Ultrasound-Responsive Biomimetic Superhydrophobic Drug-Loaded Mesoporous Silica Nanoparticles for Treating Prostate Tumor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Synthesis of MSN and F-MSN
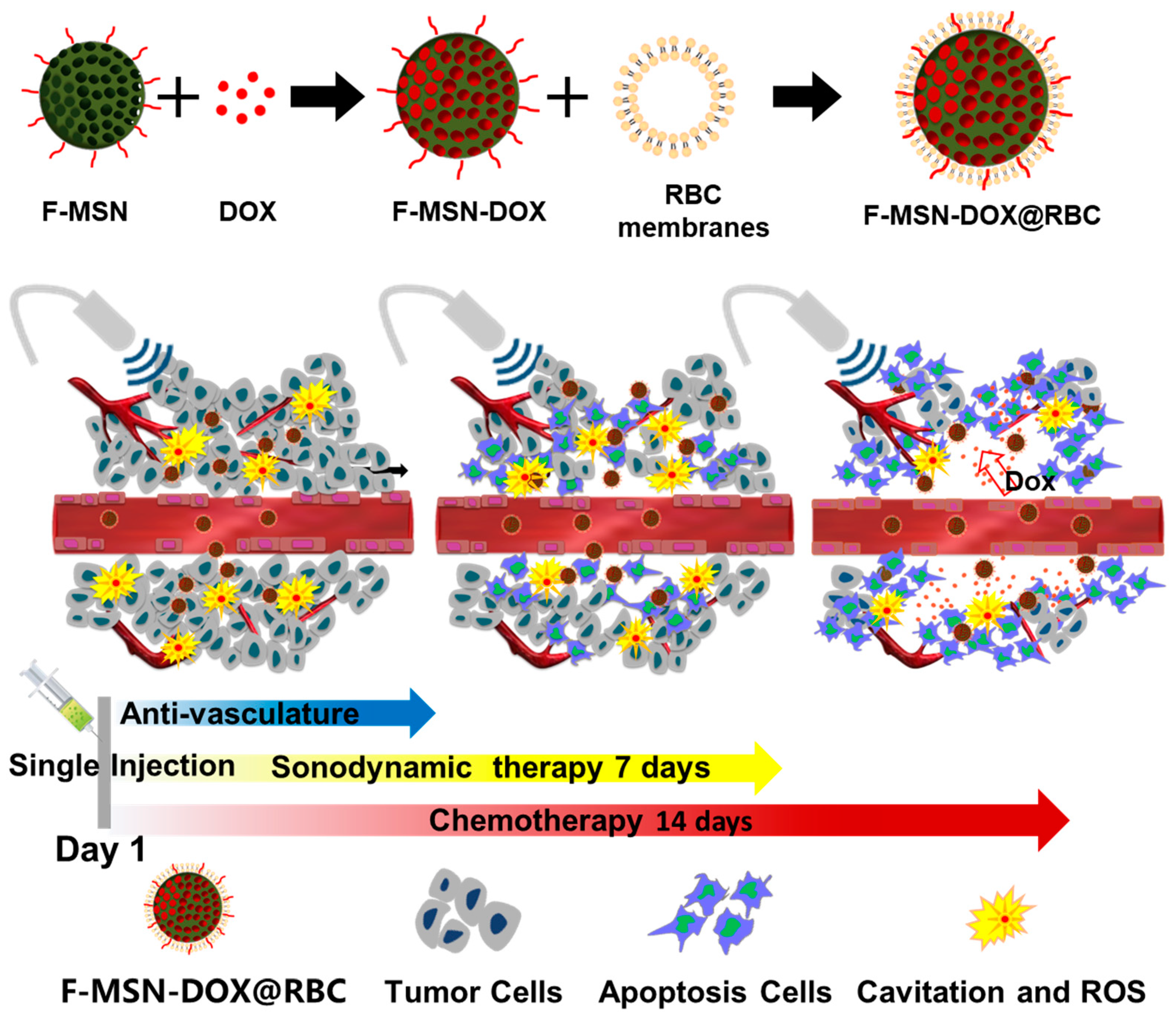
2.3. Preparation and Characterization of F-MSN-DOX@RBC
2.4. Characterization of Different NPs
2.4.1. Size Distribution, Zeta Potential and Morphology
2.4.2. Nitrogen Physisorption Isotherms
2.4.3. Drug Loading Efficiency
2.4.4. In Vitro Drug Release
2.4.5. RBC Membrane Characterization
2.5. Cell Viability
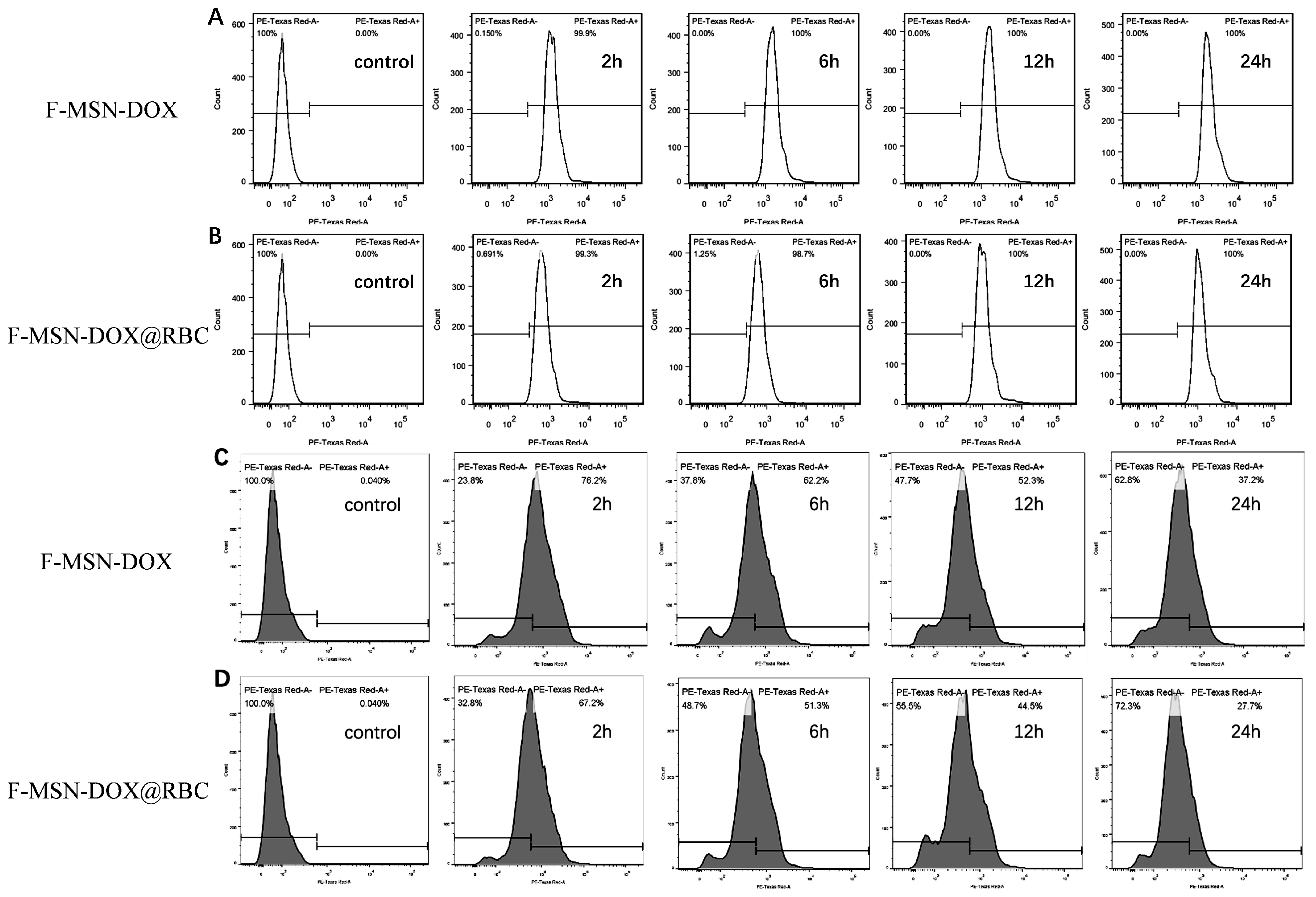
2.6. Cell Uptake
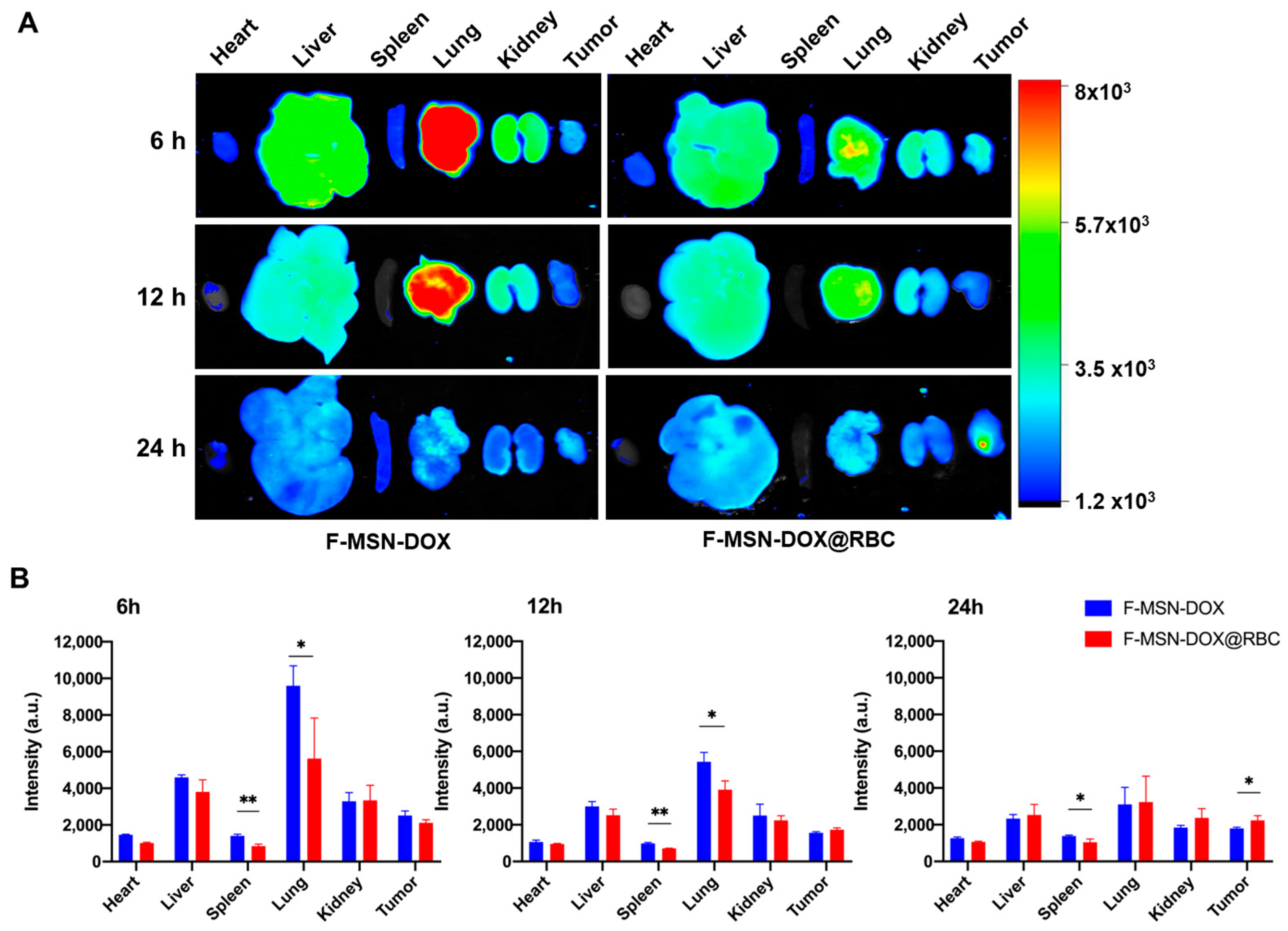
2.7. In Vivo Distribution
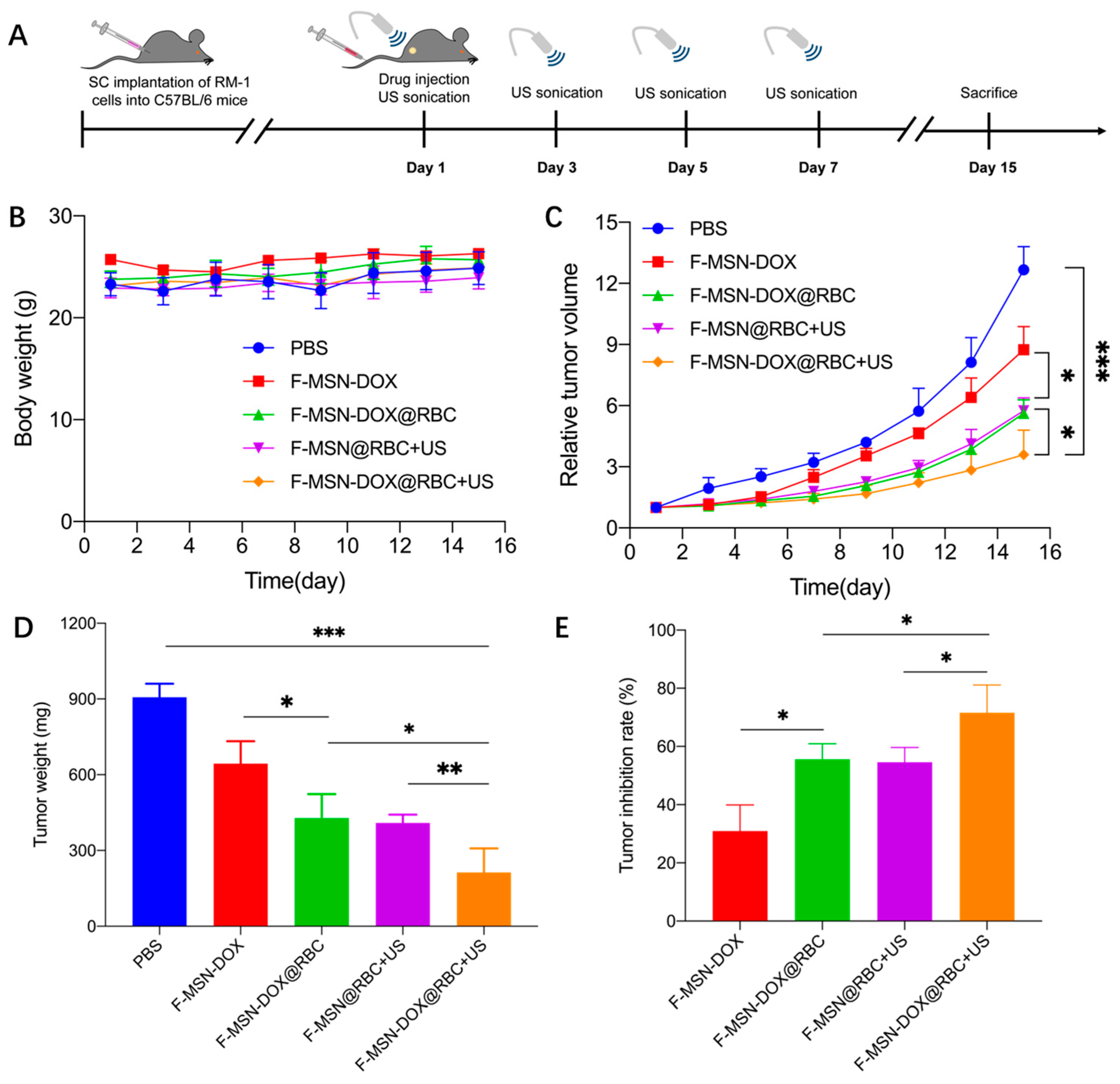
2.8. In Vivo Anti-Tumor Efficacy
2.9. Statistical Analysis
3. Results
3.1. Characterization of F-MSN-DOX@RBC
3.2. Drug-Loading, RBC Membrane Envelope Verification, and In Vitro Drug Release
3.3. Cytotoxicity Assays and Cell Uptake
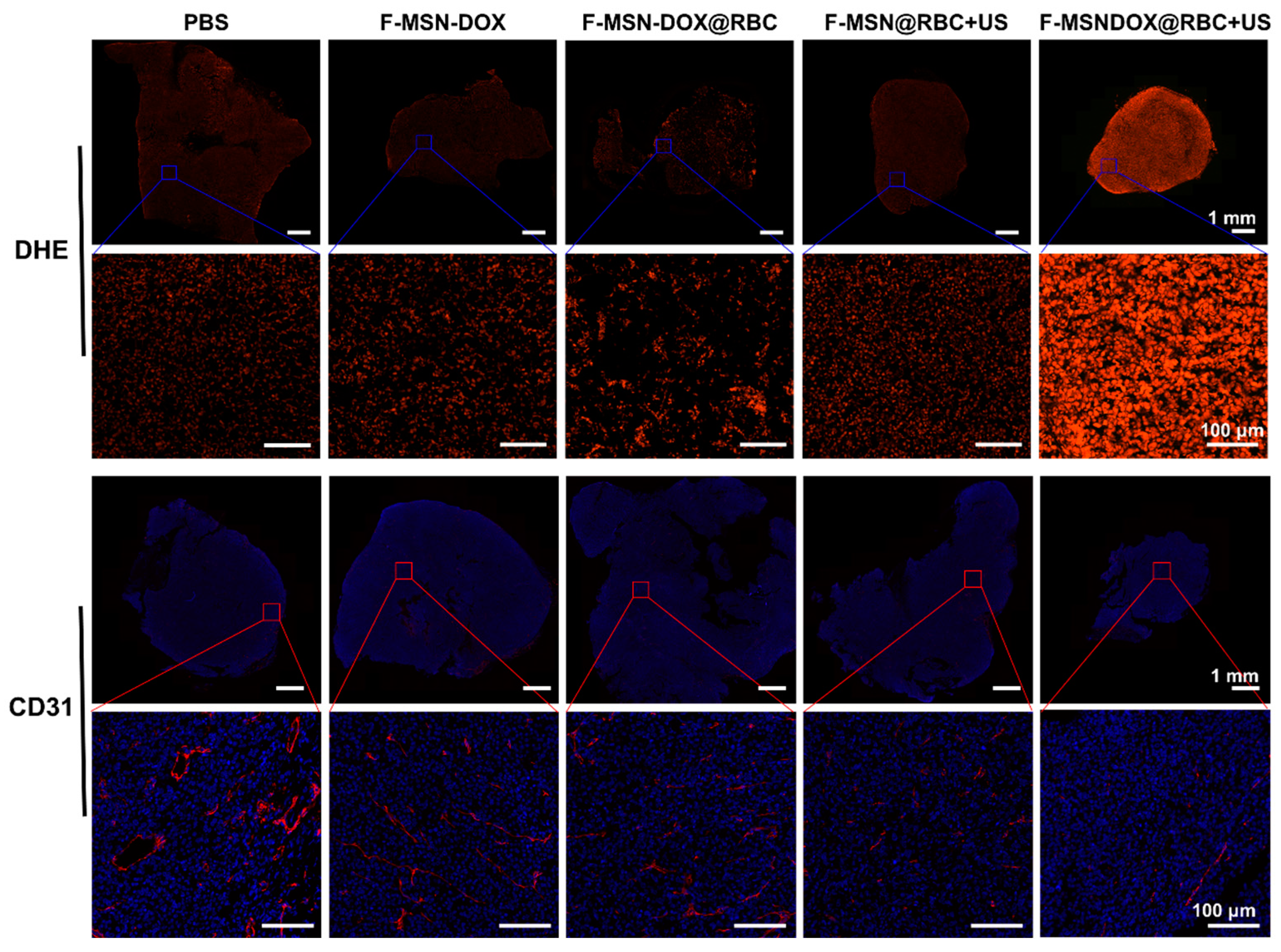
3.4. In Vivo Biodistribution
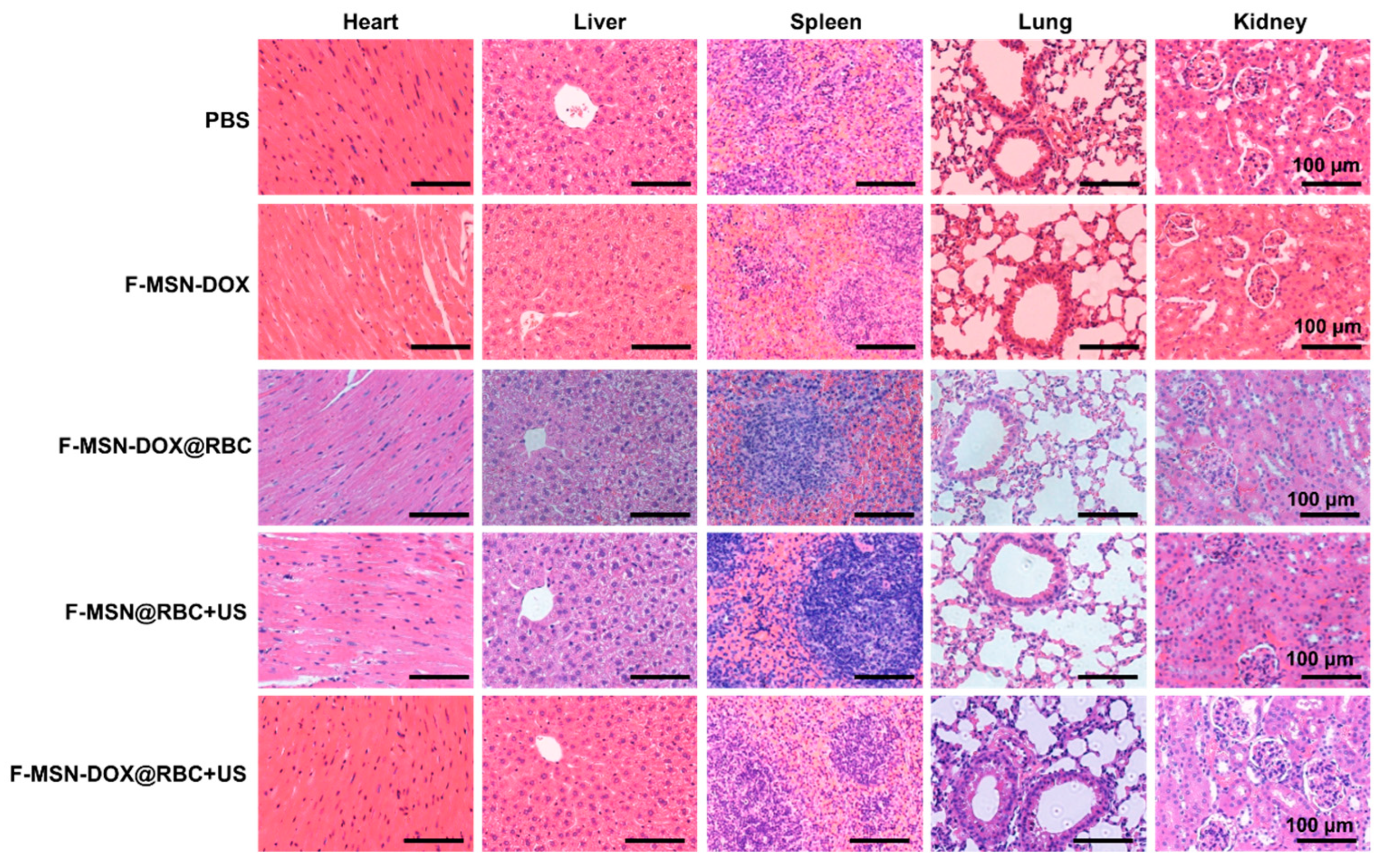
3.5. In Vivo Anti-Tumor Efficacy
4. Discussion
4.1. Ultrasound-Responsive NPs for Sonodynamic Therapy
4.2. Improved Tumor Accumulation by Decoration of NPs
4.3. Anti-Vascular Therapy
4.4. In Vivo Monitorring of F-MSN
4.5. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Lai, J.P.; Mu, X.; Xu, Y.Y.; Wu, X.L.; Wu, C.L.; Li, C.; Chen, J.B.; Zhao, Y.B. Light-responsive nanogated ensemble based on polymer grafted mesoporous silica hybrid nanoparticles. Chem. Commun. 2010, 46, 7370–7372. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.B.; Sun, X.Q.; Liu, J.J.; Feng, L.Z.; Liu, Z. Light-Responsive, Singlet-Oxygen-Triggered On-Demand Drug Release from Photosensitizer-Doped Mesoporous Silica Nanorods for Cancer Combination Therapy. Adv. Funct. Mater. 2016, 26, 4722–4732. [Google Scholar] [CrossRef]
- Chen, L.F.; Wang, W.Q.; Su, B.; Wen, Y.Q.; Li, C.B.; Zhou, Y.B.; Li, M.Z.; Shi, X.D.; Du, H.W.; Song, Y.L.; et al. A Light-Responsive Release Platform by Controlling the Wetting Behavior of Hydrophobic Surface. ACS Nano 2014, 8, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.B.; Duan, J.Z.; Lou, Y.L.; Gao, R.Y.; Yang, S.S.; Wang, P.M.; Wang, C.H.; Han, L.; Li, M.H.; Ma, C.H.; et al. Surface specifically modified NK-92 cells with CD56 antibody conjugated superparamagnetic Fe3O4 nanoparticles for magnetic targeting immunotherapy of solid tumors. Nanoscale 2021, 13, 19109–19122. [Google Scholar] [CrossRef]
