Advances in Targeted Therapy of Breast Cancer with Antibody-Drug Conjugate
Abstract
1. Introduction
2. Internalizing and Non-Internalizing ADCs
3. Development of ADC Drugs for Targeted Breast Cancer Therapy
3.1. Human Epidermal Growth Factor Receptor-Targeted ADC Therapy for Breast Cancer
3.1.1. HER2-Targeted ADCs
3.1.2. Trastuzumab Emtansine
3.1.3. Trastuzumab Deruxtecan
3.1.4. Trastuzumab Duocarmazine
3.1.5. Disitamab Vedotin
3.1.6. Sachituzumab Govitecan in HR+ and HER2-Negative Breast Cancer Therapy
3.1.7. Other ADCs Targeting Human Epidermal Growth Factor Receptor
3.2. Triple-Negative Breast Cancer-Targeted ADC Therapy
3.2.1. Sacituzumab Govitecan in TNBC Therapy
3.2.2. TNBC Therapy with Ladrituzumab Vedotin
4. ADCs Emerging as Promising Therapeutics for Breast Cancer Patients in Clinical Settings
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fang, T.; Yun, C.; Liu, X.; Cai, X. Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers. Front. Mol. Biosci. 2022, 9, 847835. [Google Scholar] [CrossRef] [PubMed]
- Kunte, S.; Abraham, J.; Montero, A.J. Novel HER2–targeted therapies for HER2–positive metastatic breast cancer. Cancer 2020, 126, 4278–4288. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody–drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef]
- Theocharopoulos, C.; Lialios, P.-P.; Samarkos, M.; Gogas, H.; Ziogas, D.C. Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines 2021, 9, 1111. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Tolaney, S.M. Clinical Development of New Antibody–Drug Conjugates in Breast Cancer: To Infinity and Beyond. BioDrugs 2021, 35, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ashman, N.; Bargh, J.D.; Spring, D.R. Non-internalizing antibody-drug conjugates. Chem. Soc. Rev. 2022, 51, 9182–9202. [Google Scholar] [CrossRef]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting cancer with antibody-drug conjugates: Promises and challenges. MABS 2021, 13, e1951427. [Google Scholar] [CrossRef]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody–drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Mckertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Joubert, N.; Beck, A.; Dumontet, C.; Denevault-Sabourin, C. Antibody–Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of antibody–drug conjugates(ADCs) for cancer therapy. Cancer Cell Int. 2022, 22, 255. [Google Scholar] [CrossRef]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- FDA Approves Sacituzumab Govitecan-Hziy for HR-Positive Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sacituzumab-govitecan-hziy-hr-positive-breast-cancer (accessed on 10 April 2023).
- Wen, Y.; Ouyang, D.; Zou, Q.; Chen, Q.; Luo, N.; He, H.; Anwar, M.; Yi, W. A literature review of the promising future of TROP2: A potential drug therapy target. Ann. Transl. Med. 2022, 10, 1403. [Google Scholar] [CrossRef]
- Available online: https://www.onclive.com/view/fda-accepts-bla-for-trastuzumab-duocarmazine-for-advanced-her2-breast-cancer (accessed on 30 January 2023).
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibodyedrug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Senter, P.D. Potent antibody drug conjugates for cancer therapy. Curr. Opin. Chem. Biol. 2009, 13, 235–244. [Google Scholar] [CrossRef]
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibodyedrug conjugates: Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef]
- Vetter, J. Toxins of amanita phalloides. Toxicon 1998, 36, 13–24. [Google Scholar] [CrossRef]
- Patterson, A.W.; Peltier, H.M.; Sasse, F.; Ellman, J.A. Design, synthesis, and biological properties of highly potent tubulysin D analogues. Chemistry 2007, 13, 9534–9541. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Vugts, D.J.; Visser, G.W.; Stigter-van Walsum, M.; Bolijn, M.; Spiga, M.; Lazzari, P.; Shankar, S.; Sani, M.; Zanda, M.; et al. Development of novel ADCs: Conjugation of tubulysin analogues to trastuzumab monitored by dual radiolabeling. Cancer Res. 2014, 74, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Geroni, C.; Fantin, M.; Battaglia, R.; Rosato, A.; Speed, W.; Zanovello, P.; Floreani, M. Formation and antitumor activity of PNU-159682, a major metabolite of nemorubicin in human liver microsomes. Clin. Cancer Res. 2005, 11, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, S.; Scaglioni, L.; Mondelli, R.; Caruso, M.; Sirtori, F.R. The interaction of nemorubicin metabolite PNU-159682 with DNA fragments d (CGTACG) 2, d (CGATCG) 2 and d (CGCGCG) 2 shows a strong but reversible binding to G:C base pairs. Bioorg. Med. Chem. 2012, 20, 6979–6988. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.V.; Audette, C.A.; Mayo, M.F.; Jones, G.E.; Doherty, H.; Maloney, E.K.; Sun, X.; Wilhelm, S.; Ab, O.; Laiet, K.C.; et al. Antibodyemaytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010, 70, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.P.; Bovee, T.D.; Doronina, S.O.; Burke, P.J.; Hunter, J.H.; Neff-LaFord, H.D.; Jonas, M.; Anderson, M.E.; Setter, J.R.; Senter, P.D.; et al. Reducing hydrophobicity of homogeneous antibody drug conjugates improves pharmacokinetics and therapeutic index. Nat. Biotechnol. 2015, 33, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Widdison, W.C.; Ponte, J.F.; Coccia, J.A.; Lanieri, L.; Setiady, Y.; Dong, L.; Skaletskaya, A.; Hong, E.E.; Wu, R.; Qiu, Q.; et al. Development of anilinoemaytansinoid ADCs that efficiently release cytotoxic metabolites in cancer cells and induce high levels of bystander killing. Bioconjug. Chem. 2015, 26, 2261–2278. [Google Scholar] [CrossRef]
- Sheng, W.; Shang, Y.; Li, L.; Zhen, Y. An EGFR/CD13 bispecific fusion protein and its enediyne-energized analog show potent antitumor activity. Anti Cancer Drugs 2014, 25, 82–91. [Google Scholar] [CrossRef]
- Thornlow, D.N.; Cox, E.C.; Walker, J.A.; Sorkin, M.; Plesset, J.B.; DeLisa, M.P.; Alabi, C.A. Dual site-specific antibody conjugates for sequential and orthogonal cargo release. Bioconjug. Chem. 2019, 30, 1702–1710. [Google Scholar] [CrossRef]
- Walker, J.A.; Bohn, J.J.; Ledesma, F.; Sorkin, M.R.; Kabaria, S.R.; Thornlow, D.N.; Alabi, C.A. Substrate design enables heterobifunctional, dual “click” antibody modification via microbial transglutaminase. Bioconjug. Chem. 2019, 30, 2452–2457. [Google Scholar] [CrossRef]
- Maruani, A.; Smith, M.E.; Miranda, E.; Chester, K.A.; Chudasama, V.; Caddick, S. A plug-and-play approach to antibody-based therapeutics via a chemoselective dual click strategy. Nat. Commun. 2015, 6, 6645. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Li, W.; Jeanty, C.; Xiang, G.; Dong, Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm. Sin. B 2020, 10, 1589–1600. [Google Scholar] [CrossRef]
- Baah, S.; Laws, M.; Rahman, K.M. Antibody-Drug Conjugates—A Tutorial Review. Molecules 2021, 26, 2943. [Google Scholar] [CrossRef]
- Deonarain, M.P.; Yahioglu, G.; Stamati, I.; Pomowski, A.; Clarke, J.; Edwards, B.M.; Diez-Posada, S.; Stewart, A.C. Small-Format Drug Conjugates: A Viable Alternative to ADCs for Solid Tumours? Antibodies 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, J.; Liao, D.; Zhang, D.; Li, X.; Jia, Y.; Kong, F. Advances in Antibody-Drug Conjugates in the Treatment of HER2-Positive Breast Cancer Breast Cancer: Targets and Therapy. Breast Cancer 2022, 14, 417–432. [Google Scholar] [PubMed]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. J. Breast Cancer Res. 2014, 6, 130–146. [Google Scholar] [CrossRef]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S.; et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol. Cancer Ther. 2015, 14, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Maomao, C.; He, L.; Dianqin, S.; Siyi, H.; Yan, X.; Yang, F.; Shaoli, Z.; Xia, C.; Lin, L.; Ji, P.; et al. Current cancer burden in China: Epidemiology, etiology, and prevention. Cancer Biol. Med. 2022, 19, 1121–1138. [Google Scholar]
- Koster, K.L.; Huober, J.; Joerger, M. New antibody-drug conjugates (ADCs) in breast cancer—An overview of ADCs recently approved and in later stages of development. Explor. Target. Anti-Tumor Ther. 2022, 3, 27–36. [Google Scholar] [CrossRef]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.H.; Conte, P.; Patre, M.; Stanzel, S.; Strasak, A.; Burris, H.A.; et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: Primary results from the phase III MARIANNE study. J. Clin. Oncol. 2017, 35, 141–148. [Google Scholar] [CrossRef]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M.F. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortes, J.; Kim, S.B.; Im, S.A.; Hegg, R.; Im, Y.H.; Roman, I.; Pedrini, J.L.; Pienkowski, T.; Swain, S.M.; et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Monturus, E.; Clark, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, F.; Yang, Y.; Zhao, L.; Zhou, H.; Dong, J.; Huang, H. Real-Time Analysis on DrugAntibody Ratio of Antibody-Drug Conjugates for Synthesis, Process Optimization, and Quality Control. Sci. Rep. 2017, 7, 7763. [Google Scholar] [CrossRef]
