Development and Characterization of Methyl-Anthranilate-Loaded Silver Nanoparticles: A Phytocosmetic Sunscreen Gel for UV Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of Silver Nanoparticles
2.3. Optimization of Silver Nanoparticles
2.4. Characterization of Silver Nanoparticles
2.4.1. UV–Visible Spectroscopy Analysis
2.4.2. Particle Size, PDI and Zeta Potential
2.4.3. Entrapment Efficiency (%)
2.4.4. Transmission Electron Microscopy (TEM)
2.4.5. Preparation and Evaluation of Silver Nanoparticles Gel
2.5. Evaluation of MA-AgNPs Gel
2.5.1. Physical Appearance
2.5.2. pH and Active Ingredient Content
2.5.3. Spreadability
2.5.4. Extrudability
2.5.5. In Vitro Active Ingredient-Releasing Activity
2.5.6. Dermatokinetics Study
2.5.7. Antioxidant Properties
2.5.8. Confocal Laser Scanning Microscope (CLSM)
2.5.9. Sunburn Protection Factors (SPF) Analysis
2.5.10. Stability Studies
2.6. Statistical Analysis
3. Results
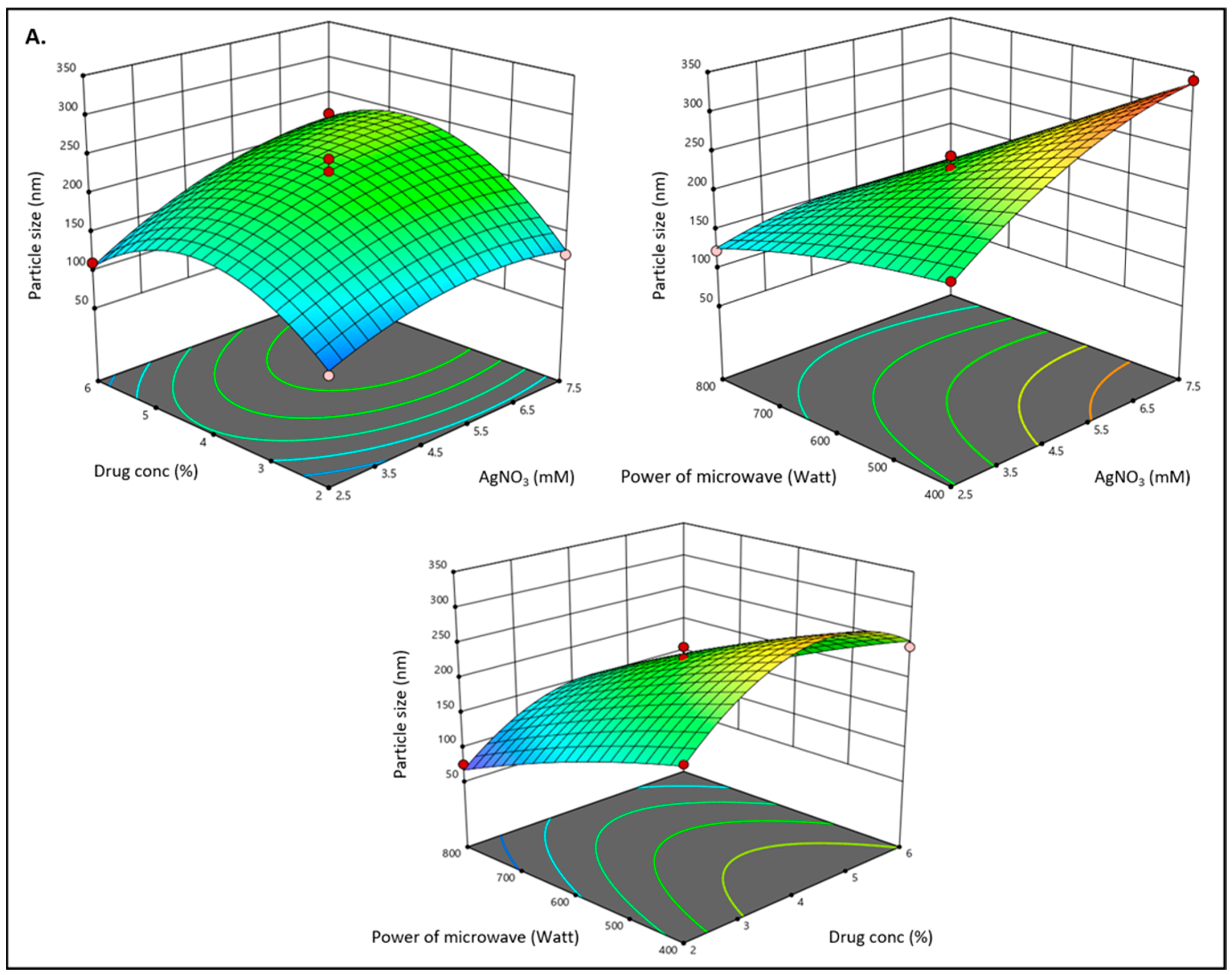
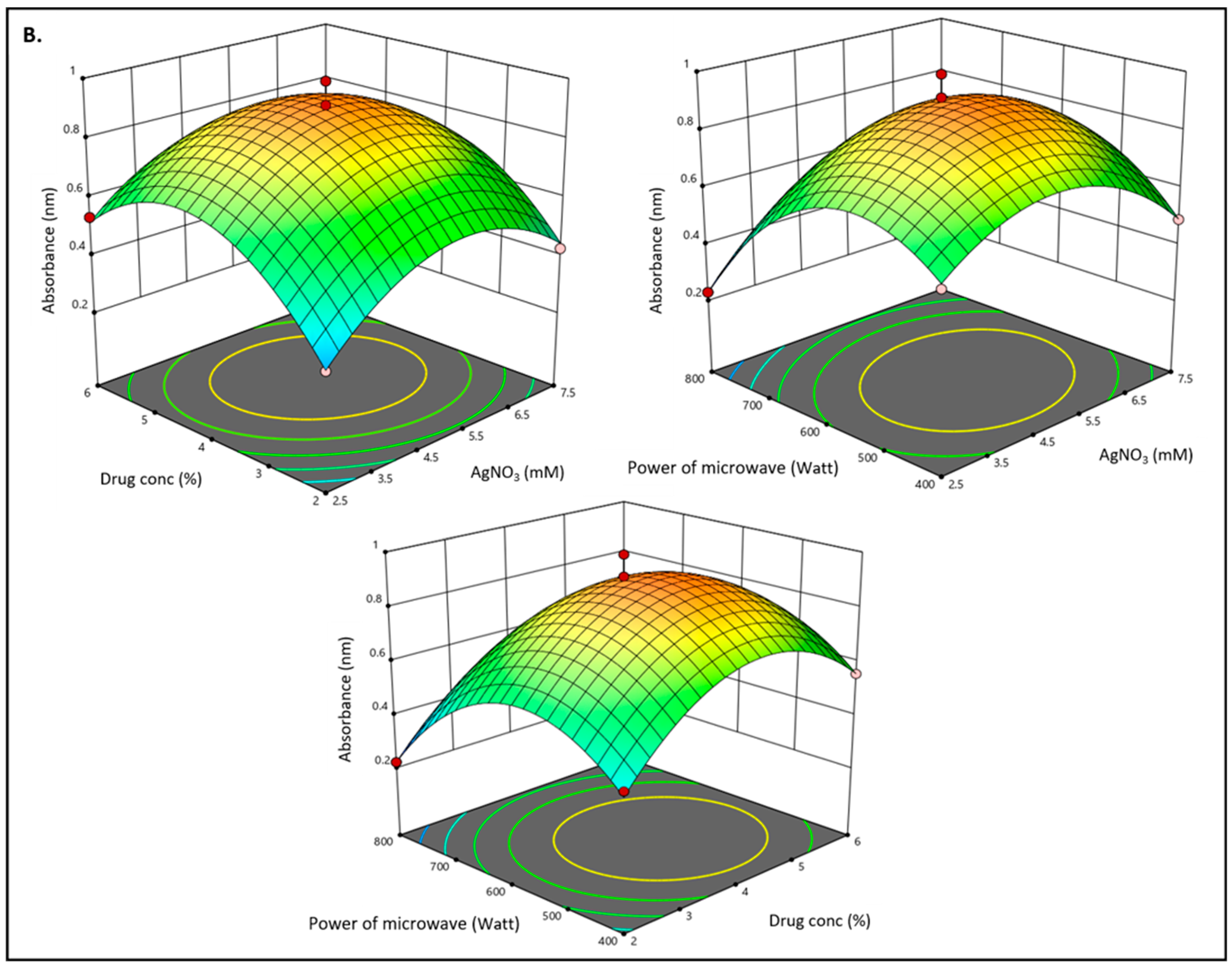
3.1. Optimization of Methyl-Anthranilate-Loaded AgNPs Formulation by Box–Behnken Design
