; Liang, Y.; Zhu, S.; Zhang, Y.; Chen, C.; et al. Biomimetic Platelet-Camouflaged Nanorobots for Binding and Isolation of Biological Threats. Adv. Mater. 2018, 30, 1704800. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Gao, W.; Xu, T.; Jurado-Sánchez, B.; Li, J.; Gao, W.; He, Q.; Zhang, L.; Wang, J. Cell-Membrane-Coated Synthetic Nanomotors for Effective Biodetoxification. Adv. Funct. Mater. 2015, 25, 3881–3887. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Wang, L.; Huang, J.; Xiang, X.; Tang, Y.; Cheng, C.; Yan, F.; Ma, L.; Qiu, L. Ultrasound-triggered perfluorocarbon-derived nanobombs for targeted therapies of rheumatoid arthritis. J. Mater. Chem. B 2019, 7, 4581–4591. [Google Scholar] [CrossRef]
- Pei, Q.; Hu, X.; Zheng, X.; Liu, S.; Li, Y.; Jing, X.; Xie, Z. Light-Activatable Red Blood Cell Membrane-Camouflaged Dimeric Prodrug Nanoparticles for Synergistic Photodynamic/Chemotherapy. ACS Nano 2018, 12, 1630–1641. [Google Scholar] [CrossRef]
- Zhang, N.; Li, M.; Sun, X.; Jia, H.; Liu, W. NIR-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery. Biomaterials 2018, 159, 25–36. [Google Scholar] [CrossRef]
- Costa Lima, S.A.; Reis, S. Temperature-responsive polymeric nanospheres containing methotrexate and gold nanoparticles: A multi-drug system for theranostic in rheumatoid arthritis. Colloids Surf. B Biointerfaces 2015, 133, 378–387. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.-M.; Park, K.-H.; Mun, C.H.; Park, Y.-B.; Yoo, K.-H. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials 2015, 61, 95–102. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Tong, C.; Huang, H.; Yi, O.; Dai, Z.; Su, Z.; Liu, B.; Cai, X. A pH-Driven indomethacin-loaded nanomedicine for effective rheumatoid arthritis therapy by combining with photothermal therapy. J. Drug Target. 2022, 30, 737–752. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, X.; Li, C. Challenges in cell membrane-camouflaged drug delivery systems: Development strategies and future prospects. Chin. Chem. Lett. 2021, 32, 2347–2358. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Fan, Z.; Zhou, H.; Li, P.Y.; Speer, J.E.; Cheng, H.J.J.o.M.C.B. Structural elucidation of cell membrane-derived nanoparticles using molecular probes. J. Mater. Chem. B 2014, 2, 8231–8238. [Google Scholar] [CrossRef]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef]
- Bagasariya, D.; Charankumar, K.; Shah, S.; Famta, P.; Khatri, D.K.; Singh Raghuvanshi, R.; Bala Singh, S.; Srivastava, S. Biomimetic nanotherapeutics: Employing nanoghosts to fight melanoma. Eur. J. Pharm. Biopharm. 2022, 177, 157–174. [Google Scholar] [CrossRef]
- Raza, F.; Zafar, H.; Zhang, S.; Kamal, Z.; Su, J.; Yuan, W.-E.; Mingfeng, Q. Recent Advances in Cell Membrane-Derived Biomimetic Nanotechnology for Cancer Immunotherapy. Adv. Healthc. Mater. 2021, 10, 2002081. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; He, R.; Xu, D.; Zang, J.; Weeranoppanant, N.; Dong, H.; Li, Y.J.B.s. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2020, 8, 552–568. [Google Scholar] [CrossRef]