- Cardillo, T.M.; Govindan, S.V.; Sharkey, R.M.; Trisal, P.; Goldenberg, D.M. Humanized anti- Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: Preclinical studies in human cancer xenograft models and monkeys. Clin. Cancer Res. 2011, 17, 3157–3169. [Google Scholar] [CrossRef]
- Aggarwal, N.; Sloane, B.F. Cathepsin B: Multiple roles in cancer. Proteom. Clin. Appl. 2014, 8, 427–437. [Google Scholar] [CrossRef]
- Ruan, J.; Zheng, H.; Fu, W.; Zhao, P.; Su, N.; Luo, R. Increased expression of cathepsin L: A novel independent prognostic marker of worse outcome in hepatocellular carcinoma patients. PLoS ONE 2014, 9, e112136. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The latest research and development into the antibody–drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, a novel HER2- targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growthfactor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Updated results from DESTINY-breast01, a phase 2 trial of trastuzumab deruxtecan (T-DXd ) in HER2 positive metastatic breast cancer. Cancer Res. 2021, 81, PD3–06. [Google Scholar] [CrossRef]
- T-DXd Yields Superior Outcomes Over Chemotherapy-Based Regimens in Patients Previously Treated With T-DM1: DESTINY-Breast02. Available online: https://ascopost.com/news/december-2022/t-dxd-yields-superior-outcomes-over-chemotherapy-based-regimens-in-patients-previously-treated-with-t-dm1-destiny-breast02/ (accessed on 10 April 2023).
- T-DXd Yields Longer Overall Survival than T-DM1 in Patients with HER2-Positive Metastatic Breast Cancer. Available online: https://www.aacr.org/about-the-aacr/newsroom/news-releases/t-dxd-yields-longer-overall-survival-than-t-dm1-in-patients-with-her2-positive-metastatic-breast-cancer/ (accessed on 9 April 2023).
- Hackshaw, M.D.; Danysh, H.E.; Singh, J.; Ritchey, M.E.; Ladner, A.; Taitt, C.; Camidge, D.R.; Iwata, H.; Powell, C.A. Incidence of pneumonitis/ interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2020, 183, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Highlights of Prescribing Information (ENHERTU®). Available online: https://www.accessdata.fda.gov/drugsatfdadocs/label/2019/761139s000lbl.pdf (accessed on 11 January 2021).
- Rozhin, J.; Sameni, M.; Ziegler, G.; Sloane, B.F. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res 1994, 54, 6517–6525. [Google Scholar]
- Eaton, J.S.; Miller, P.E.; Mannis, M.J.; Murphy, C.J. Ocular adverse events associated with antibody–drug conjugates in human clinical trials. J. Ocul. Pharmacol. Ther. 2015, 31, 589–604. [Google Scholar] [CrossRef]
- van der Lee, M.M.; Groothuis, P.G.; Ubink, R.; van der Vleuten, M.A.J.; van Achterberg, T.A.; Loosveld, E.M.; Damming, D.; Jacobs, D.C.H.; Rouwette, M.; Goedings, P.; et al. The preclinical profile of the Duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol. Cancer Ther. 2015, 14, 692–703. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Manich, C.S.; O’Shaughnessy, J.; Aftimos, P.G.; van den Tweel, E.; Oesterholt, M.; Escrivá-de-Romaní, S.; Turner, N. LBA15—Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021, 32, S1283–S1346. [Google Scholar]
- Yao, X.; Jiang, J.; Wang, X.; Huang, C.; Li, D.; Xie, K.; Xu, Q.; Li, H.; Li, Z.; Lou, L.; et al. A novel humanized anti-HER2 antibody conjugated with MMAE exerts potent anti-tumor activity. Breast Cancer Res. Treat. 2015, 153, 123–133. [Google Scholar] [CrossRef]
- Xu, B.; Wang, J.; Fang, J.; Chen, X.; Han, Y.; Li, Q.; Zhang, P.; Yuan, P.; Ma, F.; Luo, Y.; et al. Abstract PD4-06: Early clinical development of RC48-ADC in patients with HER2 positive metastatic breast cancer. Cancer Res. 2020, 80, PD4-06. [Google Scholar] [CrossRef]
- More Information about TROPiCS-02. Available online: https://clinicaltrials.gov/ct2/show/NCT03901339 (accessed on 10 April 2023).
