Living Cells and Cell-Derived Vesicles: A Trojan Horse Technique for Brain Delivery
Abstract
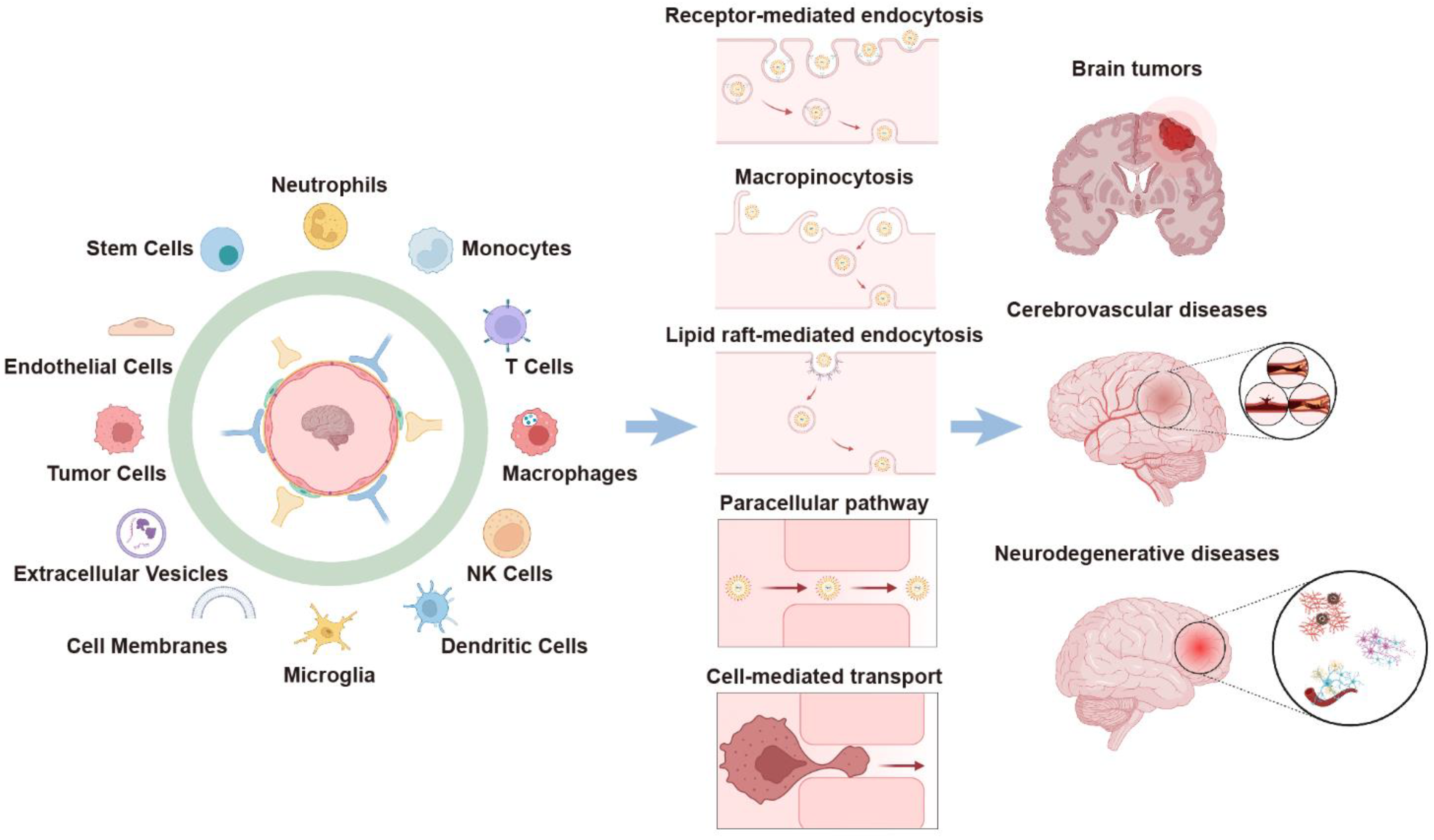
:1. Introduction
2. Cell-Mediated BBB-Crossing Delivery
2.1. Stem Cells
2.1.1. Mesenchymal Stem Cells (MSCs)
2.1.2. Neural Stem Cells (NSCs)
2.1.3. Adipose-Derived Stem Cells (ADSCs)
2.2. Immune Cells
2.2.1. Neutrophils (NEs)
2.2.2. Mononuclear Phagocytes
| Cell Types | Disease Model | Cargo | Loading Mechanism | Administration Method | Release Mechanism | In Vitro/In Vivo Improvement | Refs. |
|---|---|---|---|---|---|---|---|
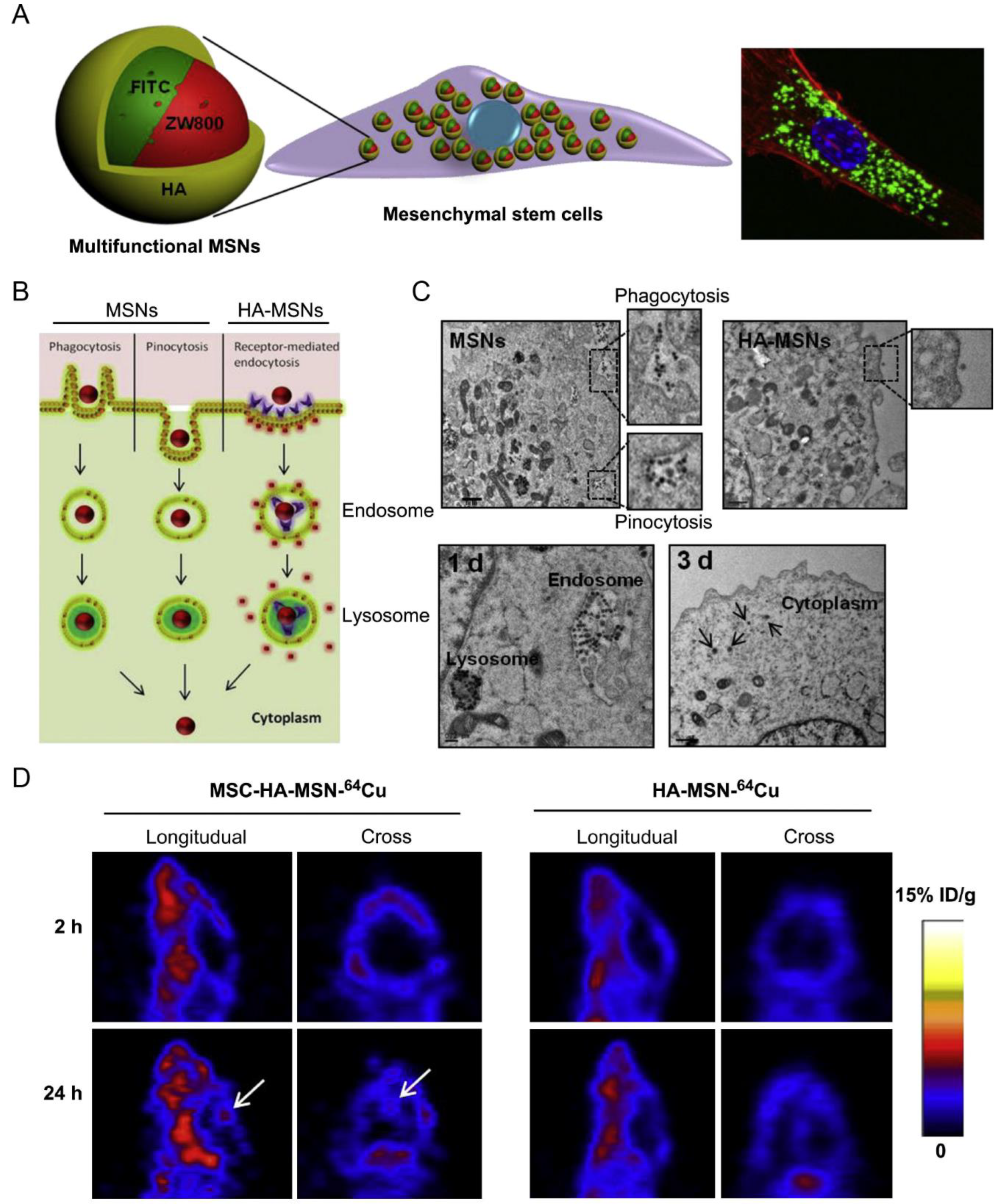
| MSC | Orthotopic glioblastoma model | MSNs incorporating FITC/NIR dye ZW800/Gd3+/64Cu | Phagocytosis, pinocytosis, and receptor-mediated endocytosis | IV | NA | Achieved 5.2-fold higher glioblastoma accumulation than the free nanoparticles | [28] |
| Human NSC line (HB1.F3.CD) | U251 glioma | Adenoviral vectors encoding rCE or hCE1m6 | Adenovirus transfection | IV or intracranial injection | NA | Allow tumor-localized conversion of irinotecan to its active metabolite and increase the therapeutic efficacy | [30] |
| ADSC | ALTS1C1 glioma | SPION/PTX-loaded NPs | Endocytosis | IV | HFMF-mediated intercellular drug transport | Exhibited a 4-fold higher therapeutic index on glioma-bearing mice compared to the typical chemotherapy using temozolomide | [32] |
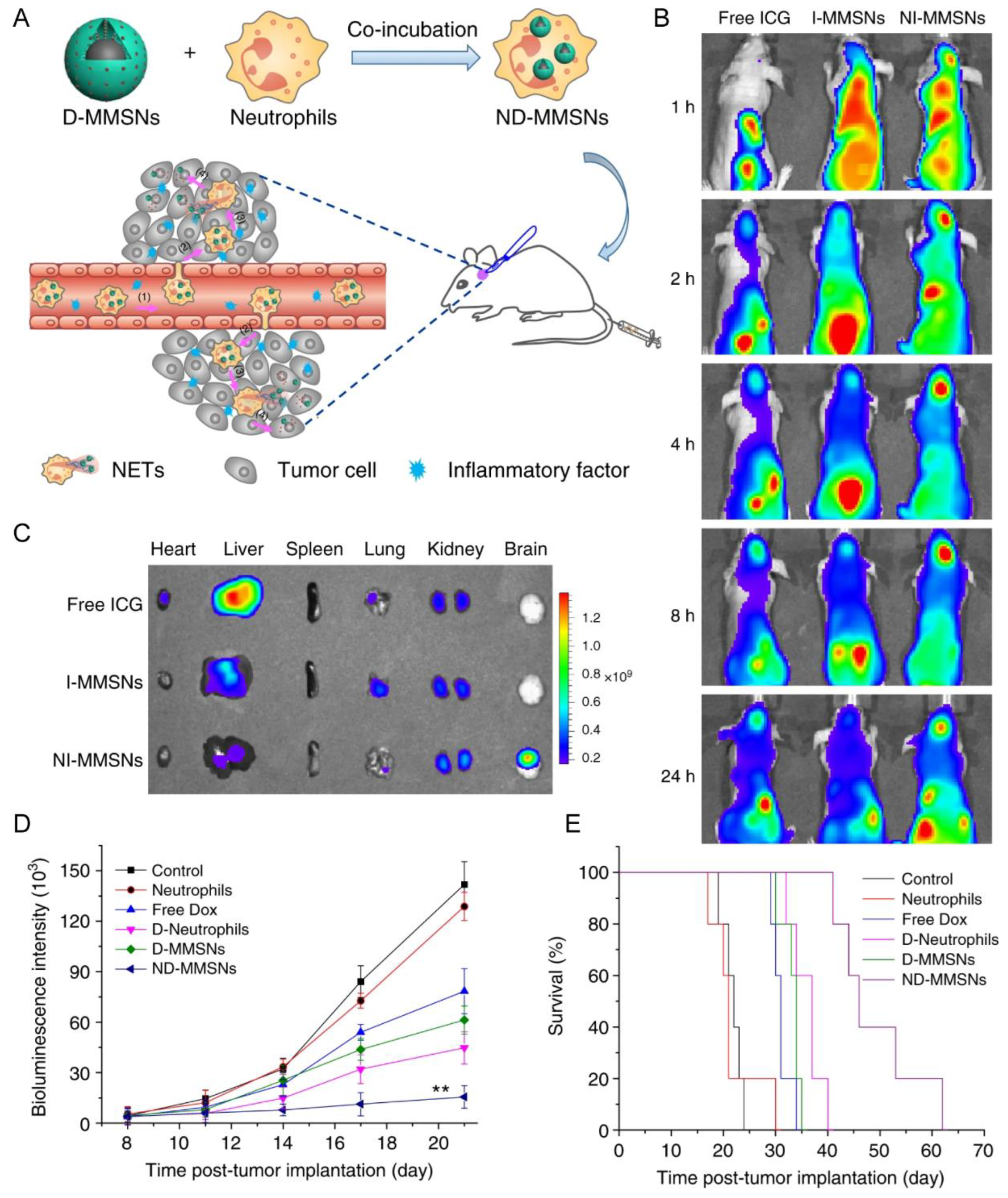
| Neutrophil | Incomplete resection glioma model | D-MMSNs | Phagocytosis | IV | NET formation | Increased accumulation in the brain tumor; improve survival rate and delay glioma relapse | [33] |
| Neutrophil | Glioma surgical resection model | PTX-CL | Endocytosis | IV | NET formation | Slowed the glioma recurrence, and improved survival rates | [17] |
| Neutrophil | Glioma surgical resection model | PTX-loaded EM@nanogels | Phagocytosis | IV | NA | Improved the accumulation of PTX at the glioma sites, and extended the median survival time | [36] |
| Neutrophil | Glioblastoma multiforme model | A molecular photoacoustic imaging probe TFML | Lipid insertion into the neutrophil membrane | IV | NA | Enhanced the photoacoustic signals for glioblastoma multiforme detection | [37] |
| Neutrophil | Focal cerebral ischemia-reperfusion | Cationic liposomes of puerarin | “Proton sponge effect” exhibited by cationic liposomes | IV | NET formation | Enhancing the therapeutic potential of puerarin to promote neuroprotection | [23] |
| Neutrophil | Cerebral ischemic | PM-camouflaged nanoparticles containing miRNA-Let-7c | Binding of P-selectin on the PM with PSGL-1 on the surface of neutrophils | IV | pH-responsive release | Achieved brain delivery of miRNA-Let-7c for the targeted regulation of neurons and microglia | [39] |
| Neutrophil | Cerebral ischemia | cl PGP-PEG-DGL/CAT-Aco NPs | NPs were internalized by circulating neutrophils via endocytosis and micropinocytosis | IV | Transient intercellular connections and exosomes | Enhanced the delivery of NPs across the BBB in vitro and in vivo, and improved the therapeutic outcome of cerebral ischemia | [20] |
| Monocyte/neutrophil | Cerebral ischemia | ER-cRGDLs | Internalization triggered by the binding of cRGD with integrin αvβ1 on the surface of monocyte/neutrophil | IV | NA | Facilitated the delivery of drugs across the BBB in vitro and in vivo, improved the neuroprotection effect of ER in late-stage of ischemia, and extended the therapeutic window | [21] |
| Monocyte | Glioblastoma | CPNs | Phagocytosis | IV | Exosome-mediated extracellular release | Enhanced the delivery of CPNs into glioblastoma spheroids and the orthotopic model, and improved the photodynamic therapy in glioblastoma spheroids | [45] |
| Monocyte | Glioblastoma | Nano-doxorubicin | NA | IV | Lysosomal exocytosis | Improve spheroids infiltration and brain tumor drug delivery | [42] |
| RAW | Acute nerve inflammation induced by LPS | UCANPs | Phagocytosis | IV | NA | UCANPs@RAW could penetrate the BBB and image the deep inflamed region in the brain | [48] |
| HSC transplantation-based macrophage | Parkinson’s Disease | Lentiviral vectors expressing GDNF | Lentiviral transfection | IV | NA | GDNF-expressing macrophages infiltrated degenerating PD-like brains in mouse models, increased GDNF levels in the midbrain, and improved motor and non-motor dysfunction via the neuroprotective effects of GNDF | [49] |
| Macrophage | C6 glioma model | Fe3O4-Cy5.5 | Phagocytosis | IV | NA | Achieved deep glioma accumulation for multimodal diagnosis, imaging-guided surgery, and photothermal therapy | [51] |
| BV2 microglial cell | Orthotopic glioblastoma mouse model | CIONPs and DiD | NA | Internal carotid artery injection; IV | NA | DiDBV2-Fe efficiently accumulated in the brain tumor for fluorescence-guided resections | [52] |
| BV2 microglial cell | U87 and GL261 glioma model | Liposomes encapsulating paclitaxel | Phagocytosis | IV | Extracellular vesicles and tunneling nanotubes | Achieved brain-targeted delivery, and suppressed tumor progression | [22] |
| M1 macrophage | U87 glioma model | DOX-loaded PLGA nanoparticles | Phagocytosis | IV | Exocytosis in exosome form | Improve brain tumor distribution and enhance the anti-glioma effect | [13] |
2.2.3. T Cells
3. EV-Mediated BBB-Crossing Delivery
3.1. Stem Cells
3.1.1. Mesenchymal Stem Cells (MSCs)
3.1.2. Neural Stem Cells (NSCs) and Neural Progenitor Cells (NPCs)
3.1.3. Other Stem Cells
3.2. Immune Cells
3.3. Tumor Cells
3.4. Plants
3.5. Other Sources
| EV Source | Surface Modification | Disease Model | Cargo | Administration Method | In Vitro/In Vivo Improvement | Refs. |
|---|---|---|---|---|---|---|
| Mesenchymal stromal cells | cRGD | Cerebral ischemia | Curcumin | IV | Showed better anti-inflammatory effects than free curcumin | [73] |
