Amorphization of Ethenzamide and Ethenzamide Cocrystals—A Case Study of Single and Binary Systems Forming Low-Melting Eutectic Phases Loaded on/in Silica Gel
Abstract
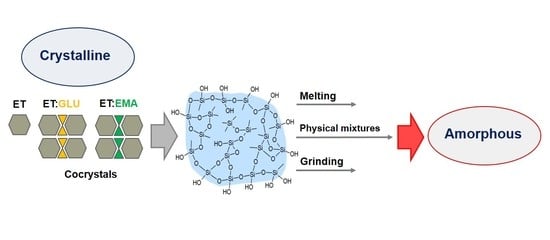
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cocrystals Preparation
2.3. Manual Mixing (MM)
2.4. Thermal Method (TM)
2.5. Ball Milling Method (BM)
2.6. PXRD Measurements
2.7. Solid-State NMR Experiments
2.8. Differential Scanning Calorimetry
2.9. Dissolution
3. Results
3.1. Solid-State NMR, Differential Scanning Calorimetry (DSC), and Powder X-ray Diffraction (PXRD) Studies of Ethenzamide with Silica
3.1.1. Analysis of Physical Mixture
3.1.2. Analysis of ET/SiO2 Systems Melted or Ground in a Ball Mill
3.2. Silica-Particles-Based Amorphization of ET:EMA and ET:GLU Cocrystals
3.3. Dissolution Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation Design for Poorly Water-Soluble Drugs Based on Biopharmaceutics Classification System: Basic Approaches and Practical Applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Shah, S.; Date, A.; Holm, R. Strategies for the Formulation Development of Poorly Soluble Drugs via Oral Route. In Methods and Principles in Medicinal Chemistry; Bachhav, Y., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 49–89. ISBN 978-3-527-34396-6. [Google Scholar]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical Particle Technologies: An Approach to Improve Drug Solubility, Dissolution and Bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble Drug Delivery Strategies: Review of Recent Advances and Business Prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Szaniawska, M. Strategies in Poorly Soluble Drug Delivery Systems. Ann. Univ. Mariae Curie-Sklodowska Sect. AA—Chem. 2019, 73, 81. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Guillarme, D.; Veuthey, J.-L.; Gurny, R. Strategies for Formulating and Delivering Poorly Water-Soluble Drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 342–351. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Varghese Gupta, S. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Molecules 2018, 23, 1719. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt Formation to Improve Drug Solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef]
- Cerreia Vioglio, P.; Chierotti, M.R.; Gobetto, R. Pharmaceutical Aspects of Salt and Cocrystal Forms of APIs and Characterization Challenges. Adv. Drug Deliv. Rev. 2017, 117, 86–110. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Liu, X.; Fahr, A. Drug Delivery Strategies for Poorly Water-Soluble Drugs: The Industrial Perspective. Expert Opin. Drug Deliv. 2011, 8, 1481–1500. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Stephen, S.; Gorain, B.; Choudhury, H.; Chatterjee, B. Exploring the Role of Mesoporous Silica Nanoparticle in the Development of Novel Drug Delivery Systems. Drug Deliv. Transl. Res. 2022, 12, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.S.; Hassaballah, M.Y.; Abdelqawy, M.A.; Emad-Eldin, M.; Farag, A.K.; Negida, A.; Ghaith, H.; Emam, S.E. An Updated Review of Mesoporous Carbon as a Novel Drug Delivery System. Drug Dev. Ind. Pharm. 2021, 47, 1029–1037. [Google Scholar] [CrossRef]
- Seljak, K.B.; Kocbek, P.; Gašperlin, M. Mesoporous Silica Nanoparticles as Delivery Carriers: An Overview of Drug Loading Techniques. J. Drug Deliv. Sci. Technol. 2020, 59, 101906. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous Materials as Elements of Modern Drug Delivery Systems for Anti-Inflammatory Agents: A Review of Recent Achievements. Pharmaceutics 2022, 14, 1542. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical Cocrystals: From Serendipity to Design to Application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Raheem Thayyil, A.; Juturu, T.; Nayak, S.; Kamath, S. Pharmaceutical Co-Crystallization: Regulatory Aspects, Design, Characterization, and Applications. Adv. Pharm. Bull. 2020, 10, 203–212. [Google Scholar] [CrossRef]
