From Batch to the Semi-Continuous Flow Hydrogenation of pNB, pNZ-Protected Meropenem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Procedure for the Synthesis of (4R,5S,6S)-(p-Nitrobenzyl)-3-[[(3S,5S)-1-(p-nitrobenzyloxycarbonyl)-5-(dimethylaminocarbonyl)-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate (4)
2.1.2. General Procedure Applied in All Batch-Mode and MW-Assisted Flow-Mode Experiments for the Synthesis of Meropenem (1)
2.2. Hydrogenation Reactions
2.2.1. Reaction in Batch Mode
2.2.2. Reaction in MW-Assisted Flow Mode
3. Results and Discussion
| Entry | Cycles | Temperature (°C) | Total Residence Time (s) | Meropenem (1) Yield in Solution (%) after Catalyst Filtration | Crude Meropenem (1) Yields (%) |
|---|---|---|---|---|---|
| 23 | 4 | 45 | 840 | 80 | 70 |
| 24 | 4 | 55 | 840 | 75 | 65 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Armstrong, T.; Fenn, S.J.; Hardie, K.R. JMM profile: Carbapenems: A broad-spectrum antibiotic. J. Med. Microbiol. 2021, 70, 001462. [Google Scholar] [CrossRef]
- Shah, P.M. Parenteral carbapenems. Clin. Microbiol. Infect. 2008, 14, 175–180. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Orsi, G.B.; Falcone, M.; Venditti, M. Surveillance and management of multidrug-resistant microorganisms. Expert Rev. Anti Infect. Ther. 2011, 9, 653–679. [Google Scholar] [CrossRef]
- Bradley, J.S.; Garau, J.; Lode, H.; Rolston, K.V.; Wilson, S.E.; Quinn, J.P. Carbapenems in clinical practice: A guide to their use in serious infection. Int. J. Antimicrob. Agents 1999, 11, 93–100. [Google Scholar] [CrossRef]
- El-Gamal, M.; Brahim, I.; Hisham, N.; Aladdin, R.; Mohammed, H.; Bahaaeldin, A. Recent updates of carbapenem antibiotics. Eur. J. Med. Chem. 2017, 5, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs). Clin. Microbiol. Infect. 2000, 6, 460–463. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Torres, J.A.; Villegas, M.V.; Quinn, J.P. Current concepts in antibiotic-resistant gram-negative bacteria. Expert Rev. Anti Infect. Ther. 2007, 5, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, G.; Russo, G.; Nicoletti, G. Recent developments in carbapenems. Expert Opin. Investig. Drugs 2002, 11, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. β-Lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.K.; Smith, C.A.; Frase, H.; Black, D.J.; Vakulenko, S.B. Kinetic and structural requirements of carbapenems activity in GES-type β-lactamases. Biochemistry 2015, 54, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-lactamases and β-lactamase inhibitors in the 21st century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Wiebe, R.; Dilay, L.; Thomson, K.; Rubinstein, E.; Hoban, D.J.; Noreddin, A.M.; Karlowsky, J.A. Comparative review of the carbapenems. Drugs 2007, 67, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P. Carbapenems: A potent class of antibiotics. Expert Opin. Pharmacother. 2008, 9, 23–37. [Google Scholar] [CrossRef]
- Kattan, J.N.; Villegas, M.V.; Quinn, J.P. New developments in carbapenems. Clin. Microbiol. Infect. 2008, 14, 1102–1111. [Google Scholar] [CrossRef]
- Baughman, R.P. The use of carbapenems in the treatment of serious infections. J. Intensive Care Med. 2009, 24, 230–241. [Google Scholar] [CrossRef]
- Breilh, D.; Texier-Maugein, J.; Allaouchiche, B.; Saux, M.C.; Boselli, E. Carbapenems. J. Chemother. 2013, 25, 1–17. [Google Scholar] [CrossRef]
