Synergistic Encapsulation of Paclitaxel and Sorafenib by Methoxy Poly(Ethylene Glycol)-b-Poly(Caprolactone) Polymeric Micelles for Ovarian Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Lines and Culture Conditions
2.3. Combination Index (CI) Analysis
2.4. HPLC Analysis
2.5. Formation of the PTX/SRF-Encapsulated Polymeric Micelles
2.6. Physicochemical Micelle Characterization
2.7. Transmission Electron Microscopy Observation
2.8. In Vitro Stability Test
2.9. In Vitro Drug Release Assay
2.10. In Vitro Cytotoxicity Assay
2.11. In Vitro Clonogenic Assay
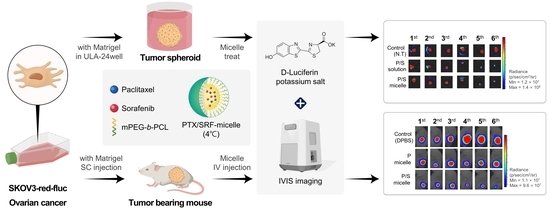
2.12. Preparation of Tumor Spheroids Using SKOV3-Red-Fluc and In Vitro 3D Tumor Spheroid Viability Assay
2.13. In Vivo Pharmacokinetic Evaluation and Biological Sample Preparation for HPLC Analysis
2.14. In Vivo Toxicity Assessment
2.15. Preparation of Xenograft Nude Mice Model Using SKOV3-Red-Fluc and Luminescence Imaging
2.16. Antitumor Evaluation in Xenograft Nude Mice and H&E Staining
2.17. Statistical Analysis
3. Results
3.1. Evaluation of the Synergistic Effect of PTX and SRF in OC Cell Lines
3.2. Evaluation of Physicochemical Properties of Micelles Encapsulated with PTX and SRF
3.3. Stability Test
3.4. In Vitro Cytotoxicity Assay
3.5. In Vitro Clonogenic Assay
3.6. In Vitro Drug Release Profile
3.7. In Vitro 3D Tumor Spheroid Viability Assay
3.8. Pharmacokinetic Evaluation
3.9. In Vivo Toxicity Assessment
3.10. Antitumor Evaluation and H&E Staining and Toxicity Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gubbels, J.A.; Claussen, N.; Kapur, A.K.; Connor, J.P.; Patankar, M.S. The detection, treatment, and biology of epithelial ovarian cancer. J. Ovarian Res. 2010, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Bast, R.C., Jr.; Lu, Z.; Han, C.Y.; Lu, K.H.; Anderson, K.S.; Drescher, C.W.; Skates, S.J. Biomarkers and Strategies for Early Detection of Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2504–2512. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef]
- Babaier, A.; Mal, H.; Alselwi, W.; Ghatage, P. Low-Grade Serous Carcinoma of the Ovary: The Current Status. Diagnostics 2022, 12, 458. [Google Scholar] [CrossRef]
- Dunton, C.J. Management of treatment-related toxicity in advanced ovarian cancer. Oncologist 2002, 7, 11–19. [Google Scholar] [CrossRef]
- Yang, C.H.; Horwitz, S.B. Taxol(®): The First Microtubule Stabilizing Agent. Int. J. Mol. Sci. 2017, 18, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.L.; Lou, Z.P.; Ma, F.Y.; Najafi, M. The interactions of paclitaxel with tumour microenvironment. Int. Immunopharmacol. 2022, 105, 108555. [Google Scholar] [CrossRef]
- Nawara, H.M.; Afify, S.M.; Hassan, G.; Zahra, M.H.; Seno, A.; Seno, M. Paclitaxel-Based Chemotherapy Targeting Cancer Stem Cells from Mono- to Combination Therapy. Biomedicines 2021, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.F.; Wang, X.Y.; Fu, Z.Q.; Peng, Q.H.; Zhang, J.Y.; Ye, F.; Fu, Y.F.; Zhou, C.Y.; Lu, W.G.; Cheng, X.D.; et al. TXNDC17 promotes paclitaxel resistance via inducing autophagy in ovarian cancer. Autophagy 2015, 11, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, A.; Kulus, M.; Wieczorkiewicz, M.; Pieńkowski, W.; Stefańska, K.; Skupin-Mrugalska, P.; Bryl, R.; Mozdziak, P.; Kempisty, B.; Piotrowska-Kempisty, H. Ovarian Cancer and Cancer Stem Cells-Cellular and Molecular Characteristics, Signaling Pathways, and Usefulness as a Diagnostic Tool in Medicine and Oncology. Cancers 2021, 13, 4178. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kim, D.; Kim, D.K.; Choi, K.U.; Suh, D.S.; Kim, J.H. Therapeutic Strategies for Targeting Ovarian Cancer Stem Cells. Int. J. Mol. Sci. 2021, 22, 5059. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Srivastava, S.K. Cancer cells stemness: A doorstep to targeted therapy. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165424. [Google Scholar] [CrossRef]