- Baeza, A.; Guisasola, E.; Ruiz-Hernandez, E.; Vallet-Regi, M. Magnetically Triggered Multidrug Release by Hybrid Mesoporous Silica Nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Sun, M.; Yue, T.; Wang, C.Y.; Fan, Z.; Gazit, E.; Du, J.Z. Ultrasound-Responsive Peptide Nanogels to Balance Conflicting Requirements for Deep Tumor Penetration and Prolonged Blood Circulation. ACS Nano 2022, 16, 9183–9194. [Google Scholar] [CrossRef]
- Ma, X.T.; Yao, M.N.; Shi, J.Y.; Li, X.D.; Gao, Y.; Luo, Q.; Hou, R.; Liang, X.L.; Wang, F. High Intensity Focused Ultrasound-Responsive and Ultrastable Cerasomal Perfluorocarbon Nanodroplets for Alleviating Tumor Multidrug Resistance and Epithelial-Mesenchymal Transition. ACS Nano 2020, 14, 15904–15918. [Google Scholar] [CrossRef]
- Long, H.; Qin, X.J.; Xu, R.; Mei, C.L.; Xiong, Z.Y.; Deng, X.; Huang, K.Y.; Liang, H.G. Non-Modified Ultrasound-Responsive Gas Vesicles from Microcystis with Targeted Tumor Accumulation. Int. J. Nanomed. 2021, 16, 8405–8416. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.Y.; Zhu, M.T.; Liang, M.L.; Zhang, L.; Zong, Y.J.; Wan, M.X. High-Performance Delivery of a CRISPR Interference System via Lipid-Polymer Hybrid Nanoparticles Combined with Ultrasound-Mediated Microbubble Destruction for Tumor-Specific Gene Repression. Adv. Healthc. Mater. 2023. [Google Scholar] [CrossRef] [PubMed]
- Schoen, S.; Kilinc, M.S.; Lee, H.; Guo, Y.T.; Degertekin, F.L.; Woodworth, G.F.; Arvanitis, C. Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv. Drug Deliv. Rev. 2022, 180, 114043. [Google Scholar] [CrossRef] [PubMed]
- Honari, A.; Merillat, D.A.; Bellary, A.; Ghaderi, M.; Sirsi, S.R. Improving Release of Liposome-Encapsulated Drugs with Focused Ultrasound and Vaporizable Droplet-Liposome Nanoclusters. Pharmaceutics 2021, 13, 609. [Google Scholar] [CrossRef]