- Moellering, R.C., Jr.; Eliopoulus, G.M.; Sentochnik, D.E. The carbapenems: New broad spectrum beta-lactam antibiotics. J. Antimicrob. Chemother. 1989, 24, 1–7. [Google Scholar] [CrossRef]
- Kahan, J.S.; Kahan, F.M.; Goegelman, R.; Currie, S.A.; Jackson, M.; Stapley, E.O.; Miller, T.W.; Miller, A.K.; Hendlin, D.; Mochales, S.; et al. Thienamycin, a new beta-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J. Antibiot. 1979, 32, 1–12. [Google Scholar] [CrossRef]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Basker, M.J.; Boon, R.J.; Hunter, P.A. Comparative antibacterial properties in vitro of seven olivanic acid derivates: MM4550, MM13902, MM17880, MM22380, MM22381, MM22382 and MM22383. J. Antibiot. 1980, 33, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Turner, P.J.; Wannop, C.; Withnell, E.S.; Grindey, A.J.; Nairn, K. In vitro antibacterial activity of SM-7338, a carbapenem antibiotic with stability to dehydropeptidase I. Antimicrob. Agents Chemother. 1989, 33, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, X.; Wang, S.; Fleming, J.; Wang, D.C.; Liu, W. The mechanism of NDM-1-catalyzed carbapenem hydrolysis is distinct from that of penicillin or cephalosporin hydrolysis. Nat. Commun. 2017, 8, 2242. [Google Scholar] [CrossRef]
- Woodward, R.B. Penems and related substances. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 239–250. [Google Scholar] [CrossRef]
- Raza, A.; Ngieng, S.C.; Sime, F.B.; Cabot, P.J.; Roberts, J.A.; Popat, A.; Kumeria, T.; Falconer, J.R. Oral meropenem for superbugs: Challenges and opportunities. Drug Discov. Today 2021, 26, 551–560. [Google Scholar] [CrossRef]
- Slama, T.G. Clinical review: Balancing the therapeutic, safety, and economic issues underlying effective antipseudomonal carbapenem use. Crit. Care 2008, 12, 233. [Google Scholar] [CrossRef]
- Cuzzolin, L.; Agostino, R. Antibiotic use in a cohort of extremely low birth weight neonates: Focus on off-label uses and prescription behaviour. Pharmacol. Pharm. 2018, 9, 382–394. [Google Scholar] [CrossRef]
- Meropenem Market Size with Emerging Trends 2022|Top Key Players Updates, Business Growing Strategies, Competitive Dynamics, Industry Segmentation and Forecast to 2027. Available online: https://www.marketwatch.com/press-release/meropenem-market-size-with-emerging-trends-2022-top-key-players-updates-business-growing-strategies-competitive-dynamics-industry-segmentation-and-forecast-to-2027-2022-11-07 (accessed on 4 February 2023).
- Carbapenem Market Share, Size, Trends, Industry Analysis Report. Available online: https://polarismarketresearch.com/industry-analysis/carbapenem-market#:~:text=The%20global%20carbapenem%20market%20was,serious%20or%20dangerous%20bacterial%20infections (accessed on 4 February 2023).
- Sunagawa, M.; Matsumura, H.; Inoue, T.; Fukasawa, M.; Kato, M. A novel carbapenem antibiotic, SM-7338 structure-activity relationships. J. Antibiot. 1990, 43, 519–532. [Google Scholar] [CrossRef]
- Sunagawa, M.; Matsumura, H.; Inoue, T.; Fukasawa, M.; Kato, M. Carboxylic Thio-Pyrrolidinyl Beta-Lactam Compounds and Production Thereof. EP 0,126,587, 9 May 1984. [Google Scholar]
- Sunagawa, M. β-Lactam Compounds. U.S. Patent 4,943,569, 24 July 1990. [Google Scholar]
- Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050706s037lbl.pdf (accessed on 5 February 2023).