- Port, A.; Almansa, C.; Enrech, R.; Bordas, M.; Plata-Salamán, C.R. Differential Solution Behavior of the New API–API Co-Crystal of Tramadol–Celecoxib (CTC) versus Its Constituents and Their Combination. Cryst. Growth Des. 2019, 19, 3172–3182. [Google Scholar] [CrossRef]
- Sekhon, B.S. Drug-Drug Co-Crystals. DARU J. Pharm. Sci. 2012, 20, 45. [Google Scholar] [CrossRef]
- Shaikh, T.R.; George, C.P.; Bhukya, P.; Shelke, N.; Pawar, K.; Garai, A.; Dandela, R.; Gonnade, R.G.; Nangia, A.K. Novel Crystal Forms of Entresto: A Supramolecular Complex of Trisodium Sacubitril/Valsartan Hemi-Pentahydrate. CrystEngComm 2022, 24, 7387–7393. [Google Scholar] [CrossRef]
- Ngilirabanga, J.B.; Samsodien, H. Pharmaceutical Co-crystal: An Alternative Strategy for Enhanced Physicochemical Properties and Drug Synergy. Nano Sel. 2021, 2, 512–526. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical Cocrystals: Walking the Talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nanda, A. Pharmaceutical Cocrystals: An Overview. Indian J. Pharm. Sci. 2017, 79, 858–871. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Tin, Y.-Y.; Soe, M.-T.-P.; Ko, B.; Park, S.; Lee, J. Recent Technologies for Amorphization of Poorly Water-Soluble Drugs. Pharmaceutics 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Tambe, S.; Jain, D.; Meruva, S.K.; Rongala, G.; Juluri, A.; Nihalani, G.; Mamidi, H.K.; Nukala, P.K.; Bolla, P.K. Recent Advances in Amorphous Solid Dispersions: Preformulation, Formulation Strategies, Technological Advancements and Characterization. Pharmaceutics 2022, 14, 2203. [Google Scholar] [CrossRef]
- Van den Mooter, G. The Use of Amorphous Solid Dispersions: A Formulation Strategy to Overcome Poor Solubility and Dissolution Rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Solid Dispersion of Poorly Water-soluble Drugs: Early Promises, Subsequent Problems, and Recent Breakthroughs. J. Pharm. Sci. 1999, 88, 1058–1066. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Co Amorphous Systems: A Product Development Perspective. Int. J. Pharm. 2016, 515, 403–415. [Google Scholar] [CrossRef]
- Han, J.; Wei, Y.; Lu, Y.; Wang, R.; Zhang, J.; Gao, Y.; Qian, S. Co-Amorphous Systems for the Delivery of Poorly Water-Soluble Drugs: Recent Advances and an Update. Expert Opin. Drug Deliv. 2020, 17, 1411–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Grohganz, H.; Löbmann, K.; Rades, T.; Hempel, N.-J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics 2021, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Chieng, N.; Aaltonen, J.; Saville, D.; Rades, T. Physical Characterization and Stability of Amorphous Indomethacin and Ranitidine Hydrochloride Binary Systems Prepared by Mechanical Activation. Eur. J. Pharm. Biopharm. 2009, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, K.; Chotera-Ouda, A.; Bak-Sypien, I.I.; Potrzebowski, M.J. Mesoporous Silica Particles as Drug Delivery Systems—The State of the Art in Loading Methods and the Recent Progress in Analytical Techniques for Monitoring These Processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous Silica Materials: From Physico-Chemical Properties to Enhanced Dissolution of Poorly Water-Soluble Drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Qian, K.K.; Bogner, R.H. Application of Mesoporous Silicon Dioxide and Silicate in Oral Amorphous Drug Delivery Systems. J. Pharm. Sci. 2012, 101, 444–463. [Google Scholar] [CrossRef]