- Lea-Banks, H.; O’Reilly, M.A.; Hynynen, K. Ultrasound-responsive droplets for therapy: A review. J. Control. Release 2019, 293, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Shang, M.M.; Sun, X.; Guo, L.; Xiao, S.; Shi, D.D.; Meng, D.; Zhao, Y.D.; Yang, L.Z.; Jiang, C.; et al. Dual-responsive nanodroplets combined with ultrasound-targeted microbubble destruction suppress tumor growth and metastasis via autophagy blockade. J. Control. Release 2022, 343, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Yeh, C.K. Concurrent anti-vascular therapy and chemotherapy in solid tumors using drug-loaded acoustic nanodroplet vaporization. Acta Biomater. 2017, 49, 472–485. [Google Scholar] [CrossRef]
- Ho, Y.J.; Wang, T.C.; Fan, C.H.; Yeh, C.K. Current progress in antivascular tumor therapy. Drug Discov. Today 2017, 22, 1503–1515. [Google Scholar] [CrossRef]
- Ho, Y.J.; Chang, Y.C.; Yeh, C.K. Improving Nanoparticle Penetration in Tumors by Vascular Disruption with Acoustic Droplet Vaporization. Theranostics 2016, 6, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Carlisle, R.; Choi, J.J.; Stevenson, M.; Shah, A.R.; Myers, R.S.; Fisher, K.; Peregrino, M.B.; Seymour, L.; Coussios, C.C. Inertial cavitation to non-invasively trigger and monitor intratumoral release of drug from intravenously delivered liposomes. J. Control. Release 2014, 178, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Morel, D.R.; Schwieger, I.; Hohn, L.; Terrettaz, J.; Llull, J.B.; Cornioley, Y.A.; Schneider, M. Human pharmacokinetics and safety evaluation of SonoVue (TM), a new contrast agent for ultrasound imaging. Investig. Radiol. 2000, 35, 80–85. [Google Scholar] [CrossRef]
- Goertz, D.E.; Wright, C.; Hynynen, K. Contrast Agent Kinetics in the Rabbit Brain during Exposure to Therapeutic Ultrasound. Ultrasound Med. Biol. 2010, 36, 916–924. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef] [Green Version]