3.2. Effect of Independent Variables on Particle Size (Y1)
3.3. Effect of Independent Variables on Absorbance (Y2)
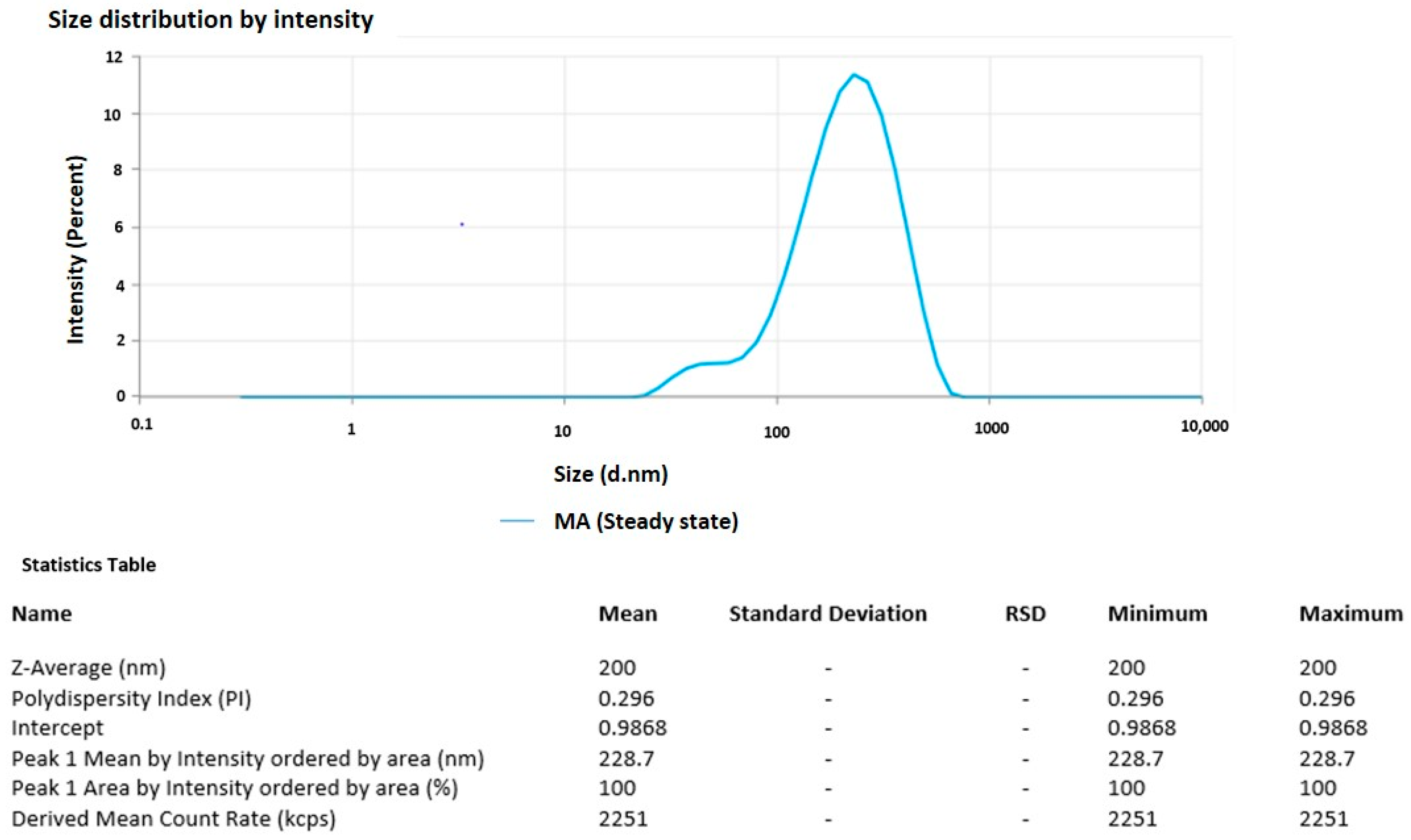
3.4. Particle Size and PDI of the Optimized Formulation
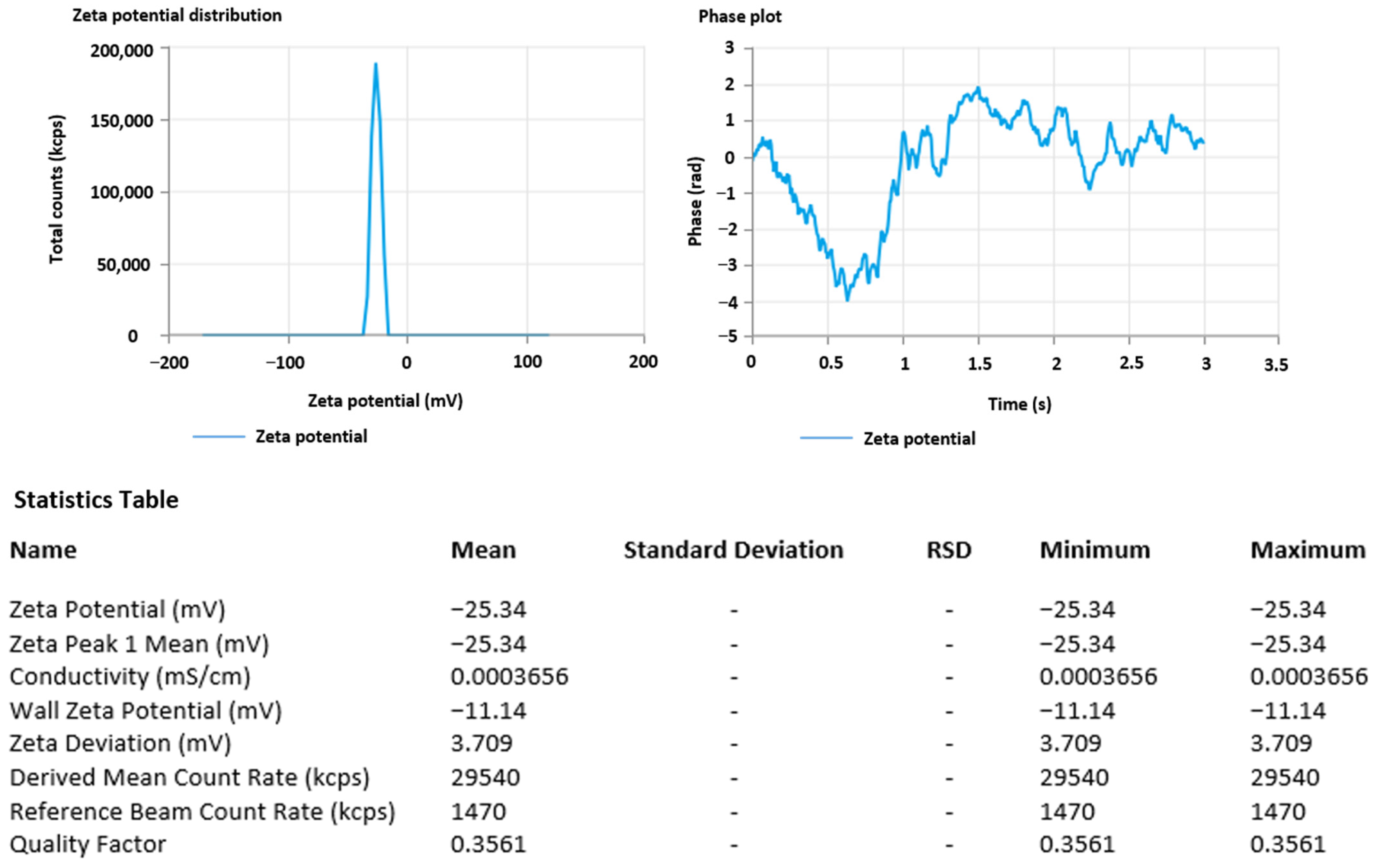
