Thermosensitive Cationic Magnetic Liposomes for Thermoresponsive Delivery of CPT-11 and SLP2 shRNA in Glioblastoma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Citric-Acid-Coated Magnetic Nanoparticle (CMNP)
2.3. Preparation of Thermosensitive Cationic Magnetic Liposomes (TCMLs)
2.4. Physico-Chemical Characterization
2.5. Drug Loading and Release
2.6. In Vitro Cell Culture
2.6.1. Cell Line and Cell Culture Condition
2.6.2. Intracellular Uptake
2.6.3. Transfection of U87 Cells and Cell Migration Assay
2.6.4. In Vitro Cytotoxicity
2.7. In Vivo Study
2.7.1. Xenograft Tumor Model
2.7.2. In Vivo Antitumor Efficacy
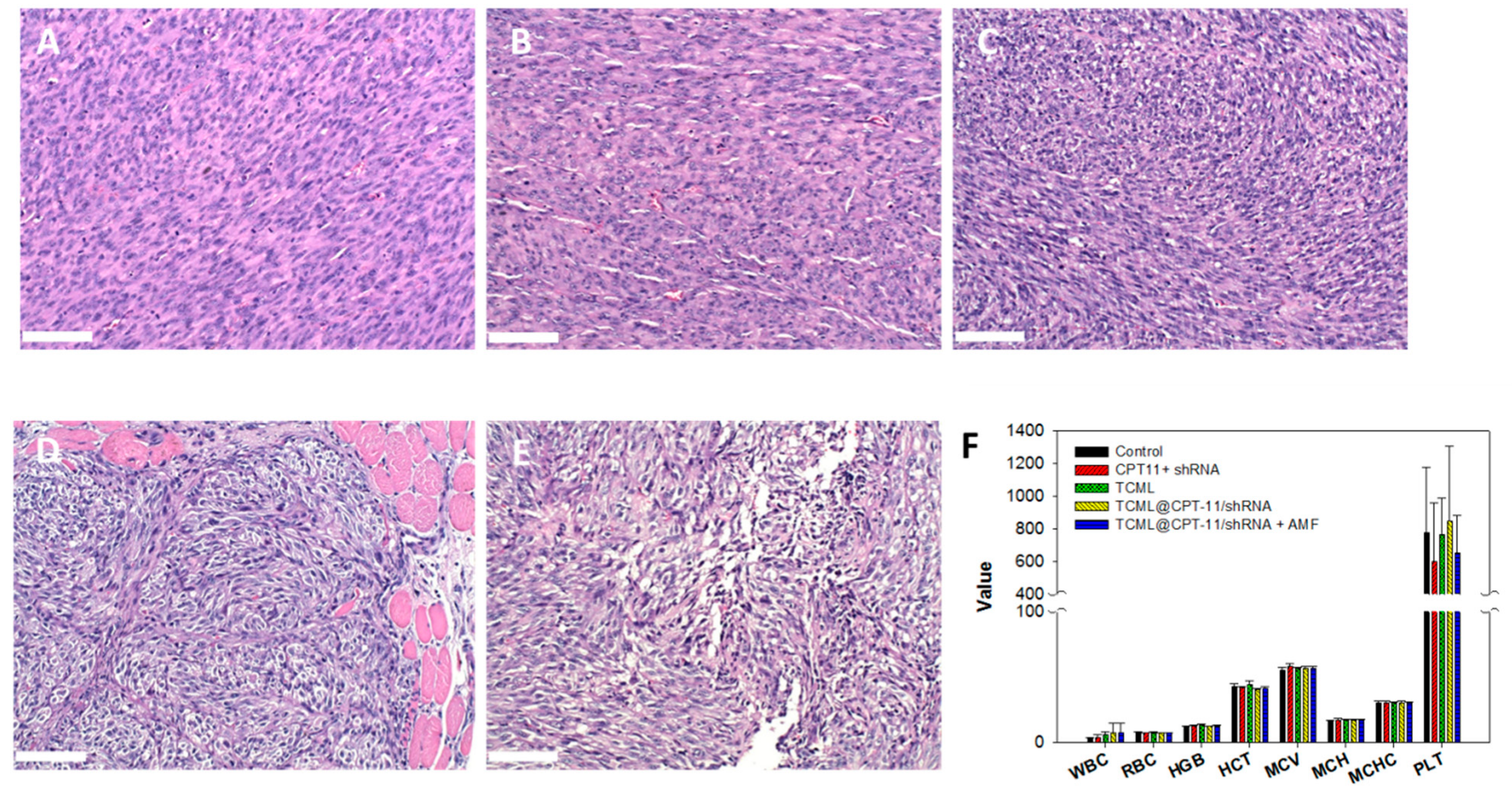
2.7.3. Histological and Hematologic Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Liposomes
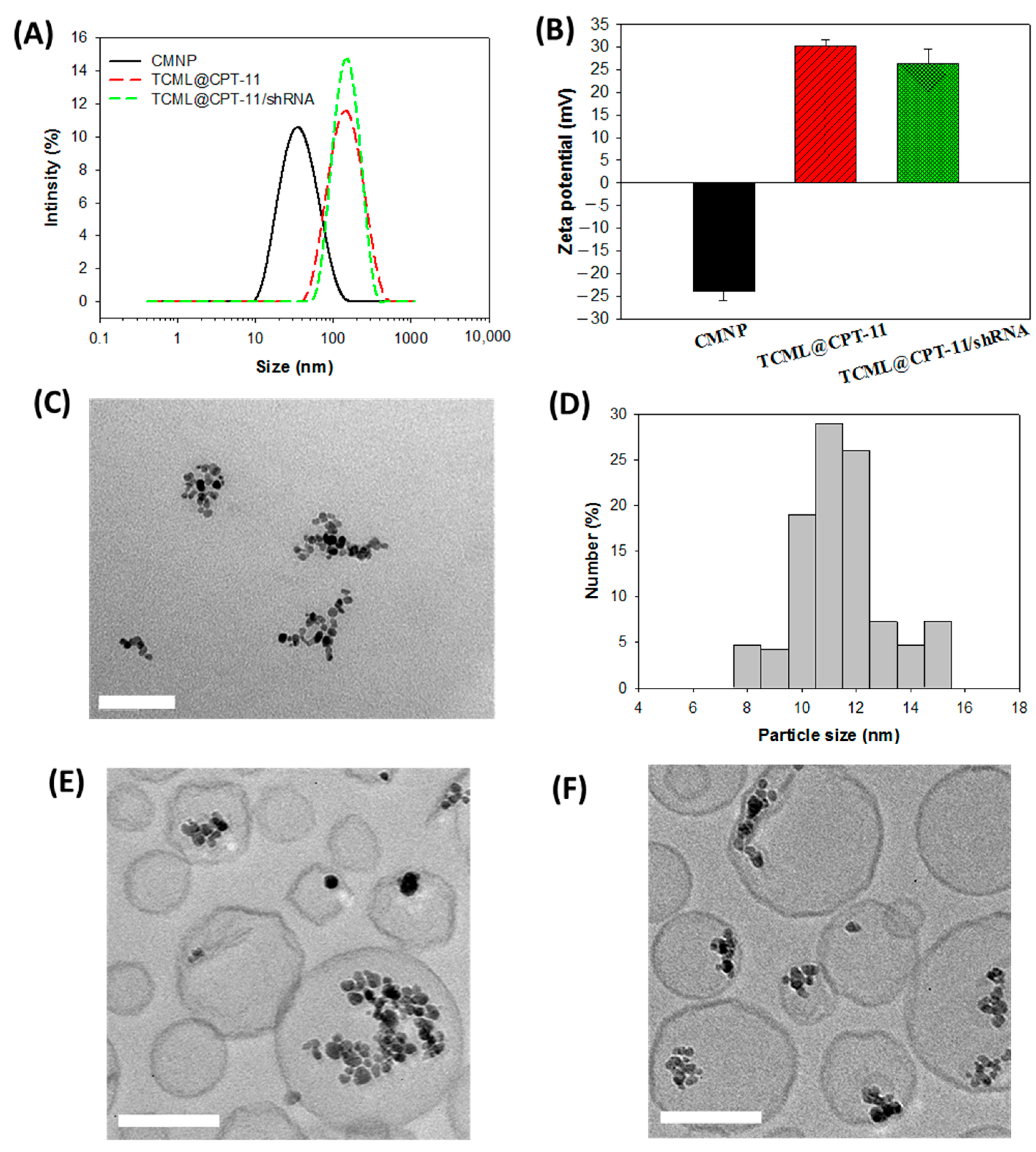
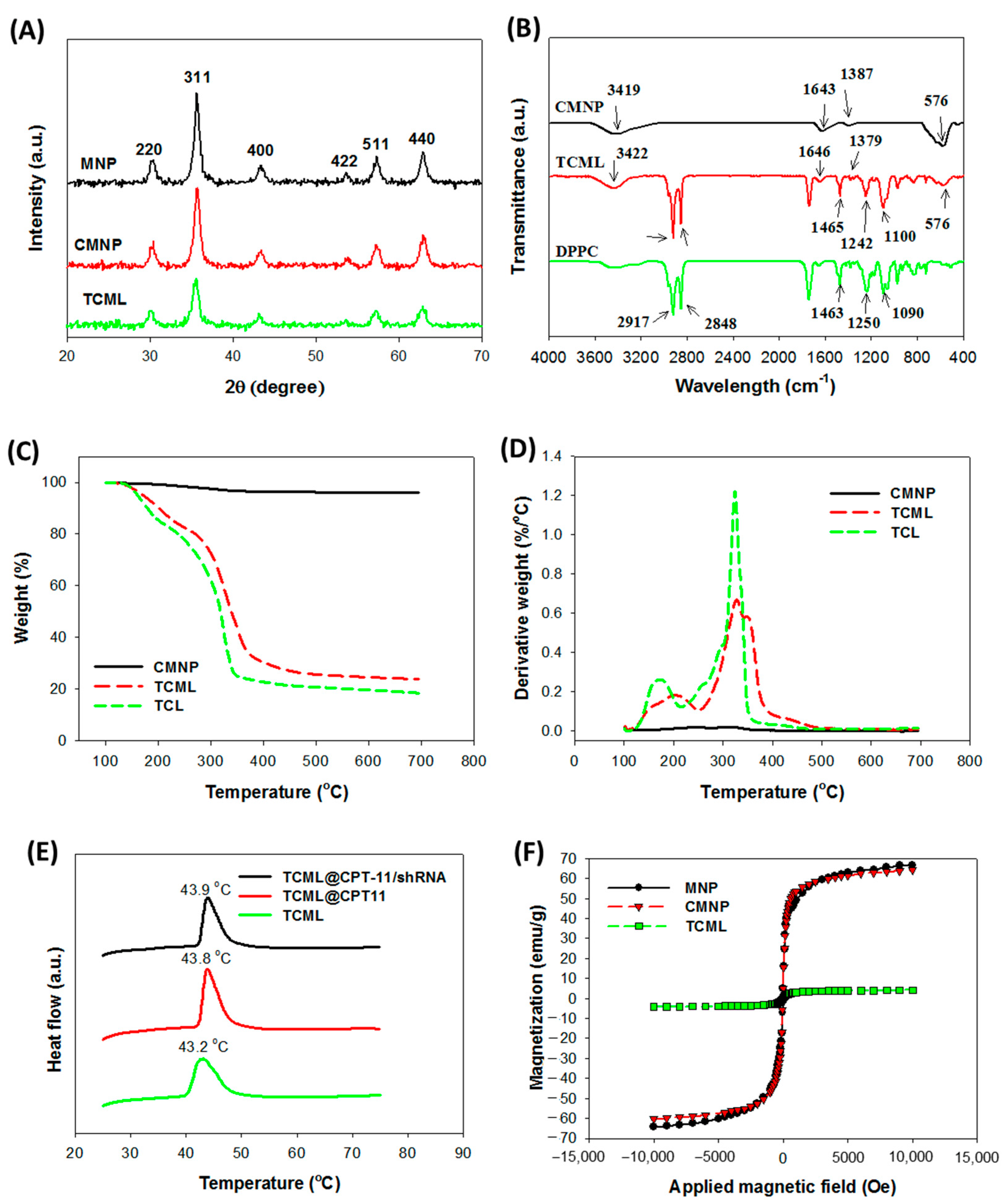
3.2. Characterization of Liposomes
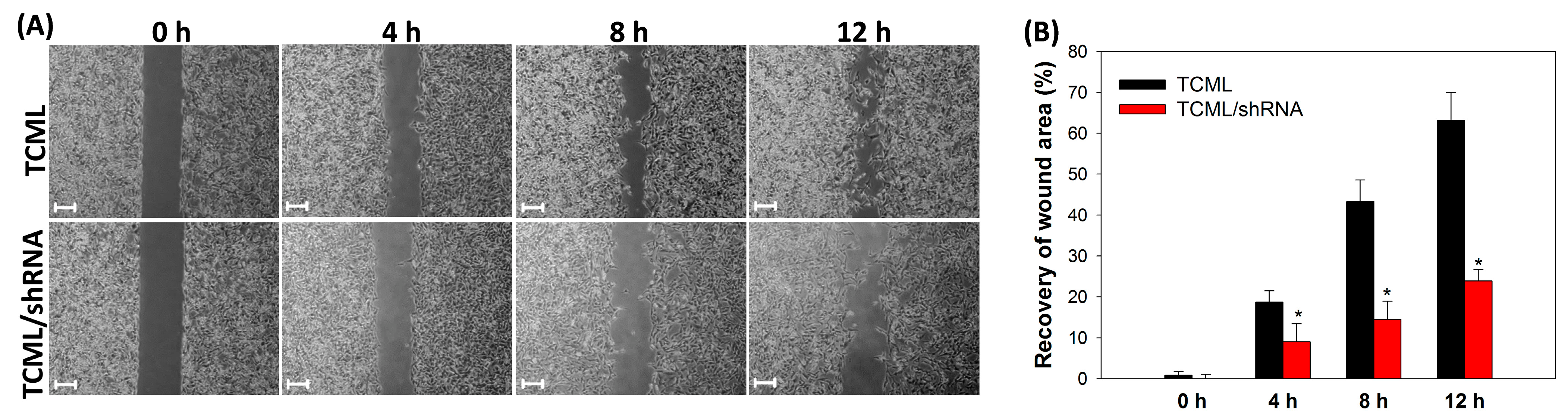
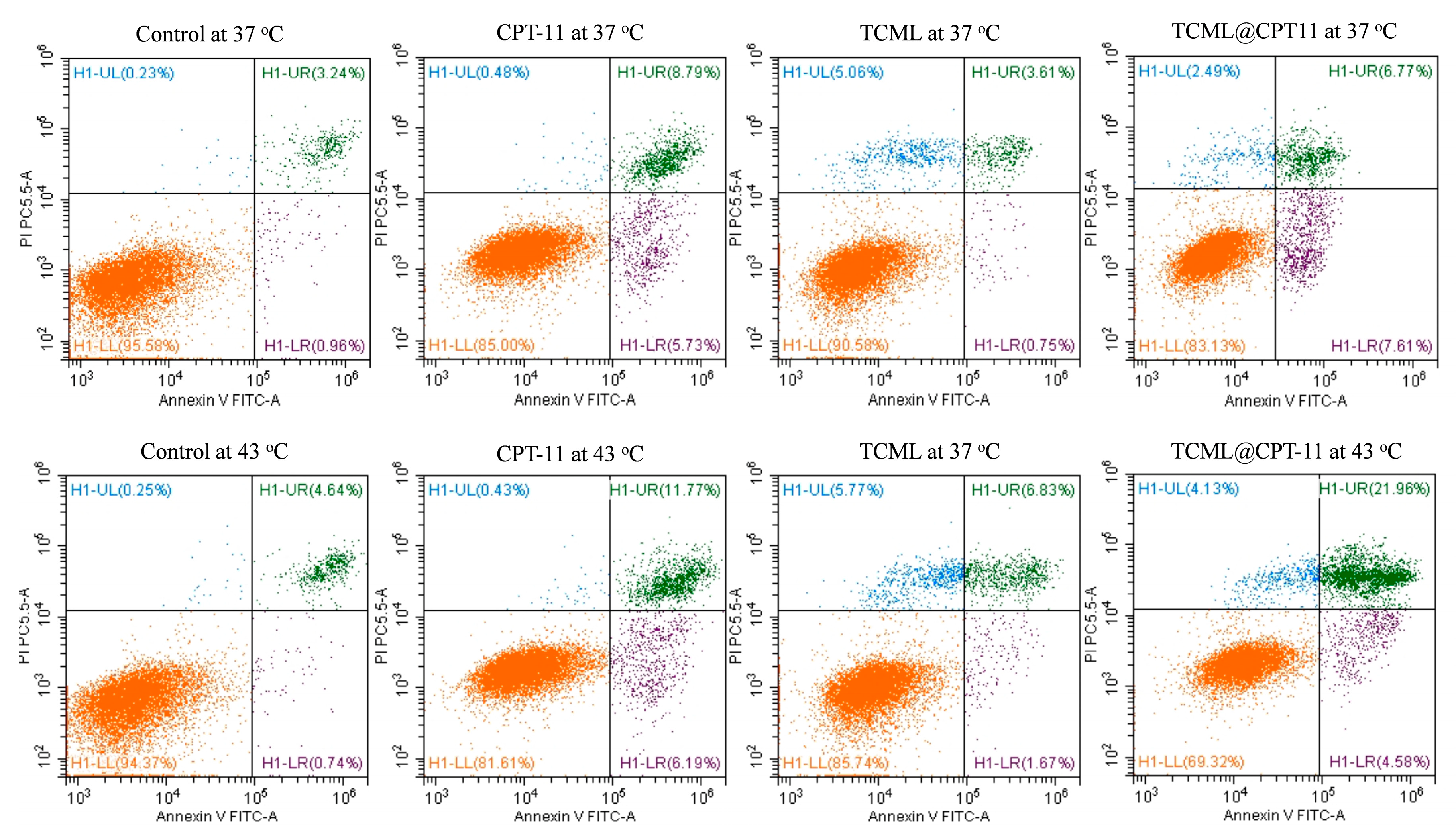
3.3. In Vitro Studies
3.4. In Vivo Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Valdebenito, S.; D’Amico, D.; Eugenin, E. Novel approaches for glioblastoma treatment: Focus on tumor heterogeneity, treatment resistance, and computational tools. Cancer Rep. 2019, 2, e1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prados, M.D.; Lamborn, K.; Yung, W.K.; Jaeckle, K.; Robins, H.I.; Mehta, M.; Fine, H.A.; Wen, P.Y.; Cloughesy, T.; Chang, S.; et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: A North American Brain Tumor Consortium study. Neuro Oncol. 2006, 8, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Abri Aghdam, M.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