- Grull, H.; Langereis, S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J. Control. Release 2012, 161, 317–327. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabanas, M.V.; Manzano, M.; Vallet-Regi, M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim, A.; Chattaraj, R.; Blum, N.T.; Shi, D.; Kumar, K.; Goodwin, A.P. Phospholipid Capped Mesoporous Nanoparticles for Targeted High Intensity Focused Ultrasound Ablation. Adv. Healthc. Mater. 2017, 6, 1700514. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Min, H.S.; Lee, H.J.; Park, D.J.; Yhee, J.Y.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Silvestre, O.F.; Chen, X.Y.; et al. pH-Controlled Gas-Generating Mineralized Nanoparticles: A Theranostic Agent for Ultrasound Imaging and Therapy of Cancers. ACS Nano 2015, 9, 134–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohse, D.; Zhang, X.H. Surface nanobubbles and nanodroplets. Rev. Mod. Phys. 2015, 87, 981–1035. [Google Scholar] [CrossRef] [Green Version]
- Borkent, B.M.; Dammer, S.M.; Schonherr, H.; Vancso, G.J.; Lohse, D. Superstability of surface nanobubbles. Phys. Rev. Lett. 2007, 98, 204502. [Google Scholar] [CrossRef] [Green Version]
- Kwan, J.J.; Myers, R.; Coviello, C.M.; Graham, S.M.; Shah, A.R.; Stride, E.; Carlisle, R.C.; Coussios, C.C. Ultrasound-Propelled Nanocups for Drug Delivery. Small 2015, 11, 5305–5314. [Google Scholar] [CrossRef] [Green Version]
- Kwan, J.J.; Graham, S.; Myers, R.; Carlisle, R.; Stride, E.; Coussios, C.C. Ultrasound-induced inertial cavitation from gas-stabilizing nanoparticles. Phys. Rev. E 2015, 92, 023019. [Google Scholar] [CrossRef]
- Jin, Q.F.; Lin, C.Y.; Kang, S.T.; Chang, Y.C.; Zheng, H.R.; Yang, C.M.; Yeh, C.K. Superhydrophobic silica nanoparticles as ultrasound contrast agents. Ultrason. Sonochem. 2017, 36, 262–269. [Google Scholar] [CrossRef]
- Yildirim, A.; Chattaraj, R.; Blum, N.T.; Goldscheitter, G.M.; Goodwin, A.P. Stable Encapsulation of Air in Mesoporous Silica Nanoparticles: Fluorocarbon-Free Nanoscale Ultrasound Contrast Agents. Adv. Healthc. Mater. 2016, 5, 1290–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim, A.; Chattaraj, R.; Blum, N.T.; Goodwin, A.P. Understanding Acoustic Cavitation Initiation by Porous Nanoparticles: Toward Nanoscale Agents for Ultrasound Imaging and Therapy. Chem. Mater. 