- Trzeciak, K.; Kaźmierski, S.; Wielgus, E.; Potrzebowski, M.J. DiSupLo—New Extremely Easy and Efficient Method for Loading of Active Pharmaceutical Ingredients into the Pores of MCM-41 Mesoporous Silica Particles. Microporous Mesoporous Mater. 2020, 308, 110506. [Google Scholar] [CrossRef]
- Trzeciak, K.; Kaźmierski, S.; Drużbicki, K.; Potrzebowski, M.J. Mapping of Guest Localization in Mesoporous Silica Particles by Solid-State NMR and Ab Initio Modeling: New Insights into Benzoic Acid and p-Fluorobenzoic Acid Embedded in MCM-41 via Ball Milling. J. Phys. Chem. C 2021, 125, 10096–10109. [Google Scholar] [CrossRef]
- Trzeciak, K.; Wielgus, E.; Kaźmierski, S.; Khalaji, M.; Dudek, M.K.; Potrzebowski, M.J. Unexpected Factors Affecting the Kinetics of Guest Molecule Release from Investigation of Binary Chemical Systems Trapped in a Single Void of Mesoporous Silica Particles. ChemPhysChem 2023, 24, e202200884. [Google Scholar] [CrossRef]
- Skorupska, E.; Jeziorna, A.; Paluch, P.; Potrzebowski, M.J. Ibuprofen in Mesopores of Mobil Crystalline Material 41 (MCM-41): A Deeper Understanding. Mol. Pharm. 2014, 11, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, E.; Kaźmierski, S.; Potrzebowski, M.J. Solid State NMR Characterization of Ibuprofen:Nicotinamide Cocrystals and New Idea for Controlling Release of Drugs Embedded into Mesoporous Silica Particles. Mol. Pharm. 2017, 14, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Lin, W.; Scholes, P.; Li, M. Investigating the Effects of Loading Factors on the In Vitro Pharmaceutical Performance of Mesoporous Materials as Drug Carriers for Ibuprofen. Materials 2017, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Cha, K.-H.; Hong, S.H.; Abuzar, S.M.; Lee, S.; Ha, E.-S.; Kim, J.-S.; Baek, I.-H.; Kim, M.-S.; Hwang, S.-J. Pharmaceutical Characterization and In Vivo Evaluation of Orlistat Formulations Prepared by the Supercritical Melt-Adsorption Method Using Carbon Dioxide: Effects of Mesoporous Silica Type. Pharmaceutics 2020, 12, 333. [Google Scholar] [CrossRef]
- Azad, M.; Moreno, J.; Davé, R. Stable and Fast-Dissolving Amorphous Drug Composites Preparation via Impregnation of Neusilin® UFL2. J. Pharm. Sci. 2018, 107, 170–182. [Google Scholar] [CrossRef]
- Kovačević, M.; German Ilić, I.; Bolko Seljak, K.; Zvonar Pobirk, A. High-Shear Wet Granulation of SMEDDS Based on Mesoporous Carriers for Improved Carvedilol Solubility. Pharmaceutics 2022, 14, 2077. [Google Scholar] [CrossRef]
- Omachi, Y. Gastroretentive Sustained-Release Tablets Combined with a Solid Self-Micro-Emulsifying Drug Delivery System Adsorbed onto Fujicalin®. AAPS PharmSciTech 2022, 23, 157. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Alotaibi, H.A.; Almehmady, A.M.; Safo, M.K.; El-Say, K.M. Influences of Glimepiride Self-Nanoemulsifying Drug Delivery System Loaded Liquisolid Tablets on the Hypoglycemic Activity and Pancreatic Histopathological Changes in Streptozotocin-Induced Hyperglycemic Rats. Nanomaterials 2022, 12, 3966. [Google Scholar] [CrossRef]
- Skorupska, E.; Jeziorna, A.; Potrzebowski, M.J. Thermal Solvent-Free Method of Loading of Pharmaceutical Cocrystals into the Pores of Silica Particles: A Case of Naproxen/Picolinamide Cocrystal. J. Phys. Chem. C 2016, 120, 13169–13180. [Google Scholar] [CrossRef]
- Galindres, D.M.; Cifuentes, D.; Tinoco, L.E.; Murillo-Acevedo, Y.; Rodrigo, M.M.; Ribeiro, A.C.F.; Esteso, M.A. A Review of the Application of Resorcinarenes and SBA-15 in Drug Delivery. Processes 2022, 10, 684. [Google Scholar] [CrossRef]