- Thomas, C.; Priano, J.; Smith, T.L. Meropenem as an antidote for intentional valproic acid overdose. Am. J. Emerg. Med. 2020, 38, 690.e1–690.e2. [Google Scholar] [CrossRef] [PubMed]
- Steffens, N.A.; Zimmermann, E.S.; Nichelle, S.M.; Brucker, N. Meropenem use and therapeutic drug monitoring in clinical practice: A literature review. J. Clin. Pharm. Ther. 2021, 46, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S. Meropenem. Pediatr. Infect. Dis. J. 1998, 17, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, L.R.; Wagstaff, A.J.; Brogden, R.N.; Bryson, H.M. Meropenem. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs 1995, 50, 73–101. [Google Scholar] [CrossRef]
- Pascale, R.; Giannella, M.; Bartoletti, M.; Viale, P.; Pea, F. Use of meropenem in treating carbapenem-resistant Enterobacteriaceae infections. Expert Rev. Anti Infect. Ther. 2019, 17, 819–827. [Google Scholar] [CrossRef]
- Dhillon, S. Meropenem/vaborbactam: A review in complicated urinary tract infections. Drugs 2018, 78, 1259–1270. [Google Scholar] [CrossRef]
- Novelli, A.; Del Giacomo, P.; Rossolini, G.M.; Tumbarello, M. Meropenem/vaborbactam: A next generation β-lactam β-lactamase inhibitor combination. Expert Rev. Anti Infect. Ther. 2020, 18, 643–655. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Rybak, M.J. Meropenem and vaborbactam: Stepping up the battle against carbapenem-resistant Enterobacteriaceae. Pharmacotherapy 2018, 38, 444–461. [Google Scholar] [CrossRef]
- Cho, J.C.; Zmarlicka, M.T.; Shaeer, K.M.; Pardo, J. Meropenem/vaborbactam, the first carbapenem/β-lactamase inhibitor combination. Ann. Pharmacother. 2018, 52, 769–779. [Google Scholar] [CrossRef]
- WHO Model List of Essential Medicines. Available online: https://en.wikipedia.org/wiki/WHO_Model_List_of_Essential_Medicines (accessed on 5 February 2023).
- Takeuchi, Y.; Inoue, T.; Sunagawa, M. Studies on the structures of meropenem (SM-7338) and it’s primary metabolite. J. Antibiot. 1993, 46, 827–832. [Google Scholar] [CrossRef]
- Sunagawa, M.; Sasaki, A.; Matsumura, H.; Goda, K.; Tamoto, K. Synthetic studies of carbapenem and penem antibiotics. V. Efficient synthesis of 1β-methylcarbapenem skeleton. Chem. Pharm. Bull. 1994, 42, 1381–1387. [Google Scholar] [CrossRef]
- Tewari, N.; Nizar, H.; Rai, B.P.; Singh, S.K.; George, V.; Prasad, M. An improved procedure for the preparation of carbapenem antibiotic: Meropenem. Org. Process Res. Dev. 2007, 11, 773–775. [Google Scholar] [CrossRef]
- Prashad, A.S.; Vlahos, N.; Fabio, P.; Feigelson, G.B. A highly refined version of the α-keto ester based carbapenem synthesis: The total synthesis of meropenem. Tetrahedron Lett. 1998, 39, 7035–7038. [Google Scholar] [CrossRef]
- Shih, D.H.; Baker, F.; Cama, L.; Christensen, B.G. Synthetic carbapenem antibiotics. I. 1-β-methylcarbapenem. Heterocycles 1984, 21, 29–40. [Google Scholar] [CrossRef]
- Iannazzo, L.; Soroka, D.; Triboulet, S.; Fonvielle, M.; Compain, F.; Dubèe, V.; Mainardi, J.L.; Hugonnet, J.E.; Braud, E.; Arthur, M.; et al. Routes of synthesis of carbapenems for optimizing both the inactivation of L,D-transpeptidase LdtMt1 of Mycobacterium tuberculosis and the stability toward hydrolysis by β-lactamse BlaC. J. Med. Chem. 2016, 59, 3427–3438. [Google Scholar] [CrossRef] [PubMed]
- Surulichamy, S.; Sekar, S.; Deshpande, P.N.; Ganpathy, P.; Sarangdhar, R.J.; Henry, S.S.; Karale, S.N.; Jangale, A.A.; Kaldate, R.D. An Improved Process for the Preparation of Beta-Lactam Antibiotic. WO 2007/031858, 22 March 2007. [Google Scholar]
- Sunagawa, M.; Isobe, Y.; Takeuchi, Y.; Matsumura, H.; Ozaki, Y.; Noguchi, Y. Carbapenem Compound and in Crystalline Form, and Its Production and Use. U.S. Patent 4,888,344, 19 December 1989. [Google Scholar]
- Nadenik, P.; Storm, O.; Kremminger, P. Meropenem Intermediate in Crystalline Form. WO 2005/118586, 15 December 2005. [Google Scholar]