2016, 28, 5962–5972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Gao, S.J.; Zhao, Y.; Li, P.J.; Liu, J.; Li, P.; Tan, K.B.; Xie, F. Disruption of Tumor Neovasculature by Microbubble Enhanced Ultrasound: A Potential New Physical Therapy of Anti-Angiogenesis. Ultrasound Med. Biol. 2012, 38, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.F.; Kang, S.T.; Chang, Y.C.; Zheng, H.R.; Yeh, C.K. Inertial cavitation initiated by polytetrafluoroethylene nanoparticles under pulsed ultrasound stimulation. Ultrason. Sonochem. 2016, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.C.; Lin, C.H.; Ku, P.H.; Chang, L.L.; Liao, K.W.; Lin, W.T.; Yang, C.M. Hollow mesoporous Ia3d silica nanospheres with singleunit-cell-thick shell: Spontaneous formation and drug delivery application. Nano Res. 2014, 7, 1439–1448. [Google Scholar] [CrossRef]
- Palanikumar, L.; Kim, H.Y.; Oh, J.Y.; Thomas, A.P.; Choi, E.S.; Jeena, M.T.; Joo, S.H.; Ryu, J.H. Noncovalent Surface Locking of Mesoporous Silica Nanoparticles for Exceptionally High Hydrophobic Drug Loading and Enhanced Colloidal Stability. Biomacromolecules 2015, 16, 2701–2714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Shi, J.L.; Shen, W.H.; Dong, X.P.; Feng, J.W.; Ruan, M.L.; Li, Y.S. Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structure. Angew. Chem. Int. Ed. 2005, 44, 5083–5087. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Wu, C.-H.; Jin, Q.-F.; Lin, C.-Y.; Chiang, P.-H.; Wu, N.; Fan, C.-H.; Yang, C.-M.; Yeh, C.-K. Superhydrophobic drug-loaded mesoporous silica nanoparticles capped with β-cyclodextrin for ultrasound image-guided combined antivascular and chemo-sonodynamic therapy. Biomaterials 2020, 232, 119723. [Google Scholar] [CrossRef]
- van Leent, M.M.T.; Priem, B.; Schrijver, D.P.; de Dreu, A.; Hofstraat, S.R.J.; Zwolsman, R.; Beldman, T.J.; Netea, M.G.; Mulder, W.J.M. Regulating trained immunity with nanomedicine. Nat. Rev. Mater. 2022, 7, 465–481. [Google Scholar] [CrossRef]
- Mirkasymov, A.B.; Zelepukin, I.V.; Nikitin, P.I.; Nikitin, M.P.; Deyev, S.M. In vivo blockade of mononuclear phagocyte system with solid nanoparticles: Efficiency and affecting factors. J. Control. Release 2021, 330, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Foy, S.P.; Jain, T.K.; Labhasetwar, V. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm. Res. 2010, 27, 2283–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Dong, H.F.; Li, M.; Cao, Y.; Yang, F.; Zhang, K.; Dai, W.H.; Wang, C.T.; Zhang, X.J. Erythrocyte-Cancer Hybrid Membrane Camouflaged Hollow Copper Sulfide Nanoparticles for Prolonged Circulation Life and Homotypic-Targeting Photothermal/Chemotherapy of Melanoma. ACS Nano 2018, 12, 5241–5252. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Gao, W.W.; Zhang, L.F. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Guo, R.R.; Yao, X.X.; Sung, S.; Pang, Z.Q.; Yang, W.L. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.L.; Xu, J.H.; Cai, B.; Yu, G.T.; Yu, X.L.; He, Z.B.; Huang, Q.Q.; Li, A.; Guo, S.S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Meng, Q.F.; Huang, Q.Q.; Liu, P.; Bu, L.L.; Kondamareddy, K.K.; Guo, S.S.; Liu, W.; Zhao, X.Z. Photocatalytic Degradation of Cell Membrane Coatings for Controlled Drug Release. Adv. Healthc. Mater. 2016, 5, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Lin, C.Y.; Chang, Y.C.; Yang, C.M.; Yeh, C.K. Roles of Textural and Surface Properties of Nanoparticles in Ultrasound-Responsive Systems. Langmuir 2018, 34, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Osminkina, L.A.; Nikolaev, A.L.; Sviridov, A.P.; Andronova, N.V.; Tamarov, K.P.; Gongalsky, M.B.; Kudryavtsev, A.A.; Treshalina, H.M.; Timoshenko, V.Y. Porous silicon nanoparticles as efficient sensitizers for sonodynamic therapy of cancer. Microporous Mesoporous Mater. 2015, 210, 169–175. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Shi, J.L. Piezocatalytic Tumor Therapy by Ultrasound-Triggered and BaTiO3-Mediated Piezoelectricity. Adv. Mater. 2020, 32, 2001976. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Zhang, T.M.; Fan, F.; Gao, F.; Ji, H.X.; Yang, L.H. Piezoelectric Materials as Sonodynamic Sensitizers to Safely Ablate Tumors: A Case Study Using Black Phosphorus. J. Phys. Chem. Lett. 2020, 11, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, S.; van der Hoef, M.; Zhang, X.; Lohse, D. Stability of Surface Nanobubbles: A Molecular Dynamics Study. Langmuir 2016, 32, 11116–11122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, J.J.; Lajoinie, G.; de Jong, N.; Stride, E.; Versluis, M.; Coussios, C.C. Ultrahigh-Speed Dynamics of Micrometer-Scale Inertial Cavitation from Nanoparticles. Phys. Rev. Appl. 2016, 6, 044004. [Google Scholar] [CrossRef]