| Mesenchymal stem cells | RVG | Alzheimer’s disease | NA | IV | Reduced plaque deposition and Aβ accumulation and improved learning and memory capabilities | [71] |
| Adipose-derived stem cells | NA | Ischemic stroke | miRNA-126 | IV | Enhanced neurogenesis, inhibited neuroinflammation, and improved functional recovery | [109] |
| Mesenchymal stem cells | CXCR4 | Brain metastasis of breast cancer | TRAIL | IV | Improved EVs delivery across the BBB and exerted cooperative therapeutic effects with carboplatin | [74] |
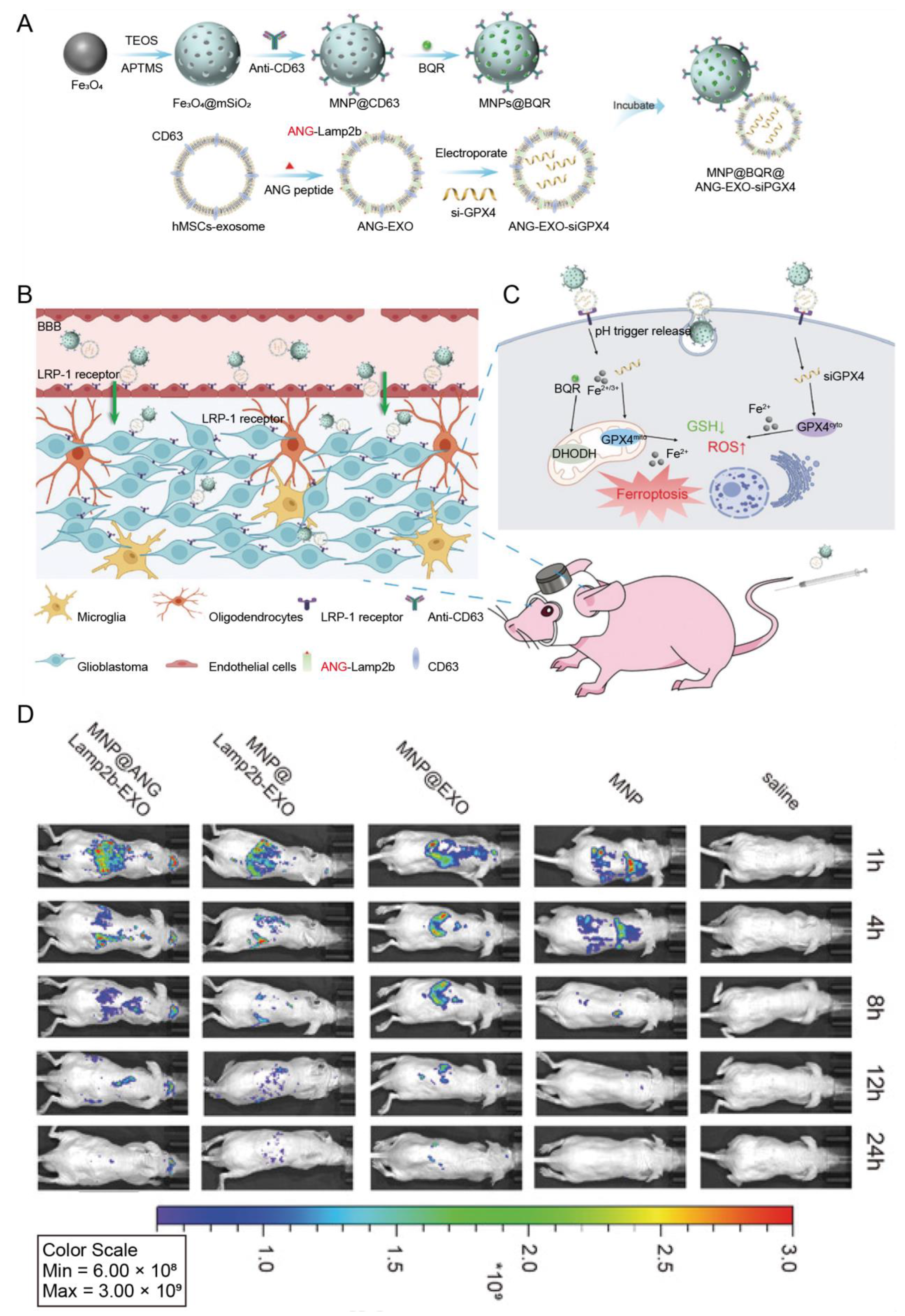
| Human mesenchymal stem cells | ANG | Glioblastoma | Magnetic nanoparticles loaded with brequinar | IV | Enabled the cargo transport through the BBB and enhanced ferroptosis induced by magnetic nanoparticles | [75] |
| Human umbilical cord mesenchymal stem cells | NA | Parkinson’s Disease | NA | IV | Reached the substantia nigra and decreased dopaminergic neuron loss and therefore upregulating dopamine levels in the striatum | [110] |
| Neural stem cells | PDGF-A | Experimental autoimmune encephalomyelitis | Bryostatin-1 | IV | Enhanced the targeting abilities toward brain lesions and showed excellent therapeutic capabilities of reducing neuroinflammation with low dosages of Bryostatin-1 | [78] |
| Neural progenitor cells | RGD-4C | Ischemic stroke | NA | IV | A set of seven miRNAs incorporated in EVReN inhibited the MAPK signaling pathway; significantly improved the targeting and therapeutic efficacy | [111] |
| Neural progenitor cells | cRGD | Glioblastoma | siRNA against PD-L1 | IV | Decreased radiation-induced PD-L1 expression on tumor-associated myeloid cells and tumor cells as well as the upregulation of CD8+ cytotoxic T cells | [80] |
| Human endometrial stem cells | NA | Parkinson’s Disease | Curcumin | IV | Suppressed α-synuclein aggregation and neural cell apoptosis | [82] |
| Embryonic stem cells | cRGD | Glioblastoma | Paclitaxel | IV | Improved the therapeutic efficacy of paclitaxel via superior glioblastoma-targeting capacity | [84] |
| Dendric cells | RVG | Parkinson’s Disease | Anti-α-synuclein shRNA | IV | Achieved long-term downregulation of target genes, inhibition of dopaminergic cell death and movement abnormalities | [88] |
| Immature dendric cells | RVG | Parkinson’s Disease | Curcumin; siRNA targeting SNCA | IV | Enhanced drug delivery via modification, improved α-synuclein clearance and neuronal recovery and cleared aberrant immune activation | [87] |
| Naive macrophages | NA | Brain inflammation | Brain-derived neurotrophic factor | IV | Interacted with endothelial ICAM-1 thus mediating the migration across the BBB, improved the cargo accumulation in the brain | [91] |
| Macrophages | RGE | Glioma | Superparamagnetic iron oxide nanoparticles and curcumin | IV | Showed significant antitumor effects synchronized with the MRI ability in vivo | [112] |
| Macrophages | NA | Alzheimer’s disease | Curcumin | IV | Activated AKT and inhibited GSK-3β, resulting in the reduction of Tau protein phosphorylation; improved cognitive function in mice model | [113] |
| Macrophages | NA | Glioma | Boron-containing carbon dots | IV | Improved the therapeutic effect of boron neutron capture therapy due to the high accumulation of boron-containing carbon dots via exosome delivery in mice brain | [114] |
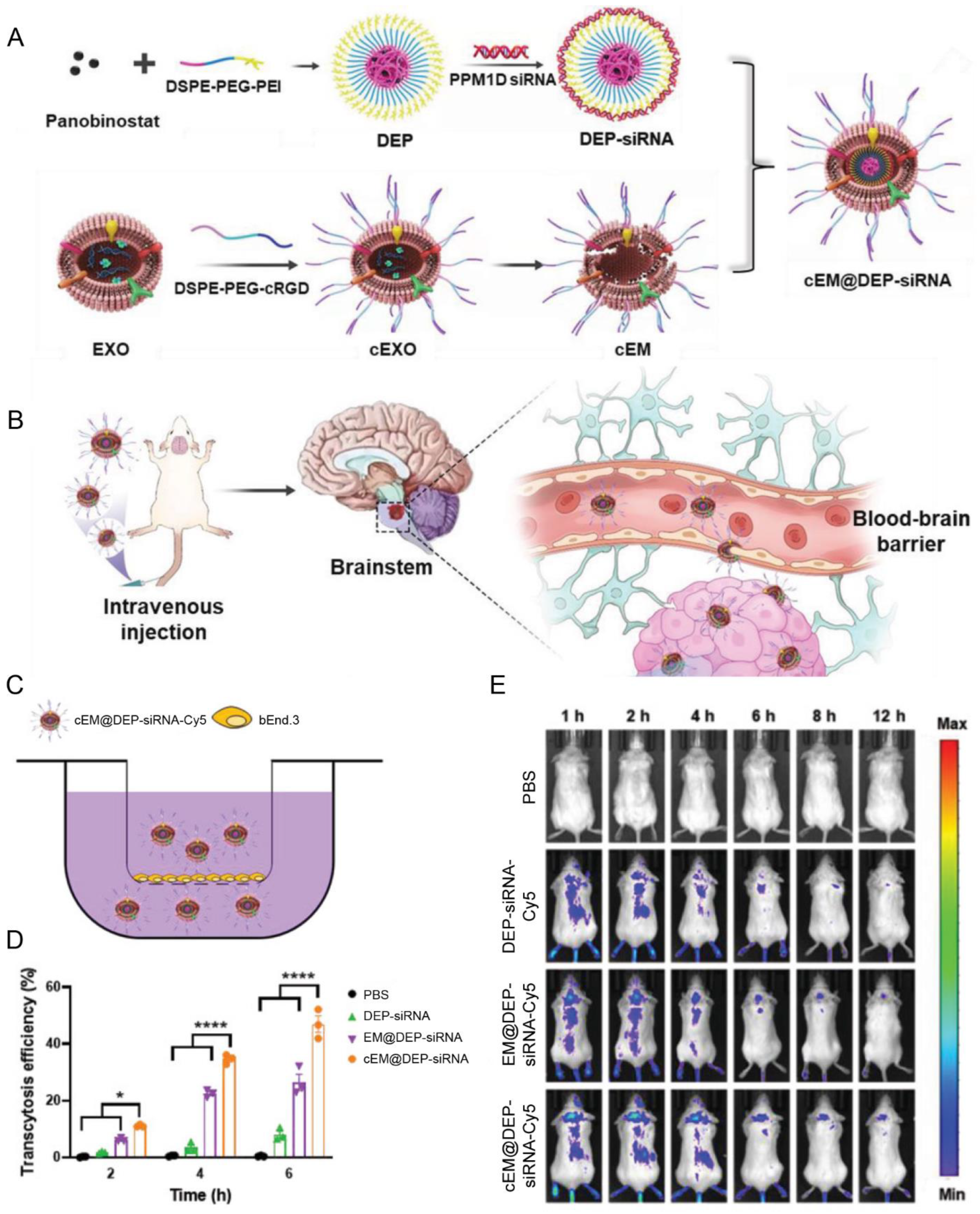
| Macrophages | cRGD | Diffuse intrinsic pontine glioma | Panobinostat; anti PPM1D siRNA | IV | Achieved excellent tumor growth inhibition ascribed to the high BBB penetration efficiency | [92] |
| M1-like macrophages | CPPO; Ce6 | Glioblastoma multiforme | Banoxantrone | IV | Induced M2 polarization to M1 for the immunomodulation of TME; combined with chemiexcited photodynamic therapy after accumulating in brain tumors | [115] |
| Macrophages | AS1411 | Glioblastoma | ICG; catalase | IV | Realized the high accumulation in the tumor site and enhanced the sonodynamic therapy of glioblastoma | [90] |
| Neutrophils | NA | Glioblastoma | Doxorubicin | IV | Penetrated the BBB and targeted tumor in response to inflammation; inhibited tumor growth and extended survival time in a glioblastoma mouse model | [93] |
| Tumor cells | NA | Parkinson’s Disease | ASO; cKGM | IV | Efficiently delivered cargos into the brain, enhanced by the transcytosis through BMECs, which is mediated by CD44v6 expressed on the membrane; reduced brain inflammation and ameliorated Parkinson’s disease symptoms | [95] |
| Tumor cells | NA | Glioblastoma multiforme | Dihydrotanshinone; temozolomide | IV | Overcome the drug resistance of temozolomide and triggered the immune response | [98] |
| Momordica charantia | NA | Glioma | NA | IV | Inhibited glioma progression via the regulation of PI3K/AKT pathway | [100] |
| Grapefruit | NA | Glioma | Doxorubicin-loaded heparin-based nanoparticles | IV | Achieved high-abundance accumulation and brain tumor uptake at glioma sites by αvβ3 receptor-mediated transcytosis and membrane fusion | [101] |
| Cerebral endothelial cells | NA | Ischemic stroke | NA | IV | Penetrated the BBB, efficiently internalized by neurons and other parenchymal cells, and improved fibrinolysis and thrombolysis combined with tPA | [106] |
| Blood | NA | Parkinson’s Disease | Dopamine | IV | Penetrated the BBB based on the transferrin receptor expressed on the surfaces; reduced systemic toxicity of dopamine | [103] |
| Blood | NA | Glioblastoma | Metformin; cPLA2 siRNA | IV | Showed great efficacy in the patient-derived glioblastoma orthotopic xenograft model by improving glioblastoma cell uptake via high expression of PTRF | [104] |
| HEK 293T | T7 peptide | Glioblastoma | Antisense miRNA oligonucleotides against miR-21 | IV | Showed more efficient delivery of cargoes than RVG-decorated exosomes and inhibited tumor growth by increasing the expression of PDCD4 and PTEN | [63] |