- Chuang, E.Y.; Lin, C.C.; Chen, K.J.; Wan, D.H.; Lin, K.J.; Ho, Y.C.; Lin, P.Y.; Sung, H.W. A FRET-guided, NIR-responsive bubble-generating liposomal system for in vivo targeted therapy with spatially and temporally precise controlled release. Biomaterials 2016, 93, 48–59. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive Polymers and Thermo-Responsive Liposomal Drug Delivery Systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef] [PubMed]
- Moosavian, S.A.; Bianconi, V.; Pirro, M.; Sahebkar, A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin. Cancer Biol. 2021, 69, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.-J.; Chuang, E.-Y.; Cheng, Y.-H.; Anilkumar, T.S.; Chen, H.-A.; Chen, J.-P. Thermosensitive magnetic liposomes for alternating magnetic field-inducible drug delivery in dual targeted brain tumor chemotherapy. Chem. Eng. J. 2019, 373, 720–733. [Google Scholar] [CrossRef]
- Anilkumar, T.S.; Shalumon, K.T.; Chen, J.P. Applications of Magnetic Liposomes in Cancer Therapies. Curr. Pharm. Des. 2019, 25, 1490–1504. [Google Scholar] [CrossRef]
- Martina, M.S.; Wilhelm, C.; Lesieur, S. The effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cells. Biomaterials 2008, 29, 4137–4145. [Google Scholar] [CrossRef]