- Tewari, N.; Meeran, H.N.P.N.; Rai, B.P.; Kumar, Y. A Process for the Preparation of Meropenem. WO 2006/035300, 6 April 2006. [Google Scholar]
- Williams, J.M.; Jobson, R.B. Process for Synthesizing Carbapenem Antibiotics. U.S. Patent 5,872,250, 16 February 1999. [Google Scholar]
- Nishino, K.; Koga, T. Improved Process for Producing Carbapenem Compound. EP 2,006,290, 24 December 2008. [Google Scholar]
- Song, Y.S.; Park, S.W.; Yoon, Y.J.; Yoon, H.K.; Moon, S.C.; Lee, B.G.; Choi, S.J.; Jun, S.A. Improved Method for Preparing Meropenem Using Zinc Powder. EP 2,407,468, 18 January 2012. [Google Scholar]
- Manca, A.; Monguzzi, R.A. Process for Synthesizing Carbapenem Using Raney Nichel. EP 2,141,167, 6 January 2010. [Google Scholar]
- Sunagawa, M.; Matsumura, H.; Inoue, T.; Fukasawa, M.; Kato, M. β-Lactam Compounds. U.S. Patent 5,122,604, 16 June 1992. [Google Scholar]
- Sunagawa, M.; Matsumura, H. Process for Preparing Carbapenem Compounds. U.S. Patent 5,578,722, 26 November 1996. [Google Scholar]
- Tewari, N.; Mane, A.; Rai, B.P.; Prasad, M. Process for the Preparation of Carbapenems. U.S. Patent 2007/0197781, 23 August 2007. [Google Scholar]
- Khemka, A.A.; Shejul, P.B.; Vyavahare, A.M.; Pandey, D.K.; Shete, S.N.; Jadhav, H.K.; Kadam, N.H. Meropenem Intermediate in Novel Crystalline Form and a Method of Manufacture of Meropenem. U.S. Patent 2009/0299057, 3 December 2009. [Google Scholar]
- Zhang, H. A Process for the Preparation of Meropenem. WO 2007/104221, 20 September 2007. [Google Scholar]
- Karale, S.N.; Jangale, A.A.; Kaldate, R.D. An Improved Process for the Preparation of Meropenem. WO 2011/141847, 17 November 2011. [Google Scholar]
- Gnanaprakasam, A.; Veermani, G.; Shahul Hameed, S.I.; Murugesan, K.; Thangavel, S.; Sekar Jeyaraj, M.; Thangavel, A.; Gautam Kumar, D. A Process for Preparation of Meropenem. WO 2012/160576, 29 November 2012. [Google Scholar]
- Cookson, J.; McNair, R.; Satoskar, D.V. Process for Preparing a Carbapenem Antibiotic. WO 2015/145161, 1 October 2015. [Google Scholar]
- Grünewald, E.; Weidlich, S.; Jantke, R. Process for the Deprotection of a Carbapenem by Heterogeneous Catalytic Hydrogenation with Hydrogen in the Presence of an Organic Amine. WO 2018/010974, 18 January 2018. [Google Scholar]
- Isidro-Llobet, A.; Guasch-Camell, J.; Alvarez, M.; Albericio, F. p-Nitrobenzyloxycarbonyl (pNZ) as a temporary Nα-protecting group in orthogonal solid-phase peptide synthesis—Avoiding diketopiperazine and aspartimide formation. Eur. J. Org. Chem. 2005, 14, 3031–3039. [Google Scholar] [CrossRef]
- Cossar, P.J.; Hizartzidis, L.; Simone, M.I.; McCluskey, A.; Gordon, C.P. The expanding utility of continuous flow hydrogenation. Org. Biomol. Chem. 2015, 13, 7119–7130. [Google Scholar] [CrossRef]
- Irfan, M.; Glasnov, T.N.; Kappe, C.O. Heterogeneous catalytic hydrogenation reactions in continuous-flow reactors. ChemSusChem 2011, 4, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jiano, J.; Song, P.; Nie, W.; Yi, C.; Zhang, Q.; Li, P. Recent progress in continuous-flow hydrogenation. ChemSusChem 2020, 13, 2876–2893. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, M.; O’Brien, M.; Ley, S.V.; Polyzos, A. Flow chemistry: Intelligent processing of gas-liquid transformations using a tube-in-tube reactor. Acc. Chem. Res. 2015, 48, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-flow technology-a tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. Engl. 2015, 54, 6688–6728. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R. The synthesis of active pharmaceuticals ingredients (APIs) using continuous flow chemistry. Beilstein J. Org. Chem. 2015, 11, 1194–1219. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.T.; Hessel, V. Micro reactor and flow chemistry for industrial applications in drug discovery and development. Green Process. Synth. 2012, 1, 149–167. [Google Scholar] [CrossRef]