3.5. Zeta Potential
3.6. Percentage Entrapment Efficiency Estimation
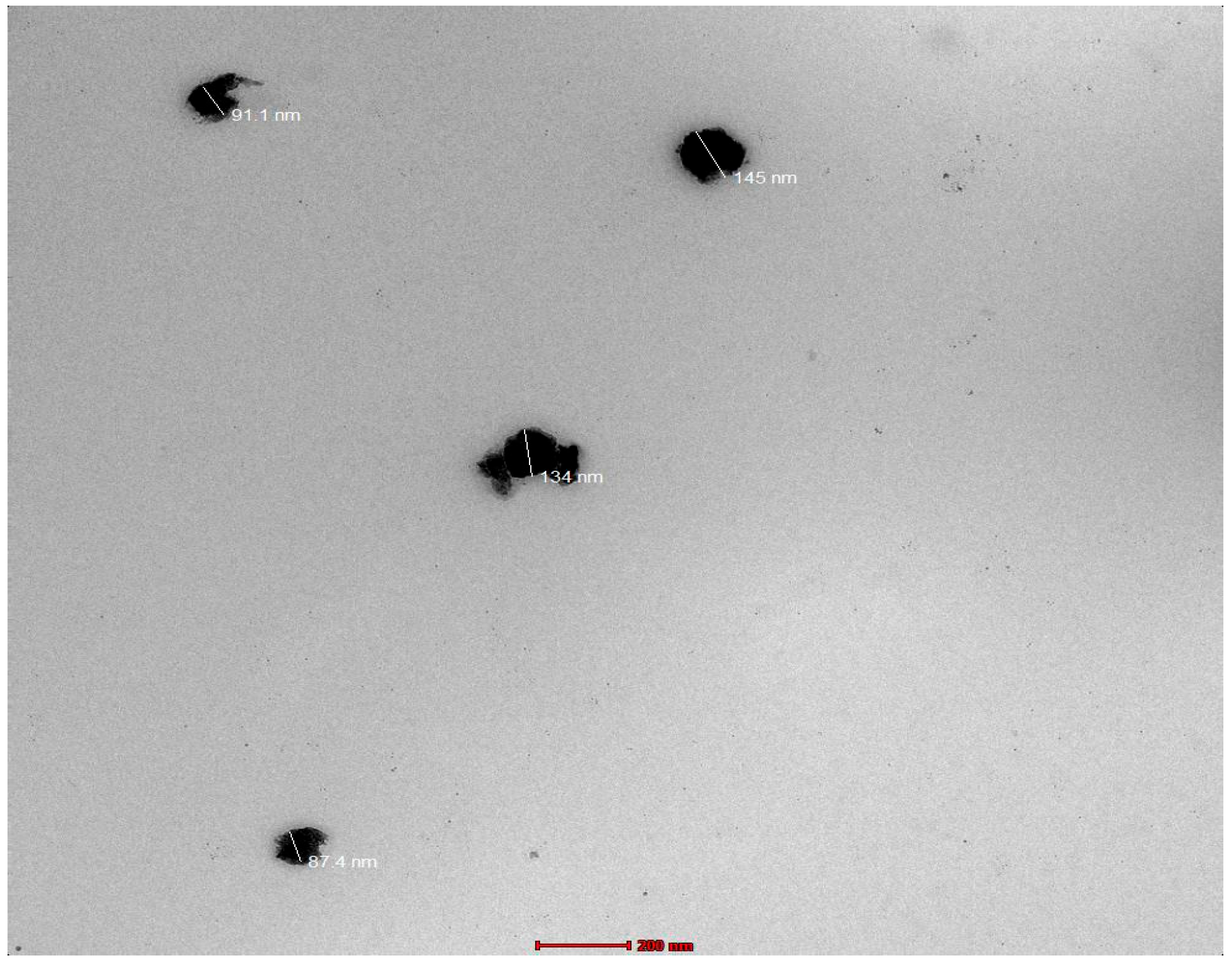
3.7. Electron Microscopy Imaging
3.8. Evaluation of MA-AgNPs Gel (MA-AgNPG)
3.9. Texture Analysis
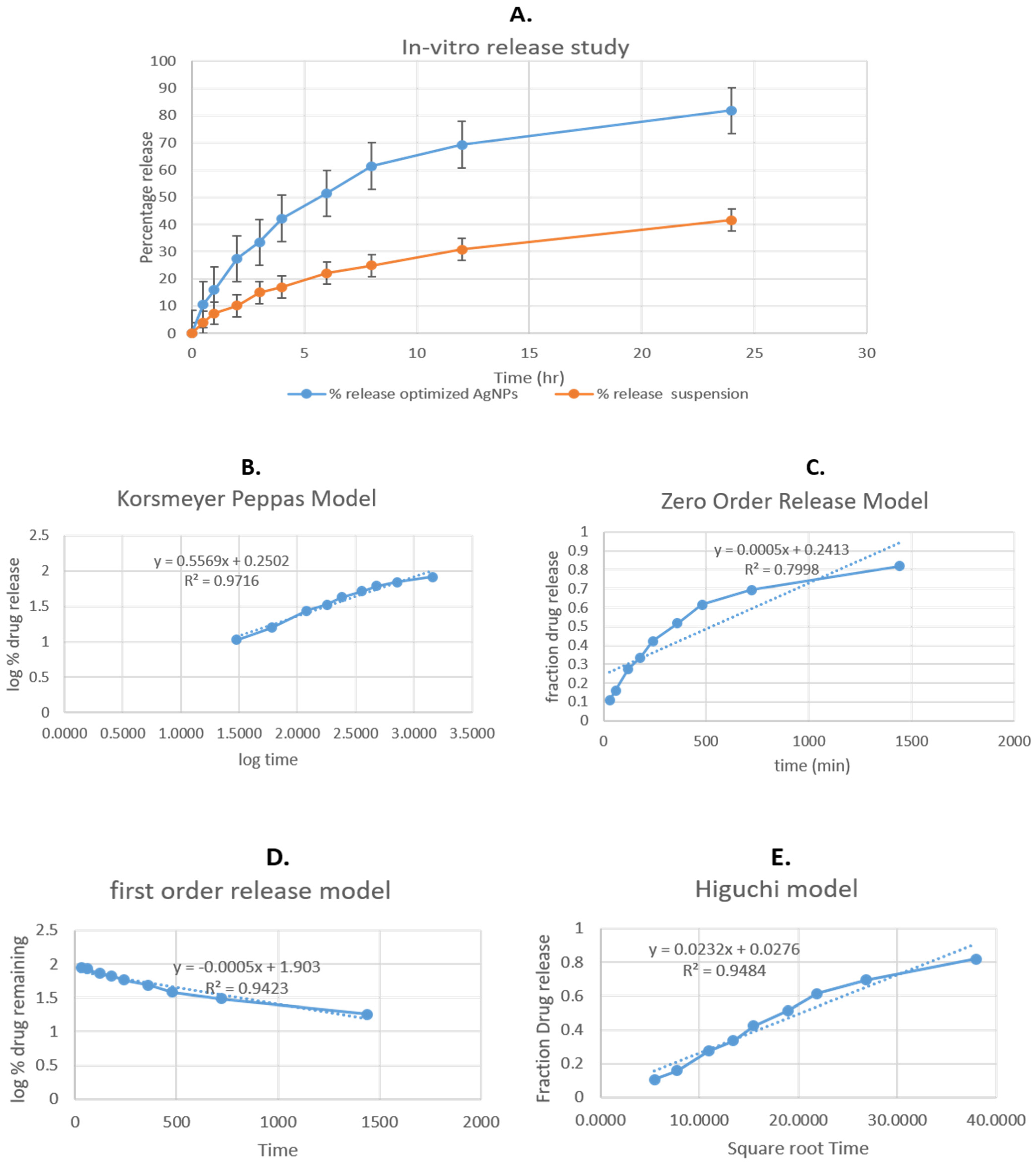
3.10. In Vitro Release Study