| HEK 293T | RVG | The chronic unpredictable stress model | CircDYM | IV | Suppressed microglia activation by bounding to TAF1; reduced the infiltration of peripheral immune cells, thereby attenuating depressive-like behaviors | [60] |
| HEK 293T | ANG and TAT peptide | Glioma | Doxorubicin | IV | Improved the efficiency to penetrate the BBB and penetrate the tumor, resulting in the high therapeutic efficacy of the chemotherapeutic drug | [62] |
4. Cell Membrane-Cloaked Nanoparticles for BBB-Crossing Delivery
4.1. Red Blood Cells (RBCs)
4.2. Immune Cells
4.2.1. Natural Killer (NK) Cells
4.2.2. Macrophages
4.2.3. Neutrophils
4.3. Tumor Cells
4.4. Stem Cells
4.5. Bacteria
4.6. Hybrid Membranes
| Cell Types | Surface Functionalization | Targets | Disease Model | Cargo | Loading Mechanism | Administration Method | Release Mechanism | In Vitro/In Vivo Improvement | Ref. |
|---|---|---|---|---|---|---|---|---|---|
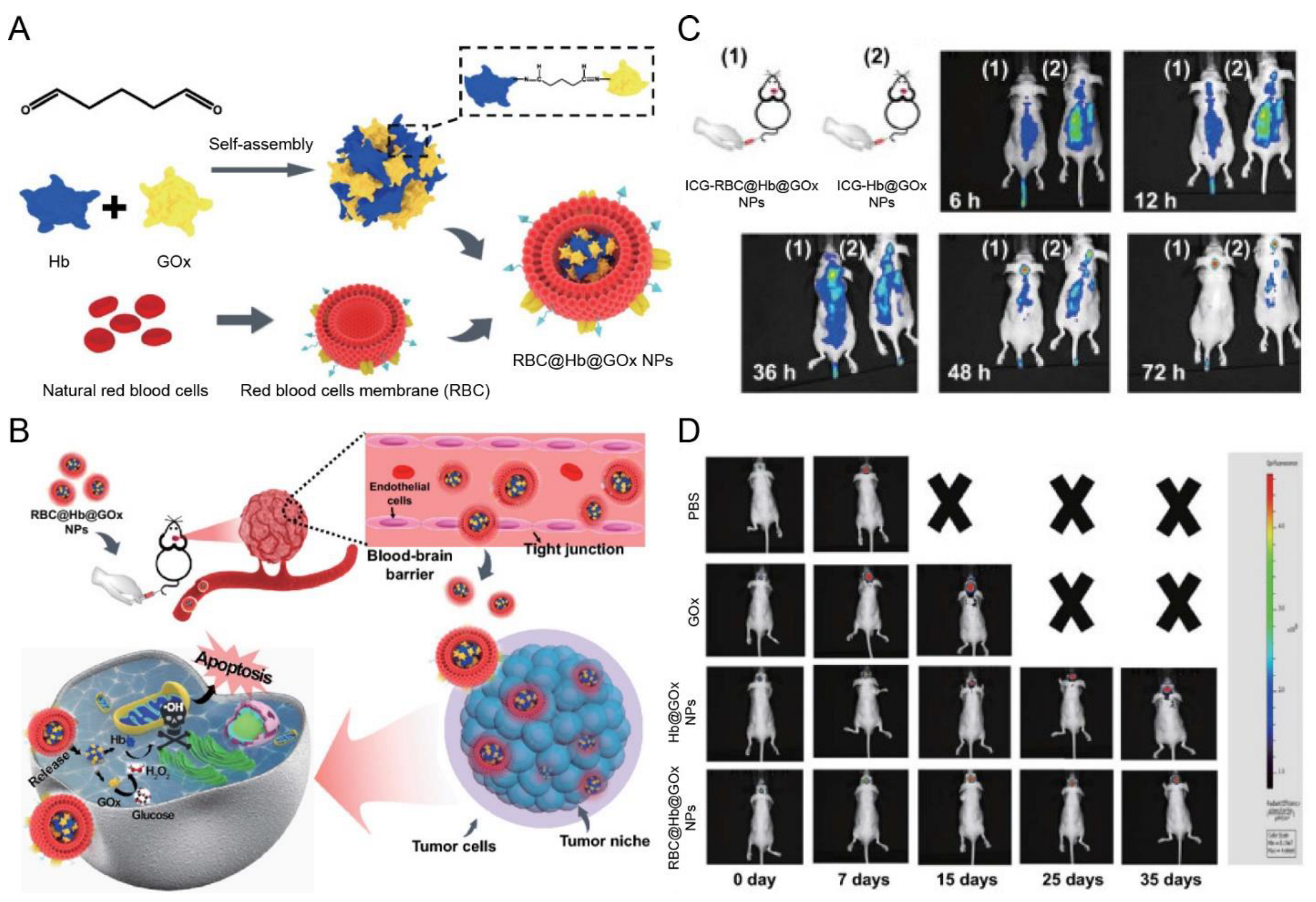
| RBC membrane | NA | NA | Orthotopic gliomas (U87 cells) | Hb@GOx NPs | Sonication | IV | In situ H2O2 causes cell membrane destruction | Exhibited notable tumor accumulation and inhibited the growth of GBM | [122] |
| RBC membrane | T807 | Phosphorylation tau | AD | Curcumin | Sonication and extrusion | IV | NA | Increased curcumin accumulation in the brain and alleviated AD progression | [123] |
| Nanoerythrocyte membrane | MG1 peptide/RVG29 peptide | M1 microglia | MCAO and EAE | NEMR | Extrusion | IV | ROS | Penetrated the BBB, targeted M1 microglia, re-educated M1 to M2 microglia, suppressed inflammation, and enhanced neuroprotection | [124] |
| NK cell membrane | NA | NA | Orthotopic gliomas (U87 cells) | AIEdots | Extrusion | IV | NA | Exhibited higher tumor accumulation, and inhibited the growth of glioma | [126] |
| Macrophage membrane (RAW264.7 cells) | NA | NA | Orthotopic gliomas (C6 cells) | Pt/MnO2@PVCL NGs | Extrusion | IV | ROS/pH | Exhibited BBB penetrating and glioma targeting ability for MR imaging-guided chemotherapy/CDT | [131] |
| Macrophage membrane (rat peritoneal macrophages) | NA | NA | tMCAO/R model | MnO2@FTY NPs | Extrusion | IV | ROS/pH | Accumulated in the ischemic region, repolarized M1 microglia to M2 microglia, reduced oxidative stress, reversed the proinflammatory microenvironment, and reinforced neuroprotective effects | [127] |
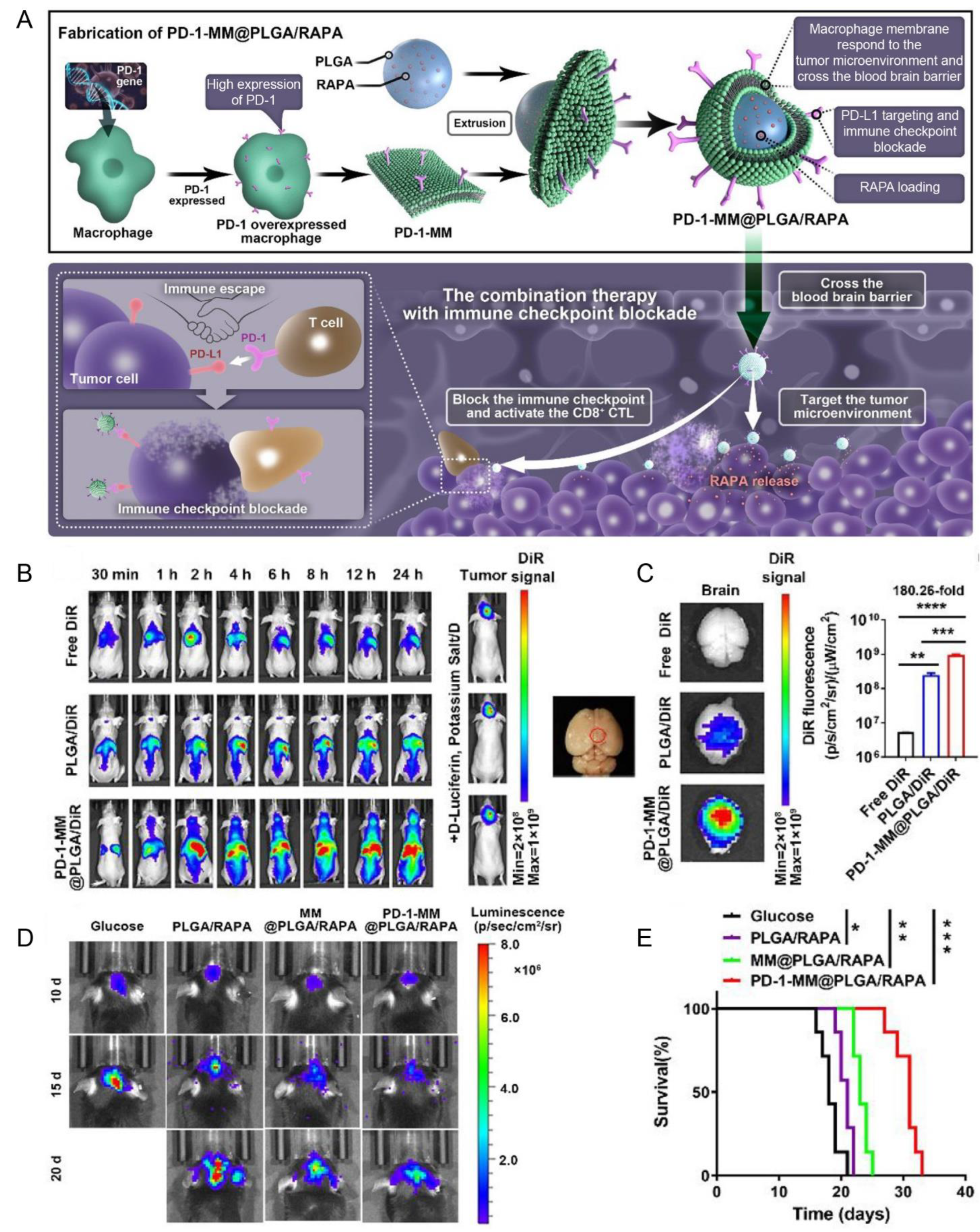
| Macrophage membrane (RAW264.7 cells) | PD-1 | PD-L1 | Orthotopic gliomas (C6 cells) | PLGA/RAPA NPs | Extrusion | IV | NA | Enhanced the BBB penetration, blocked the PD-1/PD-L1 axis, and exhibited anti-GBM efficacy | [132] |
| Neutrophil membrane (HL60 cells) | NA | NA | tMCAOmodel | MPBzyme | Extrusion | IV | NA | Elicited M2 microglia polarization, reduced neutrophil recruitment, and protected against neuronal damage | [133] |
| Neutrophil membrane (HL60 cells) | NA | NA | MCAO model | Resolvin D2 | Sonication | IV | NA | Targeted inflamed brain endothelium, and mitigated neuroinflammation | [134] |
| Tumor cell membrane (U251 glioma cells or human tumor tissue) | NA | NA | Orthotopic glioma model (U251 cells)/patient-derived xenograft tumor models (human tumor tissue) | HLPC | Extrusion | IV | NA | Enhanced glioma-targeting efficacy, induced cell apoptosis, and exhibited the antitumor effect | [135] |
| Tumor cell membrane (B16F10 cells or 4T1 cells) | NA | NA | Orthotopic glioma model (U87 cells) | PCL-ICG NPs | Sonication | IV | NA | Improved the long-circulating capacity, traversed the BBB, accumulated in glioma cells, and inhibited glioma growth. | [136] |
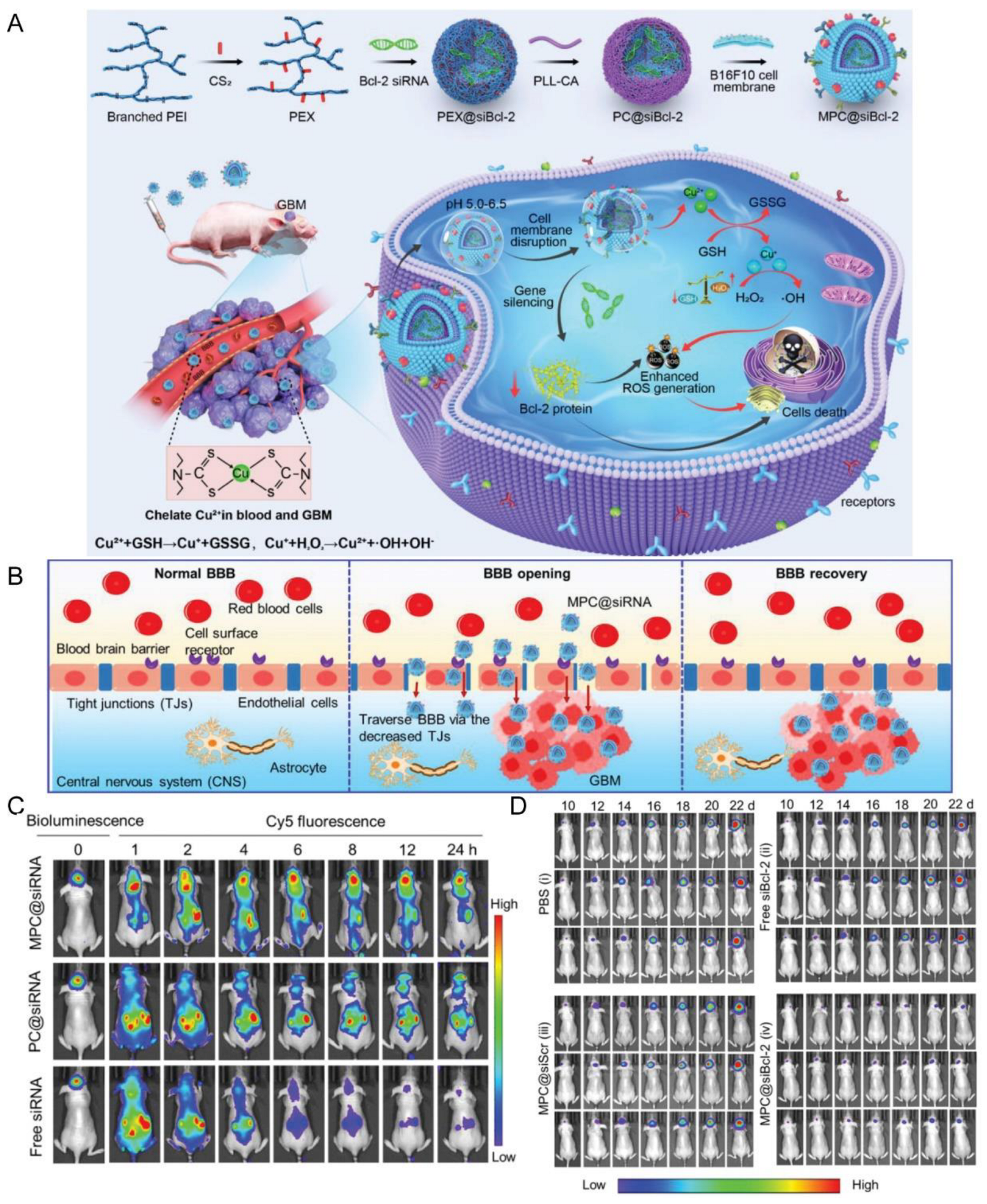
| Tumor cell membrane (B16F10 cells) | NA | NA | Local subcutaneous melanoma model (B16F10 cells)/orthotopic GBM model (U87MG, GL261 cells) | PC@siRNA | Sonication | IV | pH | Promoted BBB penetration and GBM accumulation; inhibited the growth of orthotopic GBM tumors and subcutaneous melanoma tumor | [137] |
| Tumor cell membrane (4T1 cells) | NA | NA | tMCAO model | PP/SCB | Extrusion | IV | pH | Penetrated the BBB, targeted the ischemic inflammation lesions, reduced the infarct volume, enhanced microvascular reperfusion, and promoted neuroprotective effects | [138] |
| NSC membrane | CXCR4 | SDF-1 | MCAO | Gly@PLGA NPs | Extrusion | IV | NA | Augmented the efficacy of glyburide, promoted the accumulation of nanoparticles in the ischemic region | [139] |
| LPS-free OMV from EC-K1 | NA | NA | Brain metastasis model (231Br cells) | DOX@PLGA NPs | Extrusion | IV | NA | Prolonged circulation, improved BBB penetration, enhanced brain-targeted ability, and lengthened the survival of breast cancer brain metastases | [140] |
| Hybrid membrane (C6 cells and DCs) | NA | NA | Intracranial glioma model (C6 cells) | DNS | Sonication | IV | NA | Prolonged the blood circulation time, penetrated the BBB and BBTB, delivered docetaxel to the tumor site, and enhanced the anti-tumor immune response | [141] |
| Hybrid membrane (RBC and U251 cells) | NA | NA | NA | Isoliquiritigenin | Sonication and extrusion | NA | NA | Inhibited U251 cell migration and promoted U251 cell apoptosis | [142] |
| Hybrid membrane (RAW 264.7 cells and neutrophils) | NA | NA | Mouse brain inflammatory model/glioma (C6 cells) | PLGA/RAPA NPs | Sonication and extrusion | IV | NA | Recognized the chemotactic stimuli, transported across BBB, and showed the antitumor effect | [143] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Gotz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef]
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the blood-brain barrier for the therapy of malignant brain tumor: Current status and prospects of drug delivery approaches. J. Nanobiotechnol. 2022, 20, 412. [Google Scholar] [CrossRef]