- Shivanna, A.T.; Dash, B.S.; Chen, J.-P. Functionalized Magnetic Nanoparticles for Alternating Magnetic Field- or Near Infrared Light-Induced Cancer Therapies. Micromachines 2022, 13, 1279. [Google Scholar] [CrossRef]
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef]
- Lakkadwala, S.; Dos Santos Rodrigues, B.; Sun, C.; Singh, J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J. Control. Release 2019, 307, 247–260. [Google Scholar] [CrossRef]
- Wang, W.; Song, H.; Zhang, J.; Li, P.; Li, C.; Wang, C.; Kong, D.; Zhao, Q. An injectable, thermosensitive and multicompartment hydrogel for simultaneous encapsulation and independent release of a drug cocktail as an effective combination therapy platform. J. Control. Release 2015, 203, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Tan, C. Combination of microRNA therapeutics with small-molecule anticancer drugs: Mechanism of action and co-delivery nanocarriers. Adv. Drug Deliv. Rev. 2015, 81, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, C.; Cheng, Y.; Li, D.; Gong, Y.; Liu, J.; Tian, H.; Chen, X. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 2014, 35, 8723–8734. [Google Scholar] [CrossRef]
- Kozielski, K.L.; Ruiz-Valls, A.; Tzeng, S.Y.; Guerrero-Cázares, H.; Rui, Y.; Li, Y.; Vaughan, H.J.; Gionet-Gonzales, M.; Vantucci, C.; Kim, J.; et al. Cancer-selective nanoparticles for combinatorial siRNA delivery to primary human GBM in vitro and in vivo. Biomaterials 2019, 209, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, A.; Siby, A.; Koottungal, M.J.; George, J.; John, F. Knocking Down Barriers: Advances in siRNA Delivery. ChemistrySelect 2021, 6, 13350–13362. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Xia, Y.; Tian, J.; Chen, X. Effect of surface properties on liposomal siRNA delivery. Biomaterials 2016, 79, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Ashique, S.; Almohaywi, B.; Haider, N.; Yasmin, S.; Hussain, A.; Mishra, N.; Garg, A. siRNA-based nanocarriers for targeted drug delivery to control breast cancer. Adv. Cancer Biol.-Metastasis 2022, 4, 100047. [Google Scholar] [CrossRef]
- Samadikhah, H.R.; Majidi, A.; Nikkhah, M.; Hosseinkhani, S. Preparation, characterization, and efficient transfection of cationic liposomes and nanomagnetic cationic liposomes. Int. J. Nanomed. 2011, 6, 2275–2283. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ding, F.; Cao, W.; Liu, Z.; Liu, W.; Yu, Z.; Wu, Y.; Li, W.; Li, Y.; Liu, Z. Stomatin-like protein 2 is overexpressed in cancer and involved in regulating cell growth and cell adhesion in human esophageal squamous cell carcinoma. Clin. Cancer Res. 2006, 12, 1639–1646. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Ma, K.; Gong, M.; Cui, Y.; Liu, Z.H.; Zhou, X.G.; Zhou, C.N.; Wang, T.Y. SLP-2 overexpression is associated with tumour distant metastasis and poor prognosis in pulmonary squamous cell carcinoma. Biomarkers 2010, 15, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, J.; He, H.Y.; Huang, E.W.; Cao, Q.H.; Luo, L.; Liao, Y.S.; Guo, Y. Suppression of CLC-3 chloride channel reduces the aggressiveness of glioma through inhibiting nuclear factor-κB pathway. Oncotarget 2017, 8, 63788–63798. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, L.; Wu, Z.; Lin, C.; Dai, T.; Yu, C.; Wang, X.; Wu, J.; Li, M.; Li, J. Knockdown of stomatin-like protein 2 (STOML2) reduces the invasive ability of glioma cells through inhibition of the NF-κB/MMP-9 pathway. J. Pathol. 2012, 226, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; Lan, Y.-H.; Lu, Y.-J.; Weng, Y.-L.; Chen, J.-P. Targeted delivery of irinotecan and SLP2 shRNA with GRP-conjugated magnetic graphene oxide for glioblastoma treatment. Biomater. Sci. 2022, 10, 3201–3222. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, T.; Liu, Y.; Zhang, N. Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes for the synergistic treatment of hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1374–1383. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Wang, C.; Fang, E.; Lu, X.; Wang, G.; Tong, Q. Co-delivery of doxorubicin and SATB1 shRNA by thermosensitive magnetic cationic liposomes for gastric cancer therapy. PLoS ONE 2014, 9, e92924. [Google Scholar] [CrossRef]
- Fong, Y.T.; Chen, C.H.; Chen, J.P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Sun, H.; Xu, P.; Yin, Q.; Zhang, Z.; Wang, S.; Yu, H.; Li, Y. Simultaneous inhibition of metastasis and growth of breast cancer by co-delivery of twist shRNA and paclitaxel using pluronic P85-PEI/TPGS complex nanoparticles. Biomaterials 2013, 34, 1581–1590. [Google Scholar] [CrossRef]
- Chen, H.-A.; Lu, Y.-J.; Dash, B.S.; Chao, Y.-K.; Chen, J.-P. Hyaluronic Acid-Modified Cisplatin-Encapsulated Poly(Lactic-co-Glycolic Acid) Magnetic Nanoparticles for Dual-Targeted NIR-Responsive Chemo-Photothermal Combination Cancer Therapy. Pharmaceutics 2023, 15, 290. [Google Scholar] [CrossRef]
- Yao, J.; Feng, X.; Dai, X.; Peng, G.; Guo, Z.; Liu, Z.; Wang, M.; Guo, W.; Zhang, P.; Li, Y. TMZ magnetic temperature-sensitive liposomes-mediated magnetothermal chemotherapy induces pyroptosis in glioblastoma. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102554. [Google Scholar] [CrossRef]
- Kim, H.J.; Yi, Y.; Kim, A.; Miyata, K. Small Delivery Vehicles of siRNA for Enhanced Cancer Targeting. Biomacromolecules 2018, 19, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabha, S.; Arya, G.; Chandra, R.; Ahmed, B.; Nimesh, S. Effect of size on biological properties of nanoparticles employed in gene delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 83–91. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [(14)C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Shelar, S.B.; Dey, A.; Gawali, S.L.; Dhinakaran, S.; Barick, K.C.; Basu, M.; Uppal, S.; Hassan, P.A. Spontaneous Formation of Cationic Vesicles in Aqueous DDAB-Lecithin Mixtures for Efficient Plasmid DNA Complexation and Gene Transfection. ACS Appl. Bio Mater. 2021, 4, 6005–6015. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.A.; Ma, Y.H.; Hsu, T.Y.; Chen, J.P. Preparation of Peptide and Recombinant Tissue Plasminogen Activator Conjugated Poly(Lactic-Co-Glycolic Acid) (PLGA) Magnetic Nanoparticles for Dual Targeted Thrombolytic Therapy. Int. J. Mol. Sci. 2020, 21, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kręcisz, M.; Rybka, J.D.; Strugała, A.J.; Skalski, B.; Figlerowicz, M.; Kozak, M.; Giersig, M. Interactions between magnetic nanoparticles and model lipid bilayers—Fourier transformed infrared spectroscopy (FTIR) studies of the molecular basis of nanotoxicity. J. Appl. Phys. 2016, 120, 124701. [Google Scholar] [CrossRef] [Green Version]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-H.; Hsu, H.-L.; Chen, J.-P.; Wu, T.; Ma, Y.-H. Thrombolysis induced by intravenous administration of plasminogen activator in magnetoliposomes: Dual targeting by magnetic and thermal manipulation. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101992. [Google Scholar] [CrossRef]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Mao, K.L.; Tian, F.R.; Yang, J.J.; Chen, P.P.; Xu, J.; Fan, Z.L.; Zhao, Y.P.; Li, W.F.; Zheng, L.; et al. Brain tumor-targeted delivery and therapy by focused ultrasound introduced doxorubicin-loaded cationic liposomes. Cancer Chemother. Pharmacol. 2016, 77, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Marchitto, J.; Nguyen, T.D.T.; Marasini, R.; Celia, C.; Aryal, S. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids Surf. B Biointerfaces 2020, 188, 110804. [Google Scholar] [CrossRef] [PubMed]