- Horàkovà, P.; Koči, K. Continuous-flow chemistry and photochemistry for manufacturing of active pharmaceutical ingredients. Molecules. 2022, 27, 8536. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A perspective on continuous flow chemistry in the pharmaceutical industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Burange, A.S.; Osman, S.M.; Luque, R. Understanding flow chemistry for the production of active pharmaceutical ingredients. iScience 2022, 25, 103892. [Google Scholar] [CrossRef]
- De Souza, J.M.; Galaverna, R.; De Souza, A.A.N.; Brocksom, T.J.; Pastre, J.C.; De Souza, R.O.M.A.; De Oliveira, K.T. Impact of continuous flow chemistry in the synthesis of natural products and active pharmaceutical ingredients. An. Acad. Bras. Ciệnc. 2018, 90, 1131–1174. [Google Scholar] [CrossRef]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Koenig, S.G.; Sneddon, H.F. Recent advances in flow chemistry in the pharmaceutical industry. Green Chem. 2017, 19, 1418–1419. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Comito, M.; Monguzzi, R.; Tagliapietra, S.; Palmisano, G.; Cravotto, G. Towards antibiotic synthesis in continuous-flow processes. Molecules 2023, 28, 1421. [Google Scholar] [CrossRef] [PubMed]
- Bath, A.R.; Shalla, A.H.; Dongre, R.S. Microwave assisted one-pot catalyst free green synthesis of new methyl-7-amino-4-oxo-5-phenyl-2-thioxo-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carboxylates as potent in vitro antibacterial and antifungal activity. J. Adv. Res. 2015, 6, 941–948. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006, 5, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Calcio Gaudino, E.; Manzoli, M.; Carnaroglio, D.; Wu, Z.; Grillo, G.; Rotolo, L.; Medlock, J.; Bonrath, W.; Cravotto, G. Sonochemical preparation of alumina spheres loaded with Pd nanoparticles for 2-butyne-1,4-diol semi-hydrogenation in a continuous flow microwave reactor. RSC Adv. 2018, 8, 7029–7039. [Google Scholar] [CrossRef]
- Grillo, G.; Manzoli, M.; Bucciol, F.; Tabasso, S.; Tabanelli, T.; Cavani, F.; Cravotto, G. Hydrogenation of levulinic acid to γ-valerolactone via green microwave-assisted reactions either in continuous flow or solvent-free batch processes. Ind. Eng. Chem. Res. 2021, 60, 16756–16768. [Google Scholar] [CrossRef]
- Bucciol, F.; Tabasso, S.; Grillo, G.; Menegazzo, F.; Signoretto, M.; Manzoli, M.; Cravotto, G. Boosting levulinic acid hydrogenation to value-added 1,4-pentanediol using microwave-assisted gold catalysis. J. Catal. 2019, 380, 267–277. [Google Scholar] [CrossRef]
- Grillo, G.; Cintas, P.; Colia, M.; Calcio Gaudino, E.; Cravotto, G. Process intensification in continuous flow organic synthesis with enabling and hybrid technologies. Front. Chem. Eng. 2022, 4, 966541. [Google Scholar] [CrossRef]
- Martina, K.; Cravotto, G.; Varma, R.S. Impact of microwaves on organic synthesis and strategies toward flow processes and scaling up. J. Org. Chem. 2021, 86, 13857–13872. [Google Scholar] [CrossRef]
- Rinaldi, L.; Carnaroglio, D.; Rotolo, L.; Cravotto, G. A microwave-based chemical factory in the lab: From milligram to multigram preparations. J. Chem. 2015, 2015, 879531. [Google Scholar] [CrossRef]
- Tagliapietra, S.; Calcio Gaudino, E.; Martina, K.; Barge, A.; Cravotto, G. Microwave irradiation in micro-meso-fluidic systems; hybrid technology has issued the challenge. Chem. Rec. 2018, 18, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Choedkiatsakul, I.; Ngaosuwan, K.; Assabumrungrat, S.; Mantegna, S.; Cravotto, G. Biodiesel production in a novel continuous flow microwave reactor. Renew. Energy 2015, 83, 25–29. [Google Scholar] [CrossRef]
- Quality Considerations for Continuous Manufacturing. Guidance for Industry. Available online: https://www.fda.gov/media/121314/download (accessed on 7 February 2023).