- Mohseni, M.; Samadi, N.; Ghanbari, P.; Yousefi, B.; Tabasinezhad, M.; Sharifi, S.; Nazemiyeh, H. Co-treatment by docetaxel and vinblastine breaks down P-glycoprotein mediated chemo-resistance. Iran. J. Basic Med. Sci. 2016, 19, 300. [Google Scholar]
- Samadi, N.; Ghanbari, P.; Mohseni, M.; Tabasinezhad, M.; Sharifi, S.; Nazemieh, H.; Rashidi, M.R. Combination therapy increases the efficacy of docetaxel, vinblastine and tamoxifen in cancer cells. J. Cancer Res. Ther. 2014, 10, 715–721. [Google Scholar] [PubMed]
- Della Corte, L.; Barra, F.; Foreste, V.; Giampaolino, P.; Evangelisti, G.; Ferrero, S.; Bifulco, G. Advances in paclitaxel combinations for treating cervical cancer. Expert Opin. Pharmacother. 2020, 21, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Worden, F.; Kudo, M. Sorafenib: Key lessons from over 10 years of experience. Expert Rev. Anticancer. Ther. 2019, 19, 177–189. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef] [Green Version]
- Hasskarl, J. Sorafenib: Targeting multiple tyrosine kinases in cancer. Small Mol. Oncol. 2014, 201, 145–164. [Google Scholar]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef] [Green Version]
- Nawara, H.M.; Afify, S.M.; Hassan, G.; Zahra, M.H.; Atallah, M.N.; Mansour, H.; Abu Quora, H.A.; Alam, M.J.; Osman, A.; Kakuta, H.; et al. Paclitaxel and Sorafenib: The Effective Combination of Suppressing the Self-Renewal of Cancer Stem Cells. Cancers 2020, 12, 1360. [Google Scholar] [CrossRef]
- Merz, M.; Komljenovic, D.; Zwick, S.; Semmler, W.; Bäuerle, T. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. Eur. J. Cancer 2011, 47, 277–286. [Google Scholar] [CrossRef]
- Agliano, A.; Calvo, A.; Box, C. The challenge of targeting cancer stem cells to halt metastasis. Semin. Cancer Biol. 2017, 44, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Christodoulou, M.S.; Silvani, A.; Herold-Mende, C.; Passarella, D. Chemical approaches to targeting drug resistance in cancer stem cells. Drug Discov. Today 2014, 19, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; He, B.; Qu, W.; Cui, Z.; Wang, Y.-b.; Zhang, H.; Wang, J.-C.; Zhang, Q. Preparation of the albumin nanoparticle system loaded with both paclitaxel and sorafenib and its evaluation in vitro and in vivo. J. Microencapsul. 2011, 28, 528–536. [Google Scholar] [CrossRef]
- Shord, S.S.; Camp, J.R. Intravenous administration of paclitaxel in Sprague-Dawley rats: What is a safe dose? Biopharm. Drug Dispos. 2006, 27, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, B.; Gong, X.; Wang, T.; Liu, Y.; Zhang, N. In vivo biodistribution, biocompatibility, and efficacy of sorafenib-loaded lipid-based nanosuspensions evaluated experimentally in cancer. Int. J. Nanomed. 2016, 11, 2329–2343. [Google Scholar] [CrossRef] [Green Version]
- Leone Roberti Maggiore, U.; Valenzano Menada, M.; Venturini, P.L.; Ferrero, S. Sorafenib for ovarian cancer. Expert Opin. Investig. Drugs 2013, 22, 1049–1062. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; Zhang, F.; Xu, Y.; Liang, W.; Yu, Y. Nanotechnological approaches for diagnosis and treatment of ovarian cancer: A review of recent trends. Drug Deliv. 2022, 29, 3218–3232. [Google Scholar] [CrossRef]
