A Versatile Brij-Linker for One-Step Preparation of Targeted Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
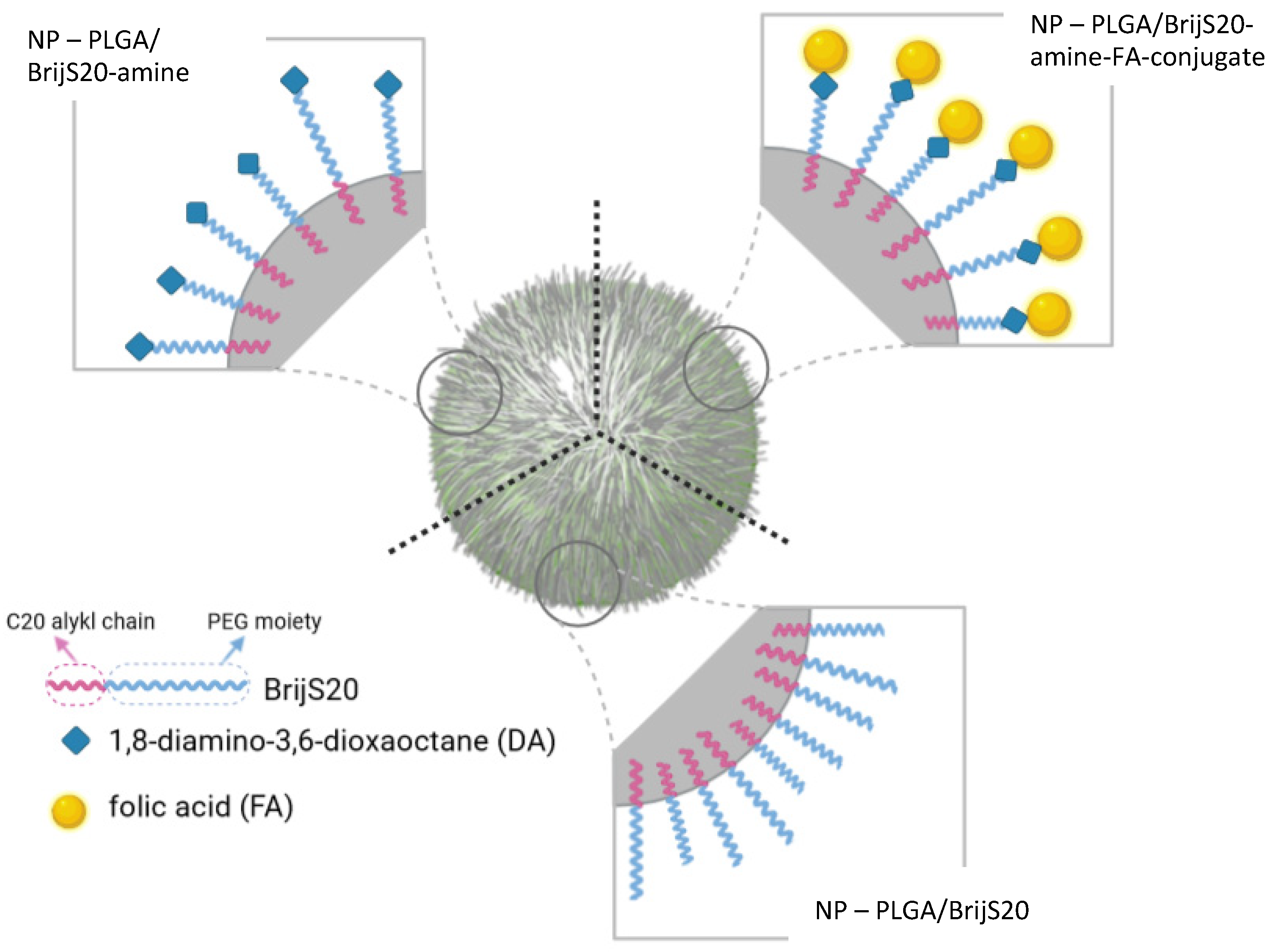
2.2. Preparation of a BrijS20-1,8-Diamino-3,6-Dioxaoctane Building Block (Brij-Amine)
2.3. Conversion of Folic Acid to a Reactive NHS-Ester and Formation of the BrijS20-Amine-Folic Acid Conjugate
2.4. Characterization of BrijS20-Amine, Folic Acid-NHS Ester and the BrijS20-Amine Folic Acid Conjugate
2.5. Preparation of PLGA Nanoparticles with the BrijS20-Amine Building Block or the BrijS20-Amine-Folic Acid-Conjugate
2.6. Characterization of Particle Size and Zeta Potential
2.7. Quantification of Primary Amine Groups and Particle-Associated Folic Acid
2.8. Binding and Internalization of BodiPy-Labelled PLGA Nanoparticles
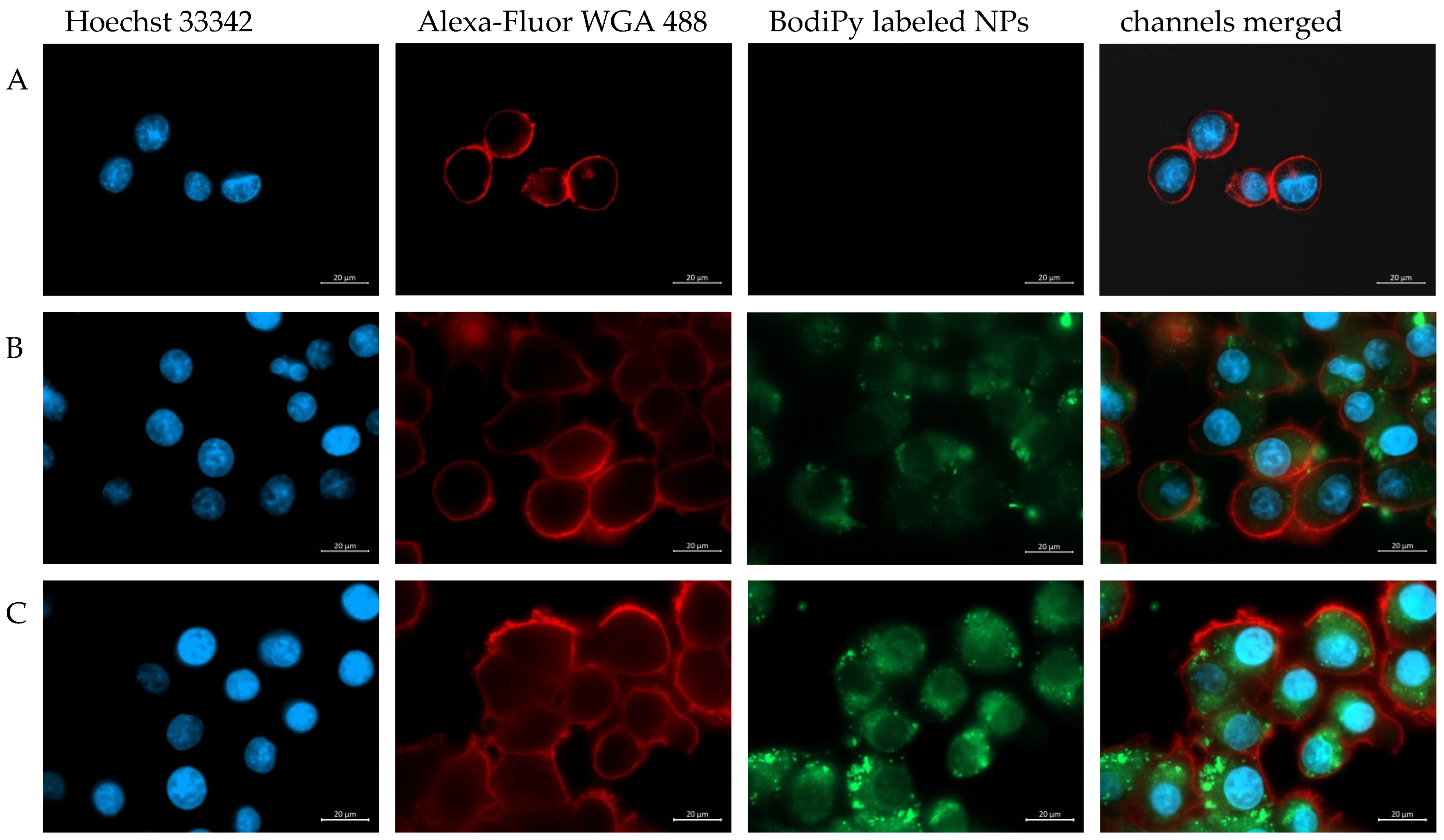
2.9. Microscopic Analysis of the Particle-Cell Interaction
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Spectroscopic Characterization of Brij-Amine and BrijS20-Amine Folic Acid Conjugate
3.2. Preparation and Characterization of Nanoparticles
3.3. Interaction of Nanoparticles with KB Cells
3.4. Microscopic Analysis of Particle Internalization in KB Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, B.; Barick, K.C.; Hassan, P.A. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv. Colloid. Interface Sci. 2021, 296, 102509. [Google Scholar] [CrossRef] [PubMed]
- Ediriwickrema, A.; Saltzman, W.M. Nanotherapy for Cancer: Targeting and Multifunctionality in the Future of Cancer Therapies. ACS Biomater. 2015, 1, 64–78. [Google Scholar] [CrossRef]
- Björnmalm, M.; Thurecht, K.J.; Michael, M.; Scott, A.M.; Caruso, F. Bridging Bio—Nano Science and Cancer Nanomedicine. ACS Nano 2017, 11, 9594–9613. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug. Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Paus, C.; van der Voort, R.; Cambi, A. Nanomedicine in cancer therapy: Promises and hurdles of polymeric nanoparticles. Explor. Med. 2021, 2, 167–185. [Google Scholar] [CrossRef]