- ICH Guideline Q13 on Continuous Manufacturing of Drug Substances and Drug Products. Available online: https://wwwema.europe.eu/en/ich-guideline-q13-continuous-manufacturing-drug-substances-drug-products (accessed on 7 February 2023).
- Comito, M.; Monguzzi, R.; Tagliapietra, S.; Palmisano, G.; Cravotto, G. Cefonicid benzathine salt: A convenient, lean, and high-performance protocol to make an old cephalosporin shine. Antibiotics 2022, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Comito, M.; Monguzzi, R.; Tagliapietra, S.; Palmisano, G.; Cravotto, G. Efficient pilot-scale synthesis of the key cefonicid intermediate at room temperature. Green Process. Synth. 2022, 11, 96–105. [Google Scholar] [CrossRef]


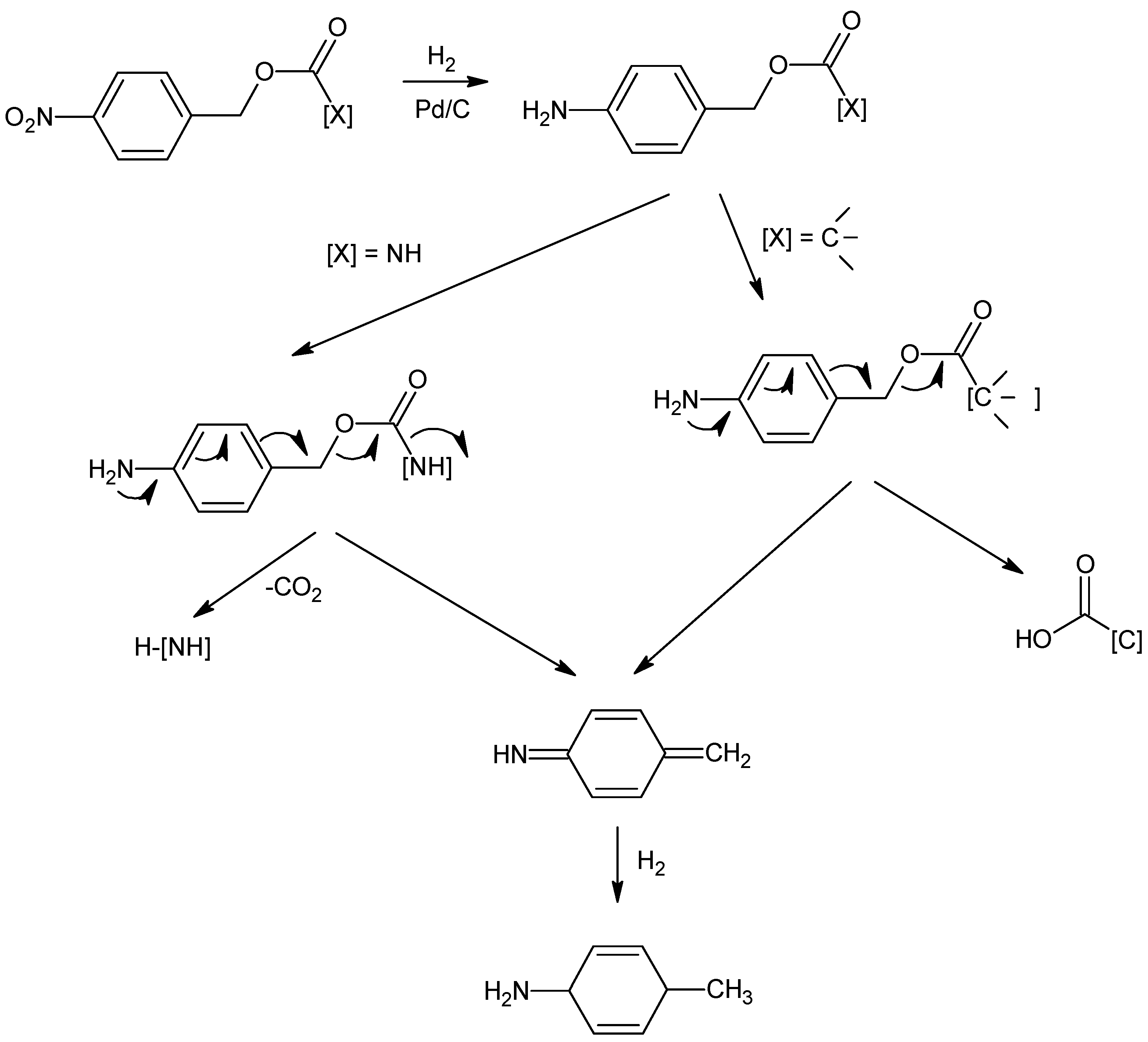
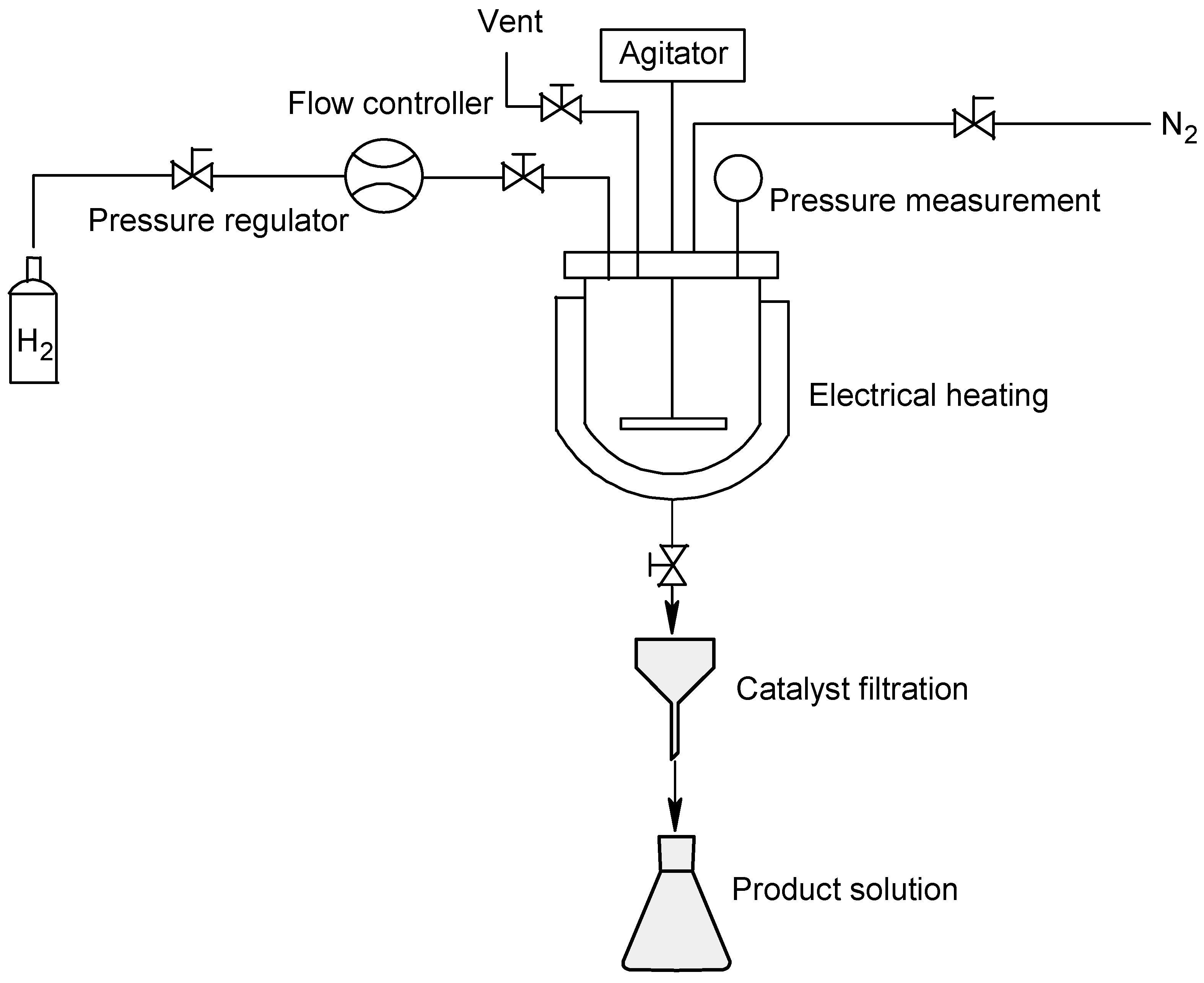


| Entry | H2 (bar) | Temperature (°C) | Catalyst a (g) | Dry Catalyst Weight/Substrate Weight (%) | Time (Min) | Meropenem (1) Yield in Solution (%) after Catalyst Filtration | Crude Meropenem (1) Yields (%) |