- Myers, R.; Coviello, C.; Erbs, P.; Foloppe, J.; Rowe, C.; Kwan, J.; Crake, C.; Finn, S.; Jackson, E.; Balloul, J.M.; et al. Polymeric Cups for Cavitation-mediated Delivery of Oncolytic Vaccinia Virus. Mol. Ther. 2016, 24, 1627–1633. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, Y.C.; Fu, J.K.; Wang, L.Z. Effective Cancer Cell Killing by Hydrophobic Nanovoid-Enhanced Cavitation under Safe Low-Energy Ultrasound. Chem. Asian J. 2014, 9, 790–796. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.C. Synergistic cytotoxicity of low-energy ultrasound and innovative mesoporous silica-based sensitive nanoagents. J. Mater. Sci. 2014, 49, 3665–3673. [Google Scholar] [CrossRef]
- Sun, Q.H.; Zhou, Z.X.; Qiu, N.S.; Shen, Y.Q. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Kopecek, J.; Kopeckova, P. HPMA copolymers: Origins, early developments, present, and future. Adv. Drug Deliv. Rev. 2010, 62, 122–149. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Guo, P.; Jiao, Z.X.; Lin, L.; Xu, C.N.; Tian, H.Y.; Chen, X.S. Poly(L-glutamic acid)-Based Zwitterionic Polymer in a Charge Conversional Shielding System for Gene Therapy of Malignant Tumors. ACS Appl. Mater. Interfaces 2020, 12, 19295–19306. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S.Y. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Sun, H.P.; Su, J.H.; Meng, Q.S.; Yin, Q.; Chen, L.L.; Gu, W.W.; Zhang, P.C.; Zhang, Z.W.; Yu, H.J.; Wang, S.L.; et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv. Mater. 2016, 28, 9581–9588. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Sun, W.J.; Qian, C.G.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef]
- Rao, L.; Meng, Q.F.; Huang, Q.Q.; Wang, Z.X.; Yu, G.T.; Li, A.; Ma, W.J.; Zhang, N.G.; Guo, S.S.; Zhao, X.Z.; et al. Platelet-Leukocyte Hybrid Membrane-Coated Immunomagnetic Beads for Highly Efficient and Highly Specific Isolation of Circulating Tumor Cells. Adv. Funct. Mater. 2018, 28, 1803531. [Google Scholar] [CrossRef]