- Mendanha, D.; Vieira de Castro, J.; Ferreira, H.; Neves, N.M. Biomimetic and cell-based nanocarriers—New strategies for brain tumor targeting. J. Control. Release 2021, 337, 482–493. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Chen, Y.; Xu, Y.; Peng, J. Cell-based drug delivery systems and their in vivo fate. Adv. Drug Deliv. Rev. 2022, 187, 114394. [Google Scholar] [CrossRef]
- Wu, J.; Ma, L.; Sun, D.; Zhang, X.; Cui, J.; Du, Y.; Guo, Y.; Wang, X.; Di, L.; Wang, R. Bioengineering extracellular vesicles as novel nanocarriers towards brain disorders. Nano Res. 2022, 16, 2635–2659. [Google Scholar] [CrossRef]
- Li, A.; Zhao, Y.; Li, Y.; Jiang, L.; Gu, Y.; Liu, J. Cell-derived biomimetic nanocarriers for targeted cancer therapy: Cell membranes and extracellular vesicles. Drug Deliv. 2021, 28, 1237–1255. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Zhu, Y.; Qin, J.; Zhao, W.; Wang, J. Primary M1 macrophages as multifunctional carrier combined with PLGA nanoparticle delivering anticancer drug for efficient glioma therapy. Drug Deliv. 2018, 25, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv. 2020, 27, 745–755. [Google Scholar] [CrossRef]
- Timin, A.S.; Litvak, M.M.; Gorin, D.A.; Atochina-Vasserman, E.N.; Atochin, D.N.; Sukhorukov, G.B. Cell-Based Drug Delivery and Use of Nano-and Microcarriers for Cell Functionalization. Adv. Healthc. Mater. 2018, 7, 1700818. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Pohl-Guimaraes, F.; Yang, C.; Dyson, K.A.; Wildes, T.J.; Drake, J.; Huang, J.; Flores, C.; Sayour, E.J.; Mitchell, D.A. RNA-Modified T Cells Mediate Effective Delivery of Immunomodulatory Cytokines to Brain Tumors. Mol. Ther. 2019, 27, 837–849. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Polajzer, T.; Miklavcic, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Zhang, C.; Ling, C.L.; Pang, L.; Wang, Q.; Liu, J.X.; Wang, B.S.; Liang, J.M.; Guo, Y.Z.; Qin, J.; Wang, J.X. Direct Macromolecular Drug Delivery to Cerebral Ischemia Area using Neutrophil-Mediated Nanoparticles. Theranostics 2017, 7, 3260–3275. [Google Scholar] [CrossRef]
- Hou, J.; Yang, X.; Li, S.; Cheng, Z.; Wang, Y.; Zhao, J.; Zhang, C.; Li, Y.; Luo, M.; Ren, H.; et al. Accessing neuroinflammation sites: Monocyte/neutrophil-mediated drug delivery for cerebral ischemia. Sci. Adv. 2019, 5, eaau8301. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, Z.; Sun, Q.; Lin, M.; Wang, R.; Peng, Y.; Chen, X.; Qi, X. Engineered Microglia Potentiate the Action of Drugs against Glioma Through Extracellular Vesicles and Tunneling Nanotubes. Adv. Healthc. Mater. 2021, 10, e2002200. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, H.; Rao, X.; Li, X.; Lu, H.; Li, F.; He, Y.; Yu, R.; Zhong, R.; Zhang, Y.; et al. Enhanced treatment for cerebral ischemia-reperfusion injury of puerarin loading liposomes through neutrophils-mediated targeted delivery. Nano Res. 2021, 14, 4634–4643. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Ransohoff, R.M. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Yang, C.-X.; Yan, X.-P. A Dual-Functional Persistently Luminescent Nanocomposite Enables Engineering of Mesenchymal Stem Cells for Homing and Gene Therapy of Glioblastoma. Adv. Funct. Mater. 2017, 27, 1604992. [Google Scholar] [CrossRef]
- Teo, G.S.; Ankrum, J.A.; Martinelli, R.; Boetto, S.E.; Simms, K.; Sciuto, T.E.; Dvorak, A.M.; Karp, J.M.; Carman, C.V. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-alpha-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells 2012, 30, 2472–2486. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Wang, H.; Niu, G.; Choi, K.Y.; Swierczewska, M.; Zhang, G.; Gao, H.; Wang, Z.; Zhu, L.; et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials 2013, 34, 1772–1780. [Google Scholar] [CrossRef]
- Aboody, K.S.; Brown, A.; Rainov, N.G.; Bower, K.A.; Liu, S.; Yang, W.; Small, J.E.; Herrlinger, U.; Ourednik, V.; Black, P.M.; et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA 2000, 97, 12846–12851. [Google Scholar] [CrossRef]
- Metz, M.Z.; Gutova, M.; Lacey, S.F.; Abramyants, Y.; Vo, T.; Gilchrist, M.; Tirughana, R.; Ghoda, L.Y.; Barish, M.E.; Brown, C.E.; et al. Neural stem cell-mediated delivery of irinotecan-activating carboxylesterases to glioma: Implications for clinical use. Stem Cells Transl. Med. 2013, 2, 983–992. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, C.; Fitch, S.; Yang, F. Targeting Tumor Hypoxia Using Nanoparticle-engineered CXCR4-overexpressing Adipose-derived Stem Cells. Theranostics 2018, 8, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lu, I.L.; Chiang, W.H.; Lin, Y.W.; Tsai, Y.C.; Chen, H.H.; Chang, C.W.; Chiang, C.S.; Chiu, H.C. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J. Control. Release 2017, 254, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, H.; Tie, C.; Yan, C.; Deng, Z.; Wan, Q.; Liu, X.; Yan, F.; Zheng, H. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 2018, 9, 4777. [Google Scholar] [CrossRef] [PubMed]
- Bernardes-Silva, M.; Anthony, D.C.; Issekutz, A.C.; Perry, V.H. Recruitment of neutrophils across the blood-brain barrier: The role of E- and P-selectins. J. Cereb. Blood Flow. Metab. 2001, 21, 1115–1124. [Google Scholar] [CrossRef]
- Fossati, G.; Ricevuti, G.; Edwards, S.W.; Walker, C.; Dalton, A.; Rossi, M.L. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999, 98, 349–354. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot. 2021, 6, eaaz9519. [Google Scholar] [CrossRef]