- Lane, L.A. Physics in nanomedicine: Phenomena governing the in vivo performance of nanoparticles Physics in nanomedicine: Phenomena governing the in vivo performance of nanoparticles. Appl. Phys. Rev. 2020, 20, 011316. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Makadia, H.; Siegel, S. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Roointan, A.; Kianpour, S.; Memari, F.; Gandomani, M.; Mohammad, S.; Hayat, G.; Mohammadi-samani, S. International Journal. of Polymeric Materials and Poly (lactic-co-glycolic acid): The most ardent and flexible candidate in biomedicine! Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 1028–1049. [Google Scholar] [CrossRef]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA / PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M.; Uskokovic, D. Poly(lactide-co-glycolide)-based Micro and Nanoparticles for the Controlled Drug Delivery of Vitamins. Curr. Nanosci. 2009, 5, 1–14. [Google Scholar]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly (lactic-o-glycolic acid) -based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug—Polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Anzengruber, M.; Wimmer, L.; Szuchar, R.; Skoll, K.; Wirth, M.; Gabor, F. LogP of N-acyl-gemcitabine and lectin-corona emerge as key parameters in nanoparticulate intravesical cancer therapy. Eur. J. Pharm. Sci. 2023, 180, 106330. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Yang, M.; Lai, S.K.; Yu, T.; Wang, Y.; Happe, C.; Zhong, W.; Zhang, M.; Anonuevo, A.; Fridley, C.; Hung, A.; et al. Nanoparticle penetration of human cervicovaginal mucus: The effect of polyvinyl alcohol. J. Control. Release 2014, 192, 202–208. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG—PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Arias, J. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef]
- Rad, A.T.; Chen, C.; Aresh, W.; Xia, Y.; Lai, P. Combinational Effects of Active Targeting Shape, and Enhanced Permeability and Retention for Cancer Theranostic Nanocarriers. ACS Appl. Mater. Interfaces 2019, 11, 10505–10519. [Google Scholar]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Friedl, J.D.; Nele, V.; De Rosa, G.; Bernkop-Schnürch, A. Bioinert, Stealth or Interactive: How Surface Chemistry of Nanocarriers Determines Their Fate In Vivo. Adv. Funct. Mater. 2021, 34, 2103347. [Google Scholar] [CrossRef]