- Kroll, A.V.; Fang, R.H.; Zhang, L.F. Biointerfacing and Applications of Cell Membrane-Coated Nanoparticles. Bioconjug. Chem. 2017, 28, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Padera, T.P.; Stoll, B.R.; Tooredman, J.B.; Capen, D.; di Tomaso, E.; Jain, R.K. Cancer cells compress intratumour vessels. Nature 2004, 427, 695. [Google Scholar] [CrossRef] [PubMed]
- Smolarczyk, R.; Czapla, J.; Jarosz-Biej, M.; Czerwinski, K.; Cichon, T. Vascular disrupting agents in cancer therapy. Eur. J. Pharmacol. 2021, 891, 173692. [Google Scholar] [CrossRef] [PubMed]
- Caissie, A.; Lee, J.; Karshafian, R.; Furukawa, M.; Kwok, S.; Giles, A.; Li, Y.Q.; Wong, S.; Czarnota, G.J. Novel Tumour Therapy Using Ultrasound-Activated Microbubbles as Vascular Disrupting Agents. Radiother. Oncol. 2010, 96, S41. [Google Scholar]



| Sample Name | Mean Size (nm) | Mean Concentration (Particles/mL) | Mean Zeta Potential (mV) |
|---|---|---|---|
| MSN | 153.7 ± 86.2 | 1.62 × 1010 ± 2.98 × 108 | −17.97 ± 0.60 |
| F-MSN | 171.1 ± 53.9 | 1.44 × 1010 ± 2.42 × 108 | −19.03 ± 0.62 |
| F-MSN-DOX | 211.2 ± 72.4 | 8.37 × 109 ± 1.16 × 108 | −21.70 ± 3.18 |
| F-MSN-DOX@RBC | 232.6 ± 78.8 | 8.19 × 109 ± 2.30 × 108 | −35.57 ± 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Q.; Chen, D.; Song, Y.; Liu, T.; Li, W.; Chen, Y.; Qin, X.; Zhang, L.; Wang, J.; Xie, M. Ultrasound-Responsive Biomimetic Superhydrophobic Drug-Loaded Mesoporous Silica Nanoparticles for Treating Prostate Tumor. Pharmaceutics 2023, 15, 1155. https://doi.org/10.3390/pharmaceutics15041155
Jin Q, Chen D, Song Y, Liu T, Li W, Chen Y, Qin X, Zhang L, Wang J, Xie M. Ultrasound-Responsive Biomimetic Superhydrophobic Drug-Loaded Mesoporous Silica Nanoparticles for Treating Prostate Tumor. Pharmaceutics. 2023; 15(4):1155. https://doi.org/10.3390/pharmaceutics15041155
Chicago/Turabian StyleJin, Qiaofeng, Dandan Chen, Yishu Song, Tianshu Liu, Wenqu Li, Yihan Chen, Xiaojuan Qin, Li Zhang, Jing Wang, and Mingxing Xie. 2023. "Ultrasound-Responsive Biomimetic Superhydrophobic Drug-Loaded Mesoporous Silica Nanoparticles for Treating Prostate Tumor" Pharmaceutics 15, no. 4: 1155. https://doi.org/10.3390/pharmaceutics15041155