- Gala, K.; Chandarlapaty, S. Molecular pathways: HER3 targeted therapy. Clin. Cancer Res. 2014, 20, 1410–1416. [Google Scholar] [CrossRef]
- Mota, J.M.; Collier, K.A.; Costa, R.L.B.; Taxter, T.; Kalyan, A.; Leite, C.A.; Chae, Y.K.; Giles, F.J.; Carneiro, B.S. A comprehensive review of heregulins, HER3, and HER4 as potential therapeutic targets in cancer. Oncotarget 2017, 8, 89284–89306. [Google Scholar] [CrossRef]
- Yonemori, K.; Masuda, N.; Takahashi, S.; Kogawa, T.; Nakayama, T.; Yamamoto, Y.; Takahashi, M.; Toyama, T.; Saeki, T.; Iwata, H. Single agent activity of U3–1402, a HER3-targeting antibody–drug conjugate, in HER3-overexpressing metastatic breast cancer: Updated results from a phase I/II trial [abstract]. Ann. Oncol. 2019, 30, III48. [Google Scholar] [CrossRef]
- Masuda, N.; Yonemori, K.; Takahashi, S.; Kogawa, T.; Nakayama, T.; Iwase, H.; Takahashi, M.; Toyama, T.; Saeki, T.; Saji, S.; et al. Abstract PD1-03: Single agent activity of U3-1402, a HER3-targeting antibody–drug conjugate, in HER3-overexpressing metastatic breast cancer: Updated results of a phase 1/2 trial [abstract]. Cancer Res. 2019, 79, PD1-03. [Google Scholar] [CrossRef]
- Krop, I.; Yonemori, K.; Takahashi, S.; Inoue, K.; Nakayama, T.; Iwata, H.; Toyama, T.; Yamamoto, Y.; Takahashi, M.; Osaki, A.; et al. Abstract PD1-09: Safety and efficacy results from the phase 1/2 study of U3-1402, a human epidermal growth factor receptor 3 (HER3)-directed antibody drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC). Cancer Res. 2021, 81, PD1-09. Available online: https://www.abstractsonline.com/pp8/#!/9223/presentation/717 (accessed on 11 January 2021). [CrossRef]
- Barok, M.; Le Joncour, V.; Martins, A.; Isola, J.; Salmikangas, M.; Laakkonen, P.; Joensuu, H. ARX788, a Novel Anti-HER2 Antibody-Drug Conjugate, Shows Anti-tumor Effects in Preclinical Models of Trastuzumab Emtansine-Resistant HER2-Positive Breast Cancer and Gastric Cancer. Cancer Lett. 2020, 473, 156–163. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lian, W.; Zhao, X.; Qi, W.; Xu, J.; Xiao, L.; Qing, Y.; Xue, T.; Wang, J. A First In-Human Study of A166 in Patients with Locally Advanced/metastatic Solid Tumors Which Are HER2-Positive or HER2-Amplified Who Did Not Respond or Stopped Responding to Approved Therapies. J. Clin. Oncol. 2020, 38, 1049. [Google Scholar] [CrossRef]
- Liu, L.F.; Desai, S.D.; Li, T.-K.; Mao, Y.; Sun, M.; Sim, S.P. Mechanism of action of camptothecin. Ann. N. Y. Acad. Sci. 2000, 922, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Xu, Z.; Li, L.; Liu, W.; Dai, Z.; Zhao, Z.; Xiao, L.; Li, H.; Hu, C. Preclinical Evaluation of MRG002, a Novel HER2-Targeting Antibody-Drug Conjugate with Potent Antitumor Activity against HER2-Positive Solid Tumors. Antib. Ther. 2021, 4, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, G.; Gampenrieder, S.; Greil, R. HER2 Directed Antibody- Drug-Conjugates beyond T-DM1 in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 1115. [Google Scholar] [CrossRef]
- Yeon, H.P.; Hee, K.A.; Ji-Yeon, K.; Jin, S.A.; Young-Hyuck, I.; Seol-Hee, K.; Lee, S.; Park, S.J.; Chung, H.-S. First-in-human Phase I Study of ALT-P7, a HER2-Targeting Antibody- Drug Conjugate in Patients with HER2-Positive Advanced Breast Cancer. J. Clin. Oncol. 2020, 38, 3551. [Google Scholar]
- Klempner, S.J.; Beeram, M.; Sabanathan, D.; Chan, A.; Hamilton, E.; Loi, S.; Oh, D.; Emens, L.A.; Patnaik, A.; Kim, J.E.; et al. Interim results of a phase I/Ib study of SBT6050 monotherapy and pembrolizumab combination in patients with advanced HER2-expressing or amplified solid tumors. Ann. Oncol. 2021, 32, S447–S456. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- Mathijssen, R.H.; Van Alphen, R.J.; Verweij, J.; Loos, W.J.; Nooter, K.; Stoter, G.; Sparreboom, A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin. Cancer Res. 2001, 7, 2182–2194. [Google Scholar]
- Sharkey, R.M.; McBride, W.J.; Cardillo, T.M.; Govindan, S.V.; Wang, Y.; Rossi, E.A.; Chang, C.-H.; Goldenberg, D.M. Enhanced delivery of SN-38 to human tumor xenografts with an anti-trop-2-SN-38 antibody conjugate (sacituzumab govitecan). Clin. Cancer Res. 2015, 21, 5131–5138. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy and safety of anti-trop-2 antibody drug conjugate Sacituzumab Govitecan (IMMU-132) in heavily pretreated patients with metastatic triplenegative breast cancer. J. Clin. Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecanhziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Bardia, A.; Tolaney, S.; Loirat, D.; Punie, K.; Oliveira, M.; Rugo, H.; Brufsky, A.; Kalinsky, K.; Cortés, J.; O’Shaughnessy, J.; et al. LBA17 ASCENT: A randomized phase III study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with previously treated metastatic triple-negative breast cancer (mTNBC). Ann. Oncol. 2020, 31, S1142–S1215. [Google Scholar] [CrossRef]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Weaver, R.; Tolaney, S.M.; Bardia, A.; Punie, K.; Brufsky, A.; Rugo, H.S.; Kalinsky, K.; Traina, T.; Klein, L.; et al. Abstract PD13-07: Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res. 2021, 81, PD13-07. [Google Scholar] [CrossRef]
- Sher, A.F.; Bruce, J.Y.; Oh, S.Y.; Anderson, I.C.; Oh, D.-Y.; Nott, L.M.; Lee, J.-S.; Lin, C.-C.; Mehra, R.; Shim, B.Y.; et al. Open-label, phase II study of ladiratuzumab vedotin (LV) for advanced gastric and gastroesophageal junction adenocarcinoma (SGNLVA-005, Trial-in-Progress). J. Clin. Oncol. 2021, 39, TPS256. [Google Scholar] [CrossRef]
- Sussman, D.; Smith, L.M.; Anderson, M.E.; Duniho, S.; Hunter, J.H.; Kostner, H.; Miyamoto, J.B.; Nesterova, A.; Westendorf, L.; Van Epps, H.A.; et al. SGN-LIV1A: A novel antibody–drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol. Cancer Ther. 2014, 13, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Sussman, D.; Smith, L.M.; Anderson, M.E.; Duniho, S.; Hunter, J.H.; Kostner, H.; Miyamoto, J.B.; Nesterova, A.; Westendorf, L.; Van Epps, H.A.; et al. Abstract 3962: SGN-LIV1A: A development stage antibody drug-conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Cancer Res 2013, 73, 3962. Available online: https://www.seagen.com/science/pipeline/ladiratuzumab-vedotin (accessed on 10 April 2023). [CrossRef]
- Modi, S.; Pusztai, L.; Forero, A.; Mita, M.; Miller, K.; Weise, A.; Krop, I.; Burris, H.; Kalinsky, K.; Tsai, M.; et al. Abstract PD3-14: Phase 1 study of the antibody-drug conjugate SGN-LIV1A in patients with heavily pretreated triple-negative metastatic breast cancer. Cancer Res 2018, 78, PD3-14. [Google Scholar] [CrossRef]
- Tsai, M.; Han, H.; Montero, A.; Tkaczuk, K.; Assad, H.; Pusztai, L.; Hurvitz, S.; Wilks, S.; Specht, J.; Nanda, R.; et al. 259P weekly ladiratuzumab vedotin monotherapy for metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, S474–S475. [Google Scholar] [CrossRef]
- Beckwith, H.; Schwab, R.; Yau, C.; Stringer-Reasor, E.; Wei, S.; Chien, A.J.; Albain, K.S.; Kalinsky, K.; Wallace, A.; Elias, A.; et al. Abstract PD1-10: Evaluation of SGN-LIV1a followed by AC in high-risk HER2 negative stage II/III breast cancer: Results from the I-SPY 2 TRIAL. Cancer Res. 2021, 81, PD1-10. Available online: https://www.abstractsonline.com/pp8/#!/9223/presentation/718 (accessed on 11 January 2021). [CrossRef]
- Ozaki, Y.; Mukohara, T.; Tsurutani, J.; Takahashi, M.; Matsumoto, K.; Futamura, M.; Masuda, N.; Kitano, S.; Yoshimura, K.; Minami, H.; et al. Abstract PD1-03: A multicenter phase II study evaluating the efficacy of nivolumab plus paclitaxel plus bevacizumab triple-combination therapy as a first-line treatment in patients with HER2-negative metastatic breast cancer: WJOG9917B NEWBEAT trial. Cancer Res. 2020, 80, PD1-03. [Google Scholar] [CrossRef]
- Najjar, M.K.; Manore, S.G.; Regua, A.T.; Lo, H.W. Antibody-Drug Conjugates for the Treatment of HER2-Positive Breast Cancer. Genes 2022, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, Y. Current Biological, Pathological and Clinical Landscape of HER2-Low Breast Cancer. Cancers 2023, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Lebeau, A.; Schildhaus, H.U.; Jackisch, C.; Rüschoff, J. New treatment options for metastatic HER2-low breast cancer. Die Pathol. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Escrivá-de-Romaní, S.; Saura, C. The change of paradigm in the treatment of HER2-positive breast cancer with the development of new generation antibody-drug conjugates. Cancer Drug Resist. 2023, 6, 45–58. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Y.; Zhou, X.; Yu, H.; Tan, Y.; Du, Y.; Zhang, Q.; Wu, Y. Current landscape of personalized clinical treatments for triple-negative breast cancer. Front. Pharmacol. 2022, 13, 977660. [Google Scholar] [CrossRef]
- Fuentes-Antrás, J.; Genta, S.; Vijenthira, A.; Siu, L.L. Antibody–drug conjugates: In search of partners of choice. Trends Cancer 2023, 9, 339–354. [Google Scholar] [CrossRef]
- Arnold, C.; Webster, P. 11 clinical trials that will shape medicine in 2022. Nat. Med. 2022, 28, 2444–2448. [Google Scholar] [CrossRef]
- Corti, C.; Giugliano, F.; Nicolò, E.; Ascione, L.; Curigliano, G. Antibody–Drug Conjugates for the Treatment of Breast Cancer. Cancers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Nakada, T. Discovery research and translation science of trastuzumab deruxtecan, from non-clinical study to clinical trial. Transl. Regul. Sci. 2021, 3, 65–71. [Google Scholar] [CrossRef]
- Makawita, S.; Meric-Bernstam, F. Antibody-Drug Conjugates: Patient and Treatment Selection. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 105–114. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef] [PubMed]
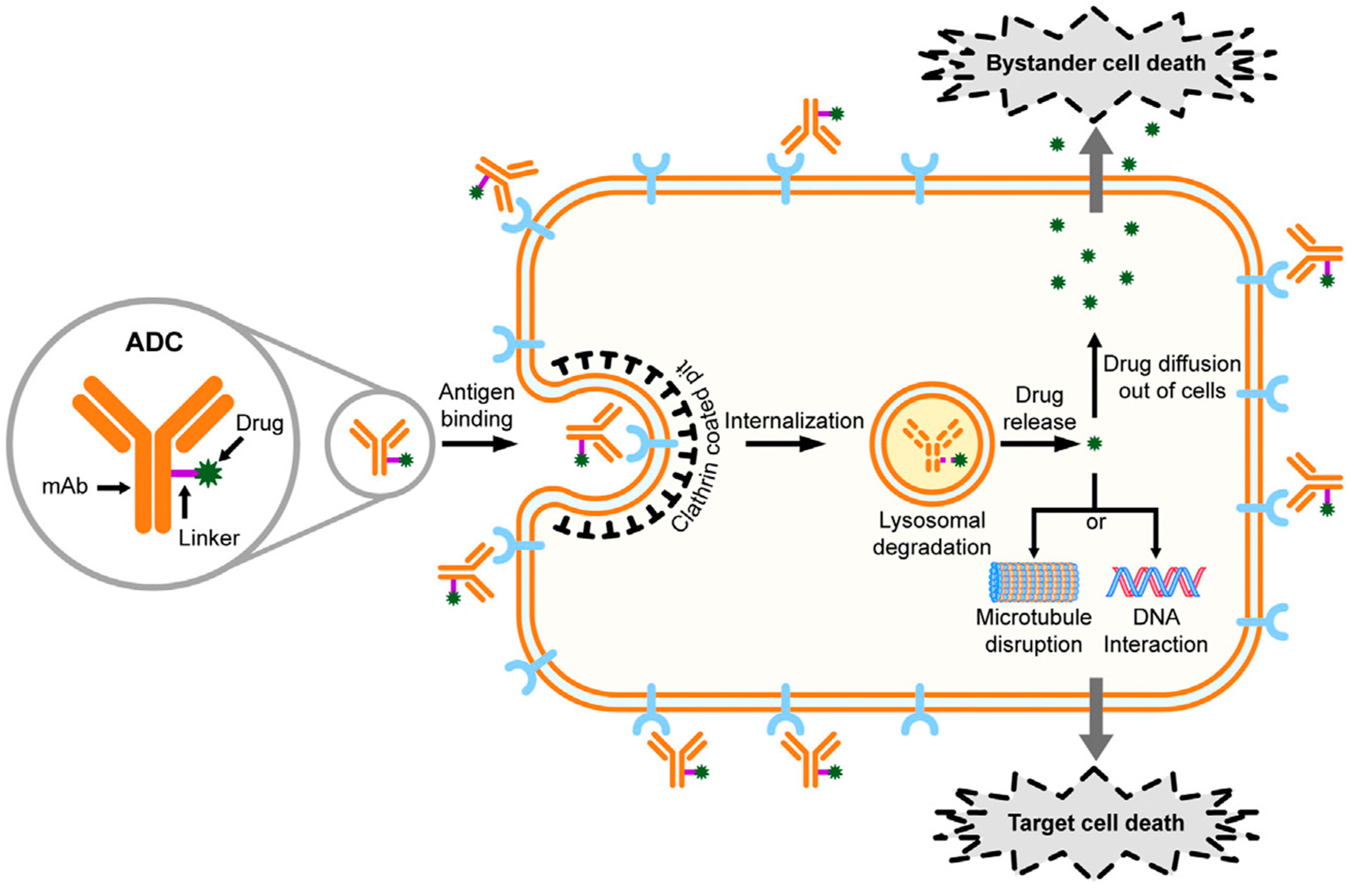

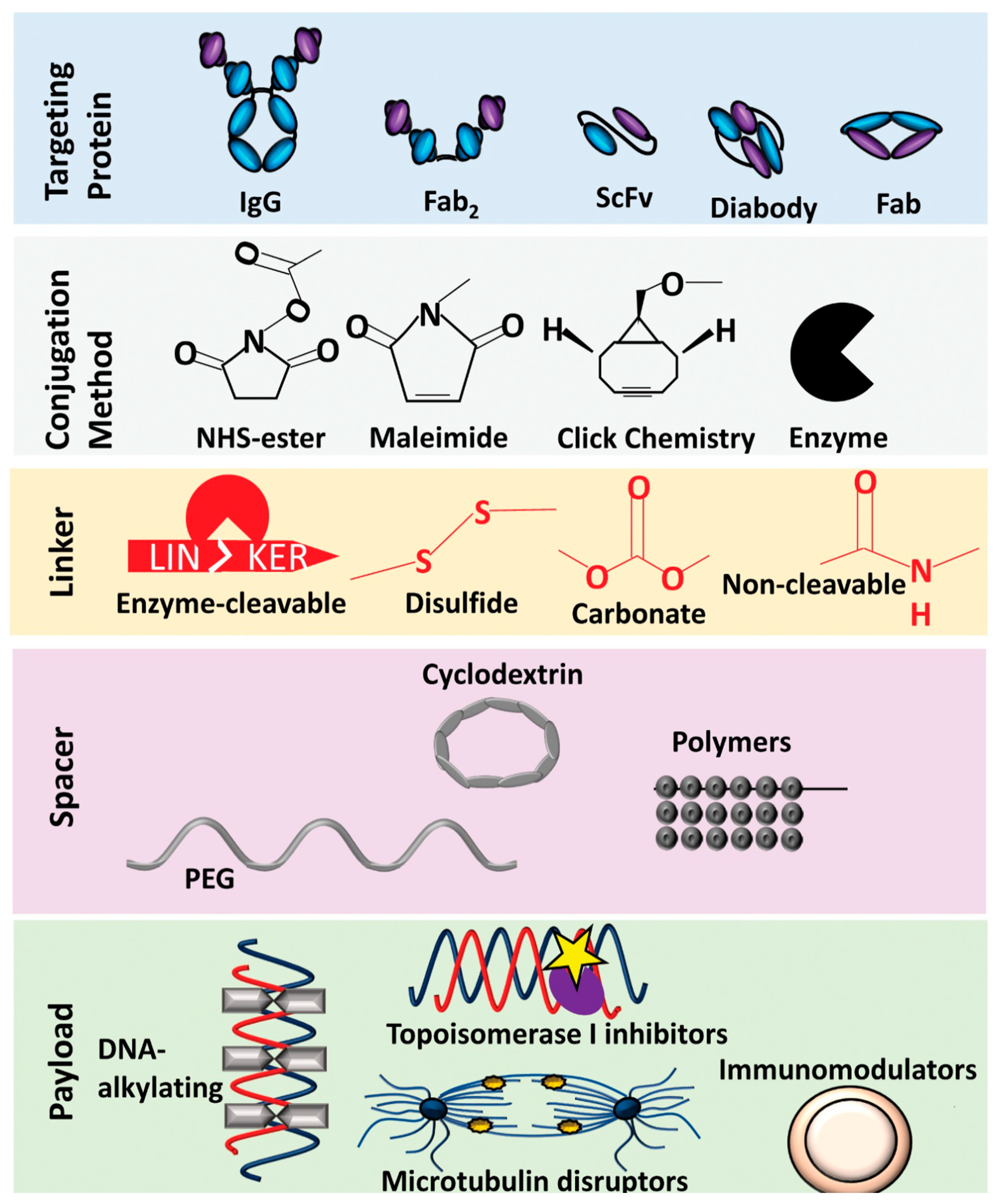


| Type | Categories of ADC Target Antigens or Other Targets | ADC Target Antigens or Other Targets |
|---|---|---|
| 1 | Well-established ADC target antigens | HER2, EGFR, EGFRvIII, c-MET, EGFR2, EGFR3 |
| 2 | ADC target antigens overexpressed on cancer cells | EpCAM, BCMA, TROP-2, LIV-1, AXL, HER3, CD166, CEACAM5, GPNMB, Mesothelin, CD70 |
| 3 | Non-internalizing ADC target cell-surface antigens | CD20, CD21, CD72, TAG72, CEACAM5, and NKA27 |
| 4 | ADC target antigens in tumor microenvironment | CD25/IL2R, B7-H3, ANTXR1 |
| 5 | ADC target antigens in cancer stem cells (CSCs) | PTK7, ROR1, 5T4 |
| 6 | Targeting secreted proteins | Gal3BP, LRG1, and MMP9. |
| 7 | Targeting abundant stromal and vasculature components containing collagen, fibrin, fibronectin, and tenascin-C | Collagen, fibrin, fibronectin, and tenascin-C |
| Internalizing ADCs | Non-Internalizing ADCs |
|---|---|
| Restricted choice of antigen targets | Broader choice of antigen targets |
| Necessitates overexpression of internalizing antigens | Circumvents inefficient internalization and trafficking to lysosomes |
| Cancer cells may acquire resistance related to internalization | Improved bystander effect and tumor penetration |
| May have limited cancer target | May be promising for targeting a wider range of cancers |
| Target antigens expressed on cancer cells | May target proteins other than those expressed in cancer cells, stromal and other tumor factors |
| ADC Drug | Target | Payload | DAR | Bystander Effect | Status | Adverse Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Ado-trastuzumab emtansine | HER2 | Matansine (Microdubule disrupting agent) | 3–4 | No | Approved in 2013, for HER2-positive MBC pretreated with trastuzumab and taxane (Adjuvant) | AST/ALT raises, thrombocytopenia, neuropathy | [2,6] |
| Trastuzumab deruxtecan | HER2 | Deruxtecan (topoisomerase I inhibitor) | 8 | Yes | Approved in 2019 for HER2-positive MBC pretreated with trastuzumab and taxane | - | [2,6,105] |
| Trastuzumab duocarmazine | HER2 | Duocarmycin prodrug | 2.8 | Yes | Not approved, phase III | Fatigue, conjunctivitis, dry eyes | [6,104] |
| Disitamab vedotin | HER2 | MMAE | 4 | No | Not approved for breast cancer yet, in phase III. Approved for gastric or gastroesophageal junction cancer by NMPA (China) | Neutropenia, AST/ALT raises | [2,6,104] |
| Sacituzumab govitecan | TROP-2 | SN-38 (topoisomerase I inhibitor) | 7.6 | Yes | Received accelerated approval for metastatic TNBC in 2020, and full approval in 2021. In 2023, received FDA approval for HER2- negative breast cancer | Neutropenia, anemia, diarrhea | [2,6,16,41,88] |
| Ladrituzumab vedotin | LIV-1 (TNBC) | MMAE | 4 | No | Not approved, phase III | Neutropenia, anemia | [2,6,104] |
| ARX-788 | HER2 | MMAF | 1.9 | – | Not approved, phase I/II | – | [2,104] |
| A166 | HER2 | Duostatin-5 | N/A | – | Not approved, phase I/II | – | [2,104] |
| MRG002 | HER2 | MMAE | 3.8 | – | Not approved, phase I/II | – | [2,104] |
| ALT-P7 | HER2 | MMAE | 2 | – | Not approved, phase I | – | [2,104] |
| GQ1001 | HER2 | DM1 | N/A | – | Not approved, phase I | – | [2,104] |
| SBT6050 | HER2 | Toll-like receptor 8 agonist (TLR8) | N/A | – | Not approved, phase I/II | – | [2] |
| ZW49 | HER2 MBC | N-acyl sulfonamide auristatin | 2 | No | Phase I | - | [2] |
| MEDI4276 | HER2 | Tubulysin-based microtubule inhibitor | 4 | Yes | Phase I/II | - | [104] |
| XMT-1522 | HER2 | dolaflexin | 12 | - | Phase I/II | _ | [6] |
| Patritumab deruxtecan | HER3 | Deruxtecan (topoisomerase I inhibitor) | 8 | – | Not approved, phase I/II | – | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhan, M.A.; Torchilin, V.P. Advances in Targeted Therapy of Breast Cancer with Antibody-Drug Conjugate. Pharmaceutics 2023, 15, 1242. https://doi.org/10.3390/pharmaceutics15041242
Subhan MA, Torchilin VP. Advances in Targeted Therapy of Breast Cancer with Antibody-Drug Conjugate. Pharmaceutics. 2023; 15(4):1242. https://doi.org/10.3390/pharmaceutics15041242
Chicago/Turabian StyleSubhan, Md Abdus, and Vladimir P. Torchilin. 2023. "Advances in Targeted Therapy of Breast Cancer with Antibody-Drug Conjugate" Pharmaceutics 15, no. 4: 1242. https://doi.org/10.3390/pharmaceutics15041242
APA StyleSubhan, M. A., & Torchilin, V. P. (2023). Advances in Targeted Therapy of Breast Cancer with Antibody-Drug Conjugate. Pharmaceutics, 15(4), 1242. https://doi.org/10.3390/pharmaceutics15041242