- Liu, X.; Duan, Y.; Hu, D.; Wu, M.; Chen, C.; Ghode, P.B.; Magarajah, G.; Thakor, N.; Liu, X.; Liu, C.; et al. Targeted Photoacoustic Imaging of Brain Tumor Mediated by Neutrophils Engineered with Lipid-Based Molecular Probe. ACS Mater. Lett. 2021, 3, 1284–1290. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Gendelman, H.E.; Kabanov, A.V. Cell-mediated drug delivery. Expert Opin. Drug Deliv. 2011, 8, 415–433. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Q.; Li, W.; Zhang, J.; Yang, C.; Chen, D. Multi-functional platelet membrane-camouflaged nanoparticles reduce neuronal apoptosis and regulate microglial phenotype during ischemic injury. Appl. Mater. Today 2022, 27, 101412. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, X.; Herting, C.J.; Garcia, V.A.; Nie, K.; Pong, W.W.; Rasmussen, R.; Dwivedi, B.; Seby, S.; Wolf, S.A.; et al. Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res. 2017, 77, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, K.; Li, T.; Chen, Z.; Wen, Y.; Liu, X.; Jia, X.; Zhang, Y.; Xu, Y.; Han, M.; et al. Monocyte-mediated chemotherapy drug delivery in glioblastoma. Nanomedicine 2018, 13, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget 2016, 7, 37081–37091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell. Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Beauge, L.; Arias-Ramos, N.; Rivarola, V.A.; Chesta, C.A.; Lopez-Larrubia, P.; Palacios, R.E. Trojan horse monocyte-mediated delivery of conjugated polymer nanoparticles for improved photodynamic therapy of glioblastoma. Nanomedicine 2020, 15, 1687–1707. [Google Scholar] [CrossRef]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhao, X.; Yan, X.P. Cell-Penetrating Peptide-Functionalized Persistent Luminescence Nanoparticles for Tracking J774A.1 Macrophages Homing to Inflamed Tissues. ACS Appl. Mater. Interfaces 2019, 11, 19894–19901. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, W.; Xu, M.; Su, X.; Li, F. Visualization of Acute Inflammation through a Macrophage-Camouflaged Afterglow Nanocomplex. ACS Appl. Mater. Interfaces 2022, 14, 259–267. [Google Scholar] [CrossRef]
- Chen, C.; Guderyon, M.J.; Li, Y.; Ge, G.; Bhattacharjee, A.; Ballard, C.; He, Z.; Masliah, E.; Clark, R.A.; O’Connor, J.C.; et al. Non-toxic HSC Transplantation-Based Macrophage/Microglia-Mediated GDNF Delivery for Parkinson’s Disease. Mol. Ther. Methods Clin. Dev. 2020, 17, 83–98. [Google Scholar] [CrossRef]
- Morantz, R.A.; Wood, G.W.; Foster, M.; Clark, M.; Gollahon, K. Macrophages in experimental and human brain tumors. Part 2: Studies of the macrophage content of human brain tumors. J. Neurosurg. 1979, 50, 305–311. [Google Scholar] [CrossRef]
- Wang, S.; Shen, H.; Mao, Q.; Tao, Q.; Yuan, G.; Zeng, L.; Chen, Z.; Zhang, Y.; Cheng, L.; Zhang, J.; et al. Macrophage-Mediated Porous Magnetic Nanoparticles for Multimodal Imaging and Postoperative Photothermal Therapy of Gliomas. ACS Appl. Mater. Interfaces 2021, 13, 56825–56837. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, X.; Wei, R.; Li, G.; Sun, B.; Zhang, H.; Liu, D.; Wang, C.; Feng, M. Engineering microglia as intraoperative optical imaging agent vehicles potentially for fluorescence-guided surgery in gliomas. Biomater. Sci. 2020, 8, 1117–1126. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Impellizzieri, D.; Basso, C.; Laroni, A.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B. T-cell trafficking in the central nervous system. Immunol. Rev. 2012, 248, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Choi, B.D.; Suryadevara, C.M.; Sanchez-Perez, L.; Yang, S.; De Leon, G.; Sayour, E.J.; McLendon, R.; Herndon, J.E., 2nd; Healy, P.; et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS ONE 2014, 9, e94281. [Google Scholar] [CrossRef] [PubMed]
- Vajkoczy, P.; Laschinger, M.; Engelhardt, B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J. Clin. Investig. 2001, 108, 557–565. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Aldinucci, A.; Amoriello, R.; Benagiano, M.; Bonechi, E.; Maggi, P.; Flori, A.; Ravagli, C.; Saer, D.; Cappiello, L.; et al. Myelin-specific T cells carry and release magnetite PGLA-PEG COOH nanoparticles in the mouse central nervous system. RSC Adv. 2018, 8, 904–913. [Google Scholar] [CrossRef]
- Ayer, M.; Schuster, M.; Gruber, I.; Blatti, C.; Kaba, E.; Enzmann, G.; Burri, O.; Guiet, R.; Seitz, A.; Engelhardt, B.; et al. T Cell-Mediated Transport of Polymer Nanoparticles across the Blood-Brain Barrier. Adv. Healthc. Mater. 2021, 10, e2001375. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Yu, X.; Bai, Y.; Han, B.; Ju, M.; Tang, T.; Shen, L.; Li, M.; Yang, L.; Zhang, Z.; Hu, G.; et al. Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours. J. Extracell. Vesicles 2022, 11, e12185. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Z.; Liu, N.; Gao, G.; Du, M.; Wang, Y.; Cheng, B.; Zhu, M.; Jia, B.; Pan, L.; et al. Kill two birds with one stone: Engineered exosome-mediated delivery of cholesterol modified YY1-siRNA enhances chemoradiotherapy sensitivity of glioblastoma. Front. Pharmacol. 2022, 13, 975291. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhai, Y.; Hao, Y.; Wang, Q.; Han, F.; Zheng, W.; Hong, J.; Cui, L.; Jin, W.; Ma, S.; et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J. Extracell. Vesicles 2022, 11, e12255. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chen, X.; Hao, Y.; Wang, J.; Xie, Q.; Wang, X. Nanoengineering facilitating the target mission: Targeted extracellular vesicles delivery systems design. J. Nanobiotechnol. 2022, 20, 431. [Google Scholar] [CrossRef]