- Xiong, W.; Sang, W.; Linghu, K.G.; Zhong, Z.F.; Cheang, W.S.; Li, J.; Hu, Y.J.; Yu, H.; Wang, Y.T. Dual-functional Brij-S20-modified nanocrystal formulation enhances the intestinal transport and oral bioavailability of berberine. Int. J. Nanomed. 2018, 13, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Voci, S.; Cristina, M.; Fresta, M.; Cosco, D. Brij-stabilized zein nanoparticles as potential drug carriers. Colloids Surfaces B Biointerfaces 2021, 201, 111647. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Hang, S.; Ling, Q.; Gang, K.; Ding, G.; John, M.; Chu, T.; Wong, G.T.C.; Li, J.; Jia, Y.; et al. Brij-functionalized chitosan nanocarrier system enhances the intestinal permeability of P-glycoprotein substrate-like drugs. Carbohydr. Polym. 2021, 266, 118112. [Google Scholar] [CrossRef]
- Tagami, T.; Ernsting, M.J.; Li, S.D. Optimization of a novel and improved thermosensitive liposome formulated with DPPC and a Brij surfactant using a robust in vitro system. J. Control. Release 2011, 154, 290–297. [Google Scholar] [CrossRef]
- Yue, L.; Yan, Z.; Li, H.; Liu, X.; Sun, P. Brij-58, a potential injectable protein-stabilizer used in therapeutic protein formulation. Eur. J. Pharm. Biopharm. 2020, 146, 73–83. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chauhan, P.; Kumar, R.; Bhasin, K.K. Toxicological responses of surfactant functionalized selenium nanoparticles: A quantitative multi-assay approach. Sci. Total. Environ. 2018, 643, 1265–1277. [Google Scholar] [CrossRef]
- Ulbrich, K.; Michaelis, M.; Rothweiler, F.; Knobloch, T.; Sithisarn, P.; Cinatl, J.; Kreuter, J. Interaction of folate-conjugated human serum albumin (HSA) nanoparticles with tumour cells. Int. J. Pharm. 2011, 406, 128–134. [Google Scholar] [CrossRef]
- Fernandez, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef]
- Jurczyk, M.; Jelonek, K.; Musiał-kulik, M.; Beberok, A.; Wrze, D. Single- versus Dual-Targeted Nanoparticles with Folic Acid and Biotin for Anticancer Drug Delivery. Pharmaceutics 2021, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Markert, S.; Lassmann, S.; Gabriel, B.; Klar, M.; Werner, M.; Gitsch, G.; Kratz, F.; Hasenburg, A. Alpha-folate Receptor Expression in Epithelial Ovarian Carcinoma and Non-neoplastic Ovarian Tissue. Anticancer Res. 2008, 28, 3567–3572. [Google Scholar]
- Paulino Da Silva Filho, O.; Ali, M.; Nabbefeld, R.; Primavessy, D.; Bovee-Geurts, P.H.; Grimm, S.; Kirchner, A.; Wiesmüller, K.H.; Schneider, M.; Walboomers, X.F.; et al. A comparison of acyl-moieties for noncovalent functionalization of PLGA and PEG-PLGA nanoparticles with a cell-penetrating peptide. RSC Adv. 2021, 11, 36116–36124. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Mok, S.; Yi, G.; Jin, Y.; Park, H.; Myung, J.; Choi, M.; Koo, H. Folate-modified PLGA nanoparticles for tumor-targeted delivery of pheophorbide a in vivo. Biochem. Biophys. Res. Commun. 2018, 498, 523–528. [Google Scholar] [CrossRef]