- Gupta, S.; Jhawat, V. Quality by design (QbD) approach of pharmacogenomics in drug designing and formulation development for optimization of drug delivery systems. J. Control. Release 2017, 245, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Nene, S.; Rangaraj, N.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Bridging the gap: Academia, industry and FDA convergence for nanomaterials. Drug Dev. Ind. Pharm. 2020, 46, 1735–1746. [Google Scholar] [CrossRef] [PubMed]




| Targets | Agents | Phases |
|---|---|---|
| Cytokine | ||
| TNF | Adalimumab | Marketed |
| Infliximab | Marketed | |
| Etanercept | Marketed | |
| Certolizumab | Marketed | |
| Golimumab | Marketed | |
| IL-1R | Anakinra | Marketed |
| IL-1 | Canakinumab | Marketed |
| Gevokizumab | Marketed | |
| Rilonacept | Terminated | |
| IL-6R | Tocilizumab | Marketed |
| IL-6a | Sarilumab | Marketed |
| Clazakizumab | Marketed | |
| Olokizumab | Marketed | |
| Sirukumab | Marketed | |
| IL-2 | MEDI5117 | Terminated |
| IL-10 | Dekavil | Phase 1 |
| IL-15 | AMG-714 | Phase 2 |
| IL-18 | rhIL-18BP | Phase 1 |
| IL-17 | Secukinumab | Phase 3 |
| Ixekizumab | Phase 2 | |
| IL-17R | Brodalumab | Terminated |
| IFN-γ | Fontolizumab | Terminated |
| Chemokines | ||
| CCL2 | p8A MCP-1 | Animal study |
| ABN912 | Phase 1 | |
| CCR9 | CCX8037 | Animal study |
| CX3CL1 | E6011 | Phase 1 |
| CCR1 | J–113863 | Animal study |
| BX147 | Animal study | |
| BAY86-5047 | Phase 2 | |
| ZK811752 | Phase 2 | |
| CCX354 | Phase 2 | |
| BMS-817399 | Phase 2 | |
| CCR2 | MK-0812 | Phase 2 |
| MC-21 | Animal study | |
| MLN1202 | Phase 2a | |
| CCR5 | SCH-X82 | Phase 2 |
| Met-RANTES | Phase 2 | |
| AZD5672 | Phase 2 | |
| Maraviroc | Terminated | |
| SCH351125 | Phase 1b | |
| CXCL10 | MDX-1100 | Phase 2 |
| CXCL12 | 30D8 | Animal study |
| CXCL13 | mAb470 | Animal study |
| CXCL16 | IgG1 12-81 | Animal study |
| CXCR1/2 | Repertaxin | Animal study |
| DF2162 | Animal study | |
| CXCR3 | SCH546738 | Animal study |
| AMG487 | Animal study | |
| JN-2 | Animal study | |
| CXCR4 | Plerixafor | Animal study |
| T140 | Animal study | |
| AMD3100 | Animal study | |
| CXCR7 | CCX733 | Animal study |
| CCR7 | 8H3-16A12 | Animal study |
| Other Proteins | ||
| TLR4 | NI-0101 | Phase 2 |
| GRK2 | Paroxetine | Phase 2 |
| MEK | ARRY-162 | Phase 2 |
| MMP-9 | Andecaliximab | Phase 2 |
| CD3 | Otelixizumab | Phase 1 |
| CD80 | Abatacept | Marketed |
| BTK | ICP-022 | Phase 1 |
| CC-292 | Phase 2 | |
| HM71224 | Phase 1 | |
| M2951 | Phase 2 | |
| GS-4059 | Phase 1 | |
| IL-23 | STA 5326 mesylate | Phase 2 |
| Guselkumab | Terminated | |
| GM-CSF | Otilimab | Phase 3 |
| Gimsilumab | Phase 1 | |
| Namilumab | Phase 2 | |
| Mavrilimumab | Phase 2 | |
| Lenzilumab | Terminated | |
| JAK | Tofacitinib | Approved |
| Baricitinib | Approved | |
| Filgotininb | Phase 3 | |
| Upadacitinib | Approved | |
| Peficitinib | Phase 3 | |
| Ruxolitinib | Phase 2 | |
| Itacitinib | Phase 2 | |
| Tasocitinib | Phase 2 | |
| INCB018424 | Phase 2 | |
| VX-509 | Phase 3 | |
| p38 MAPK | RO4402257 | Phase 2 |