|---|---|---|---|---|---|---|---|
| 1 | 20 | 37 | 5.7 | 10.0 | 30 | 66 | - |
| 2 | 20 | 37 | 2.8 | 5.0 | 60 | 63 | - |
| 3 | 20 | 37 | 1.4 | 2.5 | 120 | 53 | - |
| 4 | 6.8 | 30 | 12.0 | 21.0 | 30 | 89 | 74 |
| 5 | 6.8 | 30 | 6.0 | 10.5 | 60 | 84 | 71 |
| 6 | 6.8 | 35 | 6.0 | 10.5 | 60 | 75 | - |
| 7 | 6.8 | 45 | 6.0 | 10.5 | 60 | 60 | - |
| 8 | 6.8 | 30 | 3.0 | 5.3 | 90 | 71 | 61 |
| Entry | Cycle | Bis-Protected Meropenem (4) Residual (%) |
|---|---|---|
| 9 | 1st | 88 |
| 10 | 2nd | 69 |
| 11 | 3rd | 51 |
| 12 | 4th | 38 |
| 13 | 5th | 24 |
| 14 | 6th | 16 |
| 15 | 7th | 10 |
| 16 | 8th | 5 |
| 17 | 9th | 4 |
| 18 | 10th | 3 |
| Entry | Cycle | Bis-Protected Meropenem (4) Residual (%) |
|---|---|---|
| 19 | 1st | 66 |
| 20 | 2nd | 38 |
| 21 | 3rd | 11 |
| 22 | 4th | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comito, M.; Monguzzi, R.; Tagliapietra, S.; Maspero, A.; Palmisano, G.; Cravotto, G. From Batch to the Semi-Continuous Flow Hydrogenation of pNB, pNZ-Protected Meropenem. Pharmaceutics 2023, 15, 1322. https://doi.org/10.3390/pharmaceutics15051322
Comito M, Monguzzi R, Tagliapietra S, Maspero A, Palmisano G, Cravotto G. From Batch to the Semi-Continuous Flow Hydrogenation of pNB, pNZ-Protected Meropenem. Pharmaceutics. 2023; 15(5):1322. https://doi.org/10.3390/pharmaceutics15051322
Chicago/Turabian StyleComito, Marziale, Riccardo Monguzzi, Silvia Tagliapietra, Angelo Maspero, Giovanni Palmisano, and Giancarlo Cravotto. 2023. "From Batch to the Semi-Continuous Flow Hydrogenation of pNB, pNZ-Protected Meropenem" Pharmaceutics 15, no. 5: 1322. https://doi.org/10.3390/pharmaceutics15051322
APA StyleComito, M., Monguzzi, R., Tagliapietra, S., Maspero, A., Palmisano, G., & Cravotto, G. (2023). From Batch to the Semi-Continuous Flow Hydrogenation of pNB, pNZ-Protected Meropenem. Pharmaceutics, 15(5), 1322. https://doi.org/10.3390/pharmaceutics15051322