- Fathi Vavsari, V.; Mohammadi Ziarani, G.; Badiei, A. The Role of SBA-15 in Drug Delivery. RSC Adv. 2015, 5, 91686–91707. [Google Scholar] [CrossRef]
- Qian, K.K.; Bogner, R.H. Spontaneous Crystalline-to-Amorphous Phase Transformation of Organic or Medicinal Compounds in the Presence of Porous Media, Part 1: Thermodynamics of Spontaneous Amorphization. J. Pharm. Sci. 2011, 100, 2801–2815. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.K.; Suib, S.L.; Bogner, R.H. Spontaneous Crystalline-to-amorphous Phase Transformation of Organic or Medicinal Compounds in the Presence of Porous Media, Part 2: Amorphization Capacity and Mechanisms of Interaction. J. Pharm. Sci. 2011, 100, 4674–4686. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.K.; Wurster, D.E.; Bogner, R.H. Spontaneous Crystalline-to-Amorphous Phase Transformation of Organic or Medicinal Compounds in the Presence of Porous Media, Part 3: Effect of Moisture. Pharm. Res. 2012, 29, 2698–2709. [Google Scholar] [CrossRef]
- Lin, J.H.; Sugiyama, Y.; Awazu, S.; Hanano, M. Physiological Pharmacokinetics of Ethoxybenzamide Based on Biochemical Data Obtainedin Vitro as Well as on Physiological Data. J. Pharmacokinet. Biopharm. 1982, 10, 649–661. [Google Scholar] [CrossRef]
- Kozak, A.; Pindelska, E. Spectroscopic Analysis of the Influence of Various External Factors on Ethenzamide-Glutaric Acid (1:1) Cocrystal Formation. Eur. J. Pharm. Sci. 2019, 133, 59–68. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Conformational and Enantiotropic Polymorphism of a 1:1 Cocrystal Involving Ethenzamide and Ethylmalonic Acid. CrystEngComm 2010, 12, 3691. [Google Scholar] [CrossRef]
- Chu, P.J.; Potrzebowski, M.J.; Scott, A.I.; Gao, Y. Conformational Studies of N-Benzoyl-L-Phenylalanine by Combined Rotation and Multiple-Pulse Spectroscopy Proton Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1990, 112, 881–883. [Google Scholar] [CrossRef]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef]
- Hronský, V. Measurement of Sample Temperatures and Temperature Gradients in Magic-Angle Spinning Nmr. Acta Electrotech. Inform. 2013, 13, 95–98. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 16 February 2023).
- Gonçalves, M.C. Sol-Gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef] [PubMed]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzeciak, K.; Wielgus, E.; Kaźmierski, S.; Pawlak, T.; Potrzebowski, M.J. Amorphization of Ethenzamide and Ethenzamide Cocrystals—A Case Study of Single and Binary Systems Forming Low-Melting Eutectic Phases Loaded on/in Silica Gel. Pharmaceutics 2023, 15, 1234. https://doi.org/10.3390/pharmaceutics15041234
Trzeciak K, Wielgus E, Kaźmierski S, Pawlak T, Potrzebowski MJ. Amorphization of Ethenzamide and Ethenzamide Cocrystals—A Case Study of Single and Binary Systems Forming Low-Melting Eutectic Phases Loaded on/in Silica Gel. Pharmaceutics. 2023; 15(4):1234. https://doi.org/10.3390/pharmaceutics15041234
Chicago/Turabian StyleTrzeciak, Katarzyna, Ewelina Wielgus, Sławomir Kaźmierski, Tomasz Pawlak, and Marek J. Potrzebowski. 2023. "Amorphization of Ethenzamide and Ethenzamide Cocrystals—A Case Study of Single and Binary Systems Forming Low-Melting Eutectic Phases Loaded on/in Silica Gel" Pharmaceutics 15, no. 4: 1234. https://doi.org/10.3390/pharmaceutics15041234
APA StyleTrzeciak, K., Wielgus, E., Kaźmierski, S., Pawlak, T., & Potrzebowski, M. J. (2023). Amorphization of Ethenzamide and Ethenzamide Cocrystals—A Case Study of Single and Binary Systems Forming Low-Melting Eutectic Phases Loaded on/in Silica Gel. Pharmaceutics, 15(4), 1234. https://doi.org/10.3390/pharmaceutics15041234