- Shin, D.H.; Kwon, G.S. Epothilone B-based 3-in-1 polymeric micelle for anticancer drug therapy. Int. J. Pharm. 2017, 518, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, R.; Morott, J.; Repka, M.A.; Wang, Y.; Chen, M. mPEG-b-PCL/TPGS mixed micelles for delivery of resveratrol in overcoming resistant breast cancer. Expert Opin. Drug Deliv. 2015, 12, 361–373. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nagasaki, Y.; Kato, Y.; Sugiyama, Y.; Kataoka, K. Long-circulating poly(ethylene glycol)-poly(d,l-lactide) block copolymer micelles with modulated surface charge. J. Control. Release 2001, 77, 27. [Google Scholar] [CrossRef]
- Shaffer, C. Nanomedicine transforms drug delivery. Drug Discov. Today 2005, 10, 1581. [Google Scholar] [CrossRef] [PubMed]
- Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine 2007, 2, 669. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, B.; Xue, L.; San, W. Soluplus® micelles as a potential drug delivery system for reversal of resistant tumor. Biomed. Pharmacother. 2015, 69, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Rose Jaquilin, P.J.; Oluwafemi, O.S.; Thomas, S.; Oyedeji, A. Recent advances in drug delivery nanocarriers incorporated in temperature-sensitive Pluronic F-127–A critical review. J. Drug Deliv. Sci. Technol. 2022, 72, 103390. [Google Scholar] [CrossRef]
- Doddapaneni, B.S.; Al-Fatease, A.M.; Rao, D.A.; Alani, A.W. Dual-drug loaded micelle for combinatorial therapy targeting HIF and mTOR signaling pathways for ovarian cancer treatment. J. Control. Release 2019, 307, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.J.; Lee, Y.J.; Park, C.W.; Chung, Y.B.; Kim, J.S.; Lee, M.K.; Shin, D.H. Evaluation of the Physicochemical Properties, Pharmacokinetics, and In Vitro Anticancer Effects of Docetaxel and Osthol Encapsulated in Methoxy Poly(ethylene glycol)-b-Poly(caprolactone) Polymeric Micelles. Int. J. Mol. Sci. 2020, 22, 231. [Google Scholar] [CrossRef]
- Ma, G.; Du, X.; Zhu, J.; Xu, F.; Yu, H.; Li, J. Multi-functionalized dendrimers for targeted co-delivery of sorafenib and paclitaxel in liver cancers. J. Drug Deliv. Sci. Technol. 2021, 63, 102493. [Google Scholar] [CrossRef]
- Lei, M.; Ma, G.; Sha, S.; Wang, X.; Feng, H.; Zhu, Y.; Du, X. Dual-functionalized liposome by co-delivery of paclitaxel with sorafenib for synergistic antitumor efficacy and reversion of multidrug resistance. Drug Deliv. 2019, 26, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Ludatscher, R.M.; Stehbens, W.E. Vesicles of fenestrated and non-fenestrated endothelium. Z. Zellforsch. Mikrosk. Anat. 1969, 97, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Au, J.L.; Wientjes, M.G. Comparison of methods for evaluating drug-drug interaction. Front. Biosci. (Elite Ed.) 2010, 2, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Chou, T.-C. Frequently asked questions in drug combinations and the mass-action law-based answers. Synergy 2014, 1, 3–21. [Google Scholar] [CrossRef]
- D’Souza, S. A Review ofIn VitroDrug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar]
- Repp, L.; Unterberger, C.J.; Ye, Z.; Feltenberger, J.B.; Swanson, S.M.; Marker, P.C.; Kwon, G.S. Oligo (Lactic Acid) 8-Docetaxel Prodrug-Loaded PEG-b-PLA Micelles for Prostate Cancer. Nanomaterials 2021, 11, 2745. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Yeldag, G.; Rice, A.; del Río Hernández, A. Chemoresistance and the self-maintaining tumor microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Foltz, C.J.; Ullman-Cullere, M. Guidelines for assessing the health and condition of mice. Resource 1999, 28, 28–32. [Google Scholar]