| PH-797804 | Phase 2 | |
| VX-702 | Phase 2 | |
| BMS-582949 | Phase 2 | |
| ARRY-371797 | Phase 1 | |
| SCIO-469 | Phase 2 | |
| SB-681323 | Phase 2 | |
| IRAK-4 | PF-06650833 | Phase 2 |
| BAY1834845 | Phase 1 | |
| BAY1830839 | Phase 1 | |
| CA-4948 | Phase 2 | |
| CD20 | Rituximab | Phase 3 |
| Ocrelizumab | Terminated | |
| Ofatumumab | Phase 3 | |
| CD11a | Efalizumab | Phase 2 |
| CD19 | MDX-1342 | Phase 1 |
| Biomimetic System | Nanoformulation | Active Moiety | Size | Functionalization | Inference | Reference |
|---|---|---|---|---|---|---|
| Macrophage membrane vesicles | Prussian blue nanoparticles | siRNA | - | - | Photoacoustic-guided nanoparticles assisted better diagnosis and treatment | [89] |
| RBC-RAFLS hybrid membrane | Prussian blue nanoparticles | Schisanlactone | 141.8 ± 10 nm | Hyaluronic acid | Synergistic chemo-/photothermal therapy with controlled and targeted release | [66] |
| Macrophage-derived microvesicle (MMV) | PLGA Nanoparticles | Tacrolimus | 130 ± 14 nm | Morenhanced targeting than RBC-coated membrane | [69] | |
| Macrophage-derived microvesicle (MMV) | Zeolitic imidazolate framework-8 nanoparticles | Methotrexate | 147.7 ± 3.21 nm | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate (polyethylene glycol)-2000] | pH-sensitive release in acidic environment. | [90] |
| Neutrophil-membrane-coated | Pluronic F127 nanoparticles | Celastrol | 51.25 ± 2.086 nm | R4F peptide | Macrophages targeted formulation with reduced hepatotoxicity | [91] |
| Exosome | Nanoparticles | IL-10 pDNA and betamethasone sodium phosphate | 99.97 ± 4.77 nm | - | Combined therapy with synergistic effect | [92] |
| Extracellular vesicle (Exosome) | Nanoparticles | Dexamethasone sodium | 128.43 ± 16.27 nm | Folic acid | Biocompatibility and no hepatotoxicity | [86] |
| SPARC (secreted protein acidic and rich in cysteine) in arthritis microenvironment | Albumin nanomedicine | Methotrexate | 30.71 ± 4.62 nm | - | Longer retention and reduced systemic toxicity | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasdev, N.; Pawar, B.; Gupta, T.; Mhatre, M.; Tekade, R.K. A Bird’s Eye View of Various Cell-Based Biomimetic Nanomedicines for the Treatment of Arthritis. Pharmaceutics 2023, 15, 1150. https://doi.org/10.3390/pharmaceutics15041150
Vasdev N, Pawar B, Gupta T, Mhatre M, Tekade RK. A Bird’s Eye View of Various Cell-Based Biomimetic Nanomedicines for the Treatment of Arthritis. Pharmaceutics. 2023; 15(4):1150. https://doi.org/10.3390/pharmaceutics15041150
Chicago/Turabian StyleVasdev, Nupur, Bhakti Pawar, Tanisha Gupta, Mahi Mhatre, and Rakesh Kumar Tekade. 2023. "A Bird’s Eye View of Various Cell-Based Biomimetic Nanomedicines for the Treatment of Arthritis" Pharmaceutics 15, no. 4: 1150. https://doi.org/10.3390/pharmaceutics15041150
APA StyleVasdev, N., Pawar, B., Gupta, T., Mhatre, M., & Tekade, R. K. (2023). A Bird’s Eye View of Various Cell-Based Biomimetic Nanomedicines for the Treatment of Arthritis. Pharmaceutics, 15(4), 1150. https://doi.org/10.3390/pharmaceutics15041150