- Liu, G.; Mcennis, K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers 2022, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Brauner, B.; Schwarz, P.; Wirth, M.; Gabor, F. Micro vs. nano: PLGA particles loaded with trimethoprim for instillative treatment of urinary tract infections. Int. J. Pharm. 2020, 579, 119158. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth coating of Nanoparticles in drug-delivery systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; p. 233. [Google Scholar]
- Sonvico, F.; Dubernet, C.; Jaillard, D.; Degrouard, J.; Hoebeke, J.; Duguet, E.; Colombo, P.; Couvreur, P. Folate-Conjugated Iron Oxide Nanoparticles for Solid Tumor Targeting as Potential Specific Magnetic Hyperthermia Mediators: Synthesis, Physicochemical Characterization, and in Vitro Experiments. Bioconjug Chem. 2005, 16, 1181–1188. [Google Scholar] [CrossRef]
- Bilati, U.; Allémann, E.; Doelker, E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur. J. Pharm. Sci. 2005, 24, 67–75. [Google Scholar] [CrossRef]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Yong, K.W.; Yuen, D.; Chen, M.Z.; Johnston, A.P.R. Engineering the Orientation, Density, and Flexibility of Single-Domain Antibodies on Nanoparticles to Improve Cell Targeting. ACS Appl. Mater. Interfaces 2020, 12, 5593–5600. [Google Scholar] [CrossRef]
- Thevis, M.; Schänzer, W.; Schmickler, H. Effect of the location of hydrogen abstraction on the fragmentation of diuretics in negative electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Takayama, M. Multiple Hydrogen Loss from [M+H]+and [a]+ions of Peptides in MALDI In-Source Decay Using a Dinitro-Substituted Matrix. J. Am. Soc. Mass Spectrom. 2020, 31, 547–552. [Google Scholar] [CrossRef]
- Efiana, N.A.; Fürst, A.; Saleh, A.; Shahzadi, I.; Bernkop-Schnürch, A. Phosphate decorated lipid-based nanocarriers providing a prolonged mucosal residence time. Int. J. Pharm. 2022, 625, 122096. [Google Scholar] [CrossRef] [PubMed]