- Toth, L.A. Defining the moribund condition as an experimental endpoint for animal research. ILAR J. 2000, 41, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, K.; Jin, C.; Eshima, K.; Hong, M.H.; Eshima, K.; Fukushima, M. Anticancer activity of the intraperitoneal-delivered DFP-10825, the cationic liposome-conjugated RNAi molecule targeting thymidylate synthase, on peritoneal disseminated ovarian cancer xenograft model. Drug Des. Dev. Ther. 2018, 12, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Tuli, R.; Surmak, A.; Reyes, J.; Hacker-Prietz, A.; Armour, M.; Leubner, A.; Blackford, A.; Tryggestad, E.; Jaffee, E.M.; Wong, J.; et al. Development of a Novel Preclinical Pancreatic Cancer Research Model: Bioluminescence Image- Guided Focal Irradiation and Tumor Monitoring of Orthotopic Xenografts. Transl. Oncol. 2012, 5, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, 49. [Google Scholar] [CrossRef]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000, 21, 585–591. [Google Scholar] [CrossRef]
- Menon, U.; Griffin, M.; Gentry-Maharaj, A. Ovarian cancer screening--current status, future directions. Gynecol. Oncol. 2014, 132, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.-m.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef]
- Jo, M.J.; Shin, H.J.; Yoon, M.S.; Kim, S.Y.; Jin, C.E.; Park, C.W.; Kim, J.S.; Shin, D.H. Evaluation of pH-Sensitive Polymeric Micelles Using Citraconic Amide Bonds for the Co-Delivery of Paclitaxel, Etoposide, and Rapamycin. Pharmaceutics 2023, 15, 154. [Google Scholar] [CrossRef]
- Shin, H.; Jo, M.; Jin, I.; Park, C.-W.; Kim, J.-S.; Shin, D. Optimization and Pharmacokinetic Evaluation of Synergistic Fenbendazole and Rapamycin Co-Encapsulated in Methoxy Poly(Ethylene Glycol)-b-Poly(Caprolactone) Polymeric Micelles. Int. J. Nanomed. 2021, 16, 4873–4889. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.E.; Hsieh, C.M.; Chen, L.C.; Su, C.Y.; Liu, D.Z.; Jhan, H.J.; Ho, H.O.; Sheu, M.T. Novel application of pluronic lecithin organogels (PLOs) for local delivery of synergistic combination of docetaxel and cisplatin to improve therapeutic efficacy against ovarian cancer. Drug Deliv. 2018, 25, 632–643. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical pharmacokinetics of paclitaxel monotherapy: An updated literature review. Clin. Pharmacokinet. 2018, 57, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Trivedi, R.; Kompella, U.B. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine 2010, 5, 485–505. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Zhang, Y.; Nyström, A.M. Endocytic uptake and intracellular trafficking of bis-MPA-based hyperbranched copolymer micelles in breast cancer cells. Biomacromolecules 2012, 13, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Jo, M.J.; Jo, Y.H.; Lee, Y.J.; Park, C.W.; Kim, J.S.; Hong, J.T.; Chung, Y.B.; Lee, M.K.; Shin, D.H. Physicochemical, Pharmacokinetic, and Toxicity Evaluation of Methoxy Poly(ethylene glycol)-b-Poly(d,l-Lactide) Polymeric Micelles Encapsulating Alpinumisoflavone Extracted from Unripe Cudrania tricuspidata Fruit. Pharmaceutics 2019, 11, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feczkó, T.; Piiper, A.; Pleli, T.; Schmithals, C.; Denk, D.; Hehlgans, S.; Rödel, F.; Vogl, T.J.; Wacker, M. Theranostic Sorafenib-Loaded Polymeric Nanocarriers Manufactured by Enhanced Gadolinium Conjugation Techniques. Pharmaceutics 2019, 11, 489. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Li, Y. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]