| Group | Median (Days) | Average 1 |
|---|---|---|
| Control | 20 | 25.0 ± 8.2 |
| CPT-11 + shRNA | 29 | 29.5 ± 5.4 α |
| TCML | 22 | 25.2 ± 4.1 |
| TCML@CPT-11/shRNA | 29 | 29.6 ± 2.5 α |
| TCML@CPT-11/shRNA + AMF | 36 | 37.2 ± 4.1 α,β,γ,δ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-J.; Hsu, H.-L.; Lan, Y.-H.; Chen, J.-P. Thermosensitive Cationic Magnetic Liposomes for Thermoresponsive Delivery of CPT-11 and SLP2 shRNA in Glioblastoma Treatment. Pharmaceutics 2023, 15, 1169. https://doi.org/10.3390/pharmaceutics15041169
Lu Y-J, Hsu H-L, Lan Y-H, Chen J-P. Thermosensitive Cationic Magnetic Liposomes for Thermoresponsive Delivery of CPT-11 and SLP2 shRNA in Glioblastoma Treatment. Pharmaceutics. 2023; 15(4):1169. https://doi.org/10.3390/pharmaceutics15041169
Chicago/Turabian StyleLu, Yu-Jen, Hao-Lung Hsu, Yu-Hsiang Lan, and Jyh-Ping Chen. 2023. "Thermosensitive Cationic Magnetic Liposomes for Thermoresponsive Delivery of CPT-11 and SLP2 shRNA in Glioblastoma Treatment" Pharmaceutics 15, no. 4: 1169. https://doi.org/10.3390/pharmaceutics15041169
APA StyleLu, Y.-J., Hsu, H.-L., Lan, Y.-H., & Chen, J.-P. (2023). Thermosensitive Cationic Magnetic Liposomes for Thermoresponsive Delivery of CPT-11 and SLP2 shRNA in Glioblastoma Treatment. Pharmaceutics, 15(4), 1169. https://doi.org/10.3390/pharmaceutics15041169