- Sun, X.; Meng, H.; Wan, W.; Xie, M.; Wen, C. Application potential of stem/progenitor cell-derived extracellular vesicles in renal diseases. Stem Cell Res. Ther. 2019, 10, 8. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, D.; Lian, L.; Zhao, L.; Li, M.; Bao, H.; Xu, S. Stem Cell-derived Extracellular Vesicles: A Promising Nano Delivery Platform to the Brain? Stem Cell Rev. Rep. 2022, 19, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Do, A.D.; Kurniawati, I.; Hsieh, C.-L.; Wong, T.-T.; Lin, Y.-L.; Sung, S.-Y. Application of Mesenchymal Stem Cells in Targeted Delivery to the Brain: Potential and Challenges of the Extracellular Vesicle-Based Approach for Brain Tumor Treatment. Int. J. Mol. Sci. 2021, 22, 11187. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Casado-Diaz, A.; Quesada-Gomez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 954. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Guo, H.D.; Li, H.; Zhai, Y.; Gong, Z.B.; Wu, J.; Liu, J.S.; Dong, Y.R.; Hou, S.X.; Liu, J.R. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Liu, M.; Hu, Y.; Chen, G. The Antitumor Effect of Gene-Engineered Exosomes in the Treatment of Brain Metastasis of Breast Cancer. Front. Oncol. 2020, 10, 1453. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, X.; Qiu, W.; Zhao, R.; Duan, J.; Zhang, S.; Pan, Z.; Zhao, S.; Guo, Q.; Qi, Y.; et al. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv. Sci. 2022, 9, e2105451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Uchiyama, J.; Imami, K.; Ishihama, Y.; Kageyama, R.; Kobayashi, T. Novel Roles of Small Extracellular Vesicles in Regulating the Quiescence and Proliferation of Neural Stem Cells. Front. Cell. Dev. Biol. 2021, 9, 762293. [Google Scholar] [CrossRef]
- Vogel, A.D.; Upadhya, R.; Shetty, A.K. Neural stem cell derived extracellular vesicles: Attributes and prospects for treating neurodegenerative disorders. EBioMedicine 2018, 38, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Tian, J.; Xiao, D.; Guo, Y.X.; Xiao, Y.; Wu, X.Y.; Casella, G.; Rasouli, J.; Yan, Y.P.; Rostami, A.; et al. Engineered extracellular vesicles encapsulated Bryostatin-1 as therapy for neuroinflammation. Nanoscale 2022, 14, 2393–2410. [Google Scholar] [CrossRef]
- Martinez-Cerdeno, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104. [Google Scholar] [CrossRef]
- Tian, T.; Liang, R.; Erel-Akbaba, G.; Saad, L.; Obeid, P.J.; Gao, J.; Chiocca, E.A.; Weissleder, R.; Tannous, B.A. Immune Checkpoint Inhibition in GBM Primed with Radiation by Engineered Extracellular Vesicles. ACS Nano 2022, 16, 1940–1953. [Google Scholar] [CrossRef]
- Marinaro, F.; Gomez-Serrano, M.; Jorge, I.; Silla-Castro, J.C.; Vazquez, J.; Sanchez-Margallo, F.M.; Blazquez, R.; Lopez, E.; Alvarez, V.; Casado, J.G. Unraveling the Molecular Signature of Extracellular Vesicles From Endometrial-Derived Mesenchymal Stem Cells: Potential Modulatory Effects and Therapeutic Applications. Front. Bioeng. Biotechnol. 2019, 7, 431. [Google Scholar] [CrossRef] [PubMed]
- Mobahat, M.; Sadroddiny, E.; Nooshabadi, V.T.; Ebrahimi-Barough, S.; Goodarzi, A.; Malekshahi, Z.V.; Ai, J. Curcumin-loaded human endometrial stem cells derived exosomes as an effective carrier to suppress alpha-synuclein aggregates in 6OHDA-induced Parkinson’s disease mouse model. Cell Tissue Bank. 2022, 24, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Lakshmipathy, U.; Pelacho, B.; Sudo, K.; Linehan, J.L.; Coucouvanis, E.; Kaufman, D.S.; Verfaillie, C.M. Efficient transfection of embryonic and adult stem cells. Stem Cells 2004, 22, 531–543. [Google Scholar] [CrossRef]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic Stem Cells-Derived Exosomes Endowed with Targeting Properties as Chemotherapeutics Delivery Vehicles for Glioblastoma Therapy. Adv. Sci. 2019, 6, 1801899. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Seeger, R.C.; Fabbri, M.; Wang, L.; Wayne, A.S.; Jong, A.Y. Biological roles and potential applications of immune cell-derived extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1400370. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Peng, H.; Liu, R.; Ji, W.; Shi, Z.; Shen, J.; Ma, G.; Zhang, X. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing alpha-synuclein and immune activation of Parkinson’s disease. Sci. Adv. 2020, 6, eaba3967. [Google Scholar] [CrossRef]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y.; et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, X.; Dou, Y.; Wang, M.; Zhao, Y.; Yang, Q.; Zhao, Y. The Immuno-Modulation Effect of Macrophage-Derived Extracellular Vesicles in Chronic Inflammatory Diseases. Front. Immunol. 2021, 12, 785728. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Cao, Y.; Liu, Z. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma. Adv. Mater. 2022, 34, e2110364. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Chen, J.; Sun, Y.; Wang, Y.; Xia, B.; Tan, H.; Pan, C.; Gu, G.; Zhong, J.; Qing, G.; et al. Functionalized Macrophage Exosomes with Panobinostat and PPM1D-siRNA for Diffuse Intrinsic Pontine Gliomas Therapy. Adv. Sci. 2022, 9, e2200353. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, W.; Yang, M.; Yin, Y.; Li, H.; Hu, F.; Tang, L.; Ma, X.; Zhang, Y.; Wang, Y. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials 2021, 273, 120784. [Google Scholar] [CrossRef] [PubMed]
- Chulpanova, D.S.; Kitaeva, K.V.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Therapeutic Prospects of Extracellular Vesicles in Cancer Treatment. Front. Immunol. 2018, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, J.; Wang, Q.; Yan, L.; Wang, L.; Xing, Z.; Wang, C.; Zhang, J.; Dong, L. Delivering Antisense Oligonucleotides across the Blood-Brain Barrier by Tumor Cell-Derived Small Apoptotic Bodies. Adv. Sci. 2021, 8, 2004929. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Patil, K.; Ramm, G.A.; Ochiya, T.; Soekmadji, C. Extracellular vesicles in the development of organ-specific metastasis. J. Extracell. Vesicles 2021, 10, e12125. [Google Scholar] [CrossRef]
- Quezada, C.; Torres, A.; Niechi, I.; Uribe, D.; Contreras-Duarte, S.; Toledo, F.; San Martin, R.; Gutierrez, J.; Sobrevia, L. Role of extracellular vesicles in glioma progression. Mol. Aspects Med. 2018, 60, 38–51. [Google Scholar] [CrossRef]
- Wang, R.; Liang, Q.; Zhang, X.; Di, Z.; Wang, X.; Di, L. Tumor-derived exosomes reversing TMZ resistance by synergistic drug delivery for glioma-targeting treatment. Colloids Surf. B. Biointerfaces 2022, 215, 112505. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Yao, Z. A mini-review: Advances in plant-derived extracellular vesicles as nano-delivery systems for tumour therapy. Front. Bioeng. Biotechnol. 2022, 10, 1076348. [Google Scholar] [CrossRef]
- Wang, B.; Guo, X.-J.; Cai, H.; Zhu, Y.-H.; Huang, L.-Y.; Wang, W.; Luo, L.; Qi, S.-H. Momordica charantia-derived extracellular vesicles-like nanovesicles inhibited glioma proliferation, migration, and invasion by regulating the PI3K/AKT signaling pathway. J. Funct. Foods 2022, 90, 104968. [Google Scholar] [CrossRef]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Cui, X.; Li, X.; Yang, S.; Wang, Q.; Fang, C.; Tan, Y.; Li, L.; Xu, C.; et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro Oncol. 2022, 24, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, C.; Zhang, Y.; Alsrouji, O.K.; Chebl, A.B.; Ding, G.; Jiang, Q.; Mayer, S.A.; Lu, M.; Kole, M.K.; et al. Cerebral endothelial cell-derived small extracellular vesicles enhance neurovascular function and neurological recovery in rat acute ischemic stroke models of mechanical thrombectomy and embolic stroke treatment with tPA. J. Cereb. Blood Flow. Metab. 2021, 41, 2090–2104. [Google Scholar] [CrossRef]
- Munir, J.; Ngu, A.; Wang, H.; Ramirez, D.M.O.; Zempleni, J. Review: Milk Small Extracellular Vesicles for Use in the Delivery of Therapeutics. Pharm. Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Gupta, R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef]
- Geng, W.; Tang, H.; Luo, S.; Lv, Y.; Liang, D.; Kang, X.; Hong, W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 2019, 11, 780–792. [Google Scholar]
- Chen, H.X.; Liang, F.C.; Gu, P.; Xu, B.L.; Xu, H.J.; Wang, W.T.; Hou, J.Y.; Xie, D.X.; Chai, X.Q.; An, S.J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell. Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef]
- Tian, T.; Cao, L.; He, C.; Ye, Q.; Liang, R.; You, W.; Zhang, H.; Wu, J.; Ye, J.; Tannous, B.A.; et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics 2021, 11, 6507–6521. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kong, J.; Ma, S.; Li, J.; Mao, M.; Chen, K.; Chen, Z.; Zhang, J.; Chang, Y.; Yuan, H.; et al. Exosome-Coated B-10 Carbon Dots for Precise Boron Neutron Capture Therapy in a Mouse Model of Glioma In Situ. Adv. Funct. Mater. 2021, 31, 2100969. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Li, Z.; Peng, Y.; Tan, H.; Wang, C.; Huang, G.; Li, W.; Ma, G.; Wei, W. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal. Transduct. Target. Ther. 2022, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Cheng, P.; Pu, K. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small 2019, 15, e1804105. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, W.; Ashley, J.; Zhang, M.; Feng, X.; Shen, J.; Sun, Y. Self-Assembly Protein Superstructures as a Powerful Chemodynamic Therapy Nanoagent for Glioblastoma Treatment. Nano-Micro Lett. 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chu, X.; Gong, W.; Zheng, J.; Xie, X.; Wang, Y.; Yang, M.; Li, Z.; Gao, C.; Yang, Y. Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer’s disease. J. Nanobiotechnol. 2020, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Zhao, Y.; Liu, C.; Yang, Y.; Wang, Z.H.; Yu, W.; Zhang, K.; Zhang, Z.; Liu, J.; Zhang, Y.; et al. Engineered Nanoerythrocytes Alleviate Central Nervous System Inflammation by Regulating the Polarization of Inflammatory Microglia. Adv. Mater. 2022, 34, e2201322. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Peng, X.; Sun, Z.; Zheng, W.; Yu, J.; Du, L.; Chen, H.; Gong, P.; Zhang, P.; Cai, L.; et al. Natural-Killer-Cell-Inspired Nanorobots with Aggregation-Induced Emission Characteristics for Near-Infrared-II Fluorescence-Guided Glioma Theranostics. ACS Nano 2020, 14, 11452–11462. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, e2101526. [Google Scholar] [CrossRef]
- Cao, H.; Dan, Z.; He, X.; Zhang, Z.; Yu, H.; Yin, Q.; Li, Y. Liposomes Coated with Isolated Macrophage Membrane Can Target Lung Metastasis of Breast Cancer. ACS Nano 2016, 10, 7738–7748. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.H.; Massagué, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 2011, 20, 538–549. [Google Scholar] [CrossRef]
- Lai, J.; Deng, G.; Sun, Z.; Peng, X.; Li, J.; Gong, P.; Zhang, P.; Cai, L. Scaffolds biomimicking macrophages for a glioblastoma NIR-Ib imaging guided photothermal therapeutic strategy by crossing Blood-Brain Barrier. Biomaterials 2019, 211, 48–56. [Google Scholar] [CrossRef]
- Xiao, T.; He, M.; Xu, F.; Fan, Y.; Jia, B.; Shen, M.; Wang, H.; Shi, X. Macrophage Membrane-Camouflaged Responsive Polymer Nanogels Enable Magnetic Resonance Imaging-Guided Chemotherapy/Chemodynamic Therapy of Orthotopic Glioma. ACS Nano 2021, 15, 20377–20390. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Fan, Q.; Hu, F.; Ma, X.; Yin, Y.; Wang, B.; Kuang, L.; Hu, X.; Xu, B.; Wang, Y. Engineered Macrophage-Membrane-Coated Nanoparticles with Enhanced PD-1 Expression Induce Immunomodulation for a Synergistic and Targeted Antiglioblastoma Activity. Nano Lett. 2022, 22, 6606–6614. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Dou, C.; Xia, Y.; Li, B.; Zhao, M.; Yu, P.; Zheng, Y.; El-Toni, A.M.; Atta, N.F.; Galal, A.; et al. Neutrophil-like Cell-Membrane-Coated Nanozyme Therapy for Ischemic Brain Damage and Long-Term Neurological Functional Recovery. ACS Nano 2021, 15, 2263–2280. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Gao, J.; Zhang, C.Y.; Hayworth, C.; Frank, M.; Wang, Z. Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke. ACS Nano 2019, 13, 1272–1283. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Li, F.; Wang, S.; Zhao, J.; Wang, J.; Liu, J.; Lyu, C.; Ye, P.; Tan, H.; et al. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat. Commun. 2022, 13, 4214. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Wu, Y.; Song, X.; Zhang, S.; Liu, Z. Camouflaging Nanoparticles with Brain Metastatic Tumor Cell Membranes: A New Strategy to Traverse Blood–Brain Barrier for Imaging and Therapy of Brain Tumors. Adv. Funct. Mater. 2020, 30, 1909369. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Wang, S.; Zou, Y.; Zheng, M.; Shi, B. Brain-Targeting Metastatic Tumor Cell Membrane Cloaked Biomimetic Nanomedicines Mediate Potent Chemodynamic and RNAi Combinational Therapy of Glioblastoma. Adv. Funct. Mater. 2022, 32, 2209239. [Google Scholar] [CrossRef]
- He, W.; Mei, Q.; Li, J.; Zhai, Y.; Chen, Y.; Wang, R.; Lu, E.; Zhang, X.Y.; Zhang, Z.; Sha, X. Preferential Targeting Cerebral Ischemic Lesions with Cancer Cell-Inspired Nanovehicle for Ischemic Stroke Treatment. Nano Lett. 2021, 21, 3033–3043. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Liu, J.; Liu, F.; Du, F.; Li, M.; Chen, A.T.; Bao, Y.; Suh, H.W.; Avery, J.; et al. Targeted Drug Delivery to Stroke via Chemotactic Recruitment of Nanoparticles Coated with Membrane of Engineered Neural Stem Cells. Small 2019, 15, e1902011. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, M.; Zeng, Y.; Miao, T.; Luo, H.; Tong, Y.; Zhao, M.; Mu, R.; Gu, J.; Yang, S.; et al. Biomimetic Lipopolysaccharide-Free Bacterial Outer Membrane-Functionalized Nanoparticles for Brain-Targeted Drug Delivery. Adv. Sci. 2022, 9, e2105854. [Google Scholar] [CrossRef]
- Hao, W.; Cui, Y.; Fan, Y.; Chen, M.; Yang, G.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Yang, Y.; et al. Hybrid membrane-coated nanosuspensions for multi-modal anti-glioma therapy via drug and antigen delivery. J. Nanobiotechnol. 2021, 19, 378. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Cao, X.; Liu, Q.; Zhu, Q.; Liu, K.; Deng, T.; Yu, Q.; Deng, W.; Yu, J.; Wang, Q.; et al. Hybrid Membrane-Derived Nanoparticles for Isoliquiritin Enhanced Glioma Therapy. Pharmaceuticals 2022, 15, 1059. [Google Scholar] [CrossRef]
- Yin, Y.; Tang, W.; Ma, X.; Tang, L.; Zhang, Y.; Yang, M.; Hu, F.; Li, G.; Wang, Y. Biomimetic neutrophil and macrophage dual membrane-coated nanoplatform with orchestrated tumor-microenvironment responsive capability promotes therapeutic efficacy against glioma. Chem. Eng. J. 2022, 433, 133848. [Google Scholar] [CrossRef]
- Wang, G.G.; Calvo, K.R.; Pasillas, M.P.; Sykes, D.B.; Hacker, H.; Kamps, M.P. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 2006, 3, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.Y.; Peng, Z.; Luo, H.; Loison, F. Isolation of Human Neutrophils from Whole Blood and Buffy Coats. J. Vis. Exp. 2021, 175, e62837. [Google Scholar] [CrossRef]
- Belhadj, Z.; He, B.; Fu, J.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. Regulating Interactions Between Targeted Nanocarriers and Mononuclear Phagocyte System via an Esomeprazole-Based Preconditioning Strategy. Int. J. Nanomed. 2020, 15, 6385–6399. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Li, Y.J.; Wu, J.Y.; Liu, J.; Xu, W.; Qiu, X.; Huang, S.; Hu, X.B.; Xiang, D.X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnol. 2021, 19, 242. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.J.; Hu, X.B.; Huang, S.; Luo, S.; Tang, T.; Xiang, D.X. Exosomes and biomimetic nanovesicles-mediated anti-glioblastoma therapy: A head-to-head comparison. J. Control. Release 2021, 336, 510–521. [Google Scholar] [CrossRef]
- Zhou, X.; Miao, Y.; Wang, Y.; He, S.; Guo, L.; Mao, J.; Chen, M.; Yang, Y.; Zhang, X.; Gan, Y. Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J. Extracell. Vesicles 2022, 11, e12198. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.P.; Mastorakos, P.; Suk, J.S.; Klibanov, A.L.; Hanes, J.; Price, R.J. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J. Control. Release 2016, 223, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wan, Z.; Wang, C.; Lu, F.; Wei, M.; Wang, D.; Hao, Q. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics 2021, 11, 8185–8196. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, H.; Yoon, S.; Lee, H.; Hwang, J.; Jung, J.; Chang, J.H.; Choi, J.; Kim, H. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J. Control. Release 2021, 330, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Shao, S.; Shen, Y.; Wang, K. Macrophages as Active Nanocarriers for Targeted Early and Adjuvant Cancer Chemotherapy. Small 2016, 12, 5108–5119. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, A.; Wang, Y.; Zhang, J.; Huang, Y. Living Cells and Cell-Derived Vesicles: A Trojan Horse Technique for Brain Delivery. Pharmaceutics 2023, 15, 1257. https://doi.org/10.3390/pharmaceutics15041257
Ou A, Wang Y, Zhang J, Huang Y. Living Cells and Cell-Derived Vesicles: A Trojan Horse Technique for Brain Delivery. Pharmaceutics. 2023; 15(4):1257. https://doi.org/10.3390/pharmaceutics15041257
Chicago/Turabian StyleOu, Ante, Yuewei Wang, Jiaxin Zhang, and Yongzhuo Huang. 2023. "Living Cells and Cell-Derived Vesicles: A Trojan Horse Technique for Brain Delivery" Pharmaceutics 15, no. 4: 1257. https://doi.org/10.3390/pharmaceutics15041257
APA StyleOu, A., Wang, Y., Zhang, J., & Huang, Y. (2023). Living Cells and Cell-Derived Vesicles: A Trojan Horse Technique for Brain Delivery. Pharmaceutics, 15(4), 1257. https://doi.org/10.3390/pharmaceutics15041257