| PTX:SRF (Molar Ratio) | SKOV3-Red-Fluc | HeyA8 | ||||
|---|---|---|---|---|---|---|
| IC50 (nM) | CI Value | IC50 (nM) | CI Value | |||
| PTX | SRF | PTX | SRF | |||
| 1:1 | 226 | 226 | 0.882 | 109 | 109 | 0.447 |
| 1:1.5 | 131 | 196 | 0.511 | 60.6 | 91.0 | 0.255 |
| 1:2.3 | 127 | 297 | 0.501 | 75.8 | 177 | 0.331 |
| 1:4 | 107 | 427 | 0.427 | 71.0 | 284 | 0.332 |
| 1:9 | 105 | 946 | 0.438 | 67.3 | 606 | 0.377 |
| Polymer Category | Amount of Polymer Used (mg) | Amount of PTX Used (mg) | Amount of SRF Used (mg) | PTX Encapsulation Efficiency (EE, %) | SRF Encapsulation Efficiency (EE, %) | PTX Drug Loading (DL, %) | SRF Drug Loading (DL, %) | Particle Size (nm) | PolyDispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|---|---|---|
| mPEG-b-PLA | 100 | 3 | 3 | 83.0 ± 4.83 | 84.6 ± 5.90 | 2.35 ± 0.16 | 2.48 ± 0.13 | 65.3 ± 12.3 | 0.35 ± 0.01 | −9.90 ± 8.31 |
| F-127 | 100 | 3 | 3 | 81.5 ± 1.45 | 81.0 ± 0.99 | 2.31 ± 0.03 | 2.38 ± 0.15 | 39.9 ± 7.43 | 0.28 ± 0.04 | −5.58 ± 5.83 |
| Soluplus® | 100 | 3 | 3 | 14.6 ± 9.22 | 15.7 ± 11.1 | 0.41 ± 0.31 | 0.45 ± 0.25 | 76.1 ± 10.1 | 0.12 ± 0.04 | −3.92 ± 3.83 |
| mPEG-b-PCL | 60 | 3 | 3 | 94.4 ± 4.14 | 91.6 ± 5.30 | 4.29 ± 0.18 | 4.17 ± 0.24 | 33.1 ± 2.15 | 0.23 ± 0.03 | −0.12 ± 0.18 |
| 100 | 3 | 3 | 92.1 ± 7.76 | 90.2 ± 9.21 | 2.61 ± 0.26 | 2.64 ± 0.22 | 39.2 ± 1.15 | 0.28 ± 0.02 | −8.42 ± 2.93 | |
| 120 | 3 | 3 | 87.6 ± 6.46 | 86.1 ± 4.06 | 2.09 ± 0.15 | 2.05 ± 0.09 | 40.3 ± 4.36 | 0.26 ± 0.02 | −11.3 ± 2.74 |
| Parameter | PTX Solution | PTX Micelle | PTX in PTX/SRF Solution | PTX In PTX/SRF Micelle |
|---|---|---|---|---|
| Dose (µg∙kg−1) | 1000 | 1000 | 1000 | 1000 |
| AUC0–8h (µg∙min∙mL−1) | 440 ± 237 | 1020 ± 628 | 531 ± 281 | 1320 ± 712 |
| CLt (mL∙(kg∙min)−1) | 23.8 ± 13.0 | 11.2 ± 6.79 | 19.4 ± 10.3 | 7.90 ± 4.34 |
| Relative bioavailability | - | 232 | - | 249 |
| Parameter | SRF Solution | SRF Micelle | SRF in PTX/SRF Solution | SRF in PTX/SRF Micelle |
|---|---|---|---|---|
| Dose (µg∙kg−1) | 1000 | 1000 | 1000 | 1000 |
| AUC0–8h (µg∙min∙mL−1) | 3960 ± 200 | 4210 ± 2110 | 3720 ± 1880 | 4580 ± 2360 |
| CLt (mL∙(kg∙min)−1) | 2.54 ± 1.28 | 2.38 ± 1.19 | 2.70 ± 1.36 | 2.22 ± 1.14 |
| Relative bioavailability | - | 106 | - | 123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.E.; Yoon, M.S.; Jo, M.J.; Kim, S.Y.; Lee, J.M.; Kang, S.J.; Park, C.-W.; Kim, J.-S.; Shin, D.H. Synergistic Encapsulation of Paclitaxel and Sorafenib by Methoxy Poly(Ethylene Glycol)-b-Poly(Caprolactone) Polymeric Micelles for Ovarian Cancer Therapy. Pharmaceutics 2023, 15, 1206. https://doi.org/10.3390/pharmaceutics15041206
Jin CE, Yoon MS, Jo MJ, Kim SY, Lee JM, Kang SJ, Park C-W, Kim J-S, Shin DH. Synergistic Encapsulation of Paclitaxel and Sorafenib by Methoxy Poly(Ethylene Glycol)-b-Poly(Caprolactone) Polymeric Micelles for Ovarian Cancer Therapy. Pharmaceutics. 2023; 15(4):1206. https://doi.org/10.3390/pharmaceutics15041206
Chicago/Turabian StyleJin, Chae Eun, Moon Sup Yoon, Min Jeong Jo, Seo Yeon Kim, Jae Min Lee, Su Jeong Kang, Chun-Woong Park, Jin-Seok Kim, and Dae Hwan Shin. 2023. "Synergistic Encapsulation of Paclitaxel and Sorafenib by Methoxy Poly(Ethylene Glycol)-b-Poly(Caprolactone) Polymeric Micelles for Ovarian Cancer Therapy" Pharmaceutics 15, no. 4: 1206. https://doi.org/10.3390/pharmaceutics15041206