- Esim, O.; Bakirhan, N.K.; Sarper, M.; Savaser, A.; Ozkan, S.A.; Ozkan, Y. Influence of emulsifiers on the formation and in vitro anticancer activity of epirubicin loaded PLGA nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 60, 102027. [Google Scholar] [CrossRef]
- Robin, B.; Albert, C.; Beladjine, M.; Legrand, F.X.; Geiger, S.; Moine, L.; Nicolas, V.; Canette, A.; Trichet, M.; Tsapis, N.; et al. Tuning morphology of Pickering emulsions stabilised by biodegradable PLGA nanoparticles: How PLGA characteristics influence emulsion properties. J. Colloid Interface Sci. 2021, 595, 202–211. [Google Scholar] [CrossRef]
- Kim, H.; Röth, D.; Isoe, Y.; Hayashi, K.; Mochizuki, C.; Kalkum, M.; Nakamura, M. Protein corona components of polyethylene glycol-conjugated organosilica nanoparticles modulates macrophage uptake. Colloids Surfaces B Biointerfaces 2021, 199, 111527. [Google Scholar] [CrossRef]
- Shen, Z.; Fisher, A.; Liu, W.K.; Li, Y. PEGylated “stealth” nanoparticles and liposomes. In Engineering of Biomaterials for Drug Delivery Systems: Beyond Polyethylene Glycol; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kojima, H.; Yamamoto, H.; Kawashima, Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J. Control. Release 2001, 75, 83–91. [Google Scholar] [CrossRef]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Coullerez, G.; Hofmann-Amtenbrink, M.; Vries, M.; Motazacker, M.; Rezaee, F.; Hofmann, H. Significance of surface charge and shell material of superparamagnetic iron oxide nanoparticle (SPION) based core/shell nanoparticles on the composition of the protein corona. Biomater. Sci. 2015, 3, 265–278. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzengruber, M.; Nepustil, L.M.; Kurtaj, F.; Tahir, A.; Skoll, K.; Sami, H.; Wirth, M.; Gabor, F. A Versatile Brij-Linker for One-Step Preparation of Targeted Nanoparticles. Pharmaceutics 2023, 15, 1403. https://doi.org/10.3390/pharmaceutics15051403
Anzengruber M, Nepustil LM, Kurtaj F, Tahir A, Skoll K, Sami H, Wirth M, Gabor F. A Versatile Brij-Linker for One-Step Preparation of Targeted Nanoparticles. Pharmaceutics. 2023; 15(5):1403. https://doi.org/10.3390/pharmaceutics15051403
Chicago/Turabian StyleAnzengruber, Maria, Lisa Marie Nepustil, Fatlinda Kurtaj, Ammar Tahir, Katharina Skoll, Haider Sami, Michael Wirth, and Franz Gabor. 2023. "A Versatile Brij-Linker for One-Step Preparation of Targeted Nanoparticles" Pharmaceutics 15, no. 5: 1403. https://doi.org/10.3390/pharmaceutics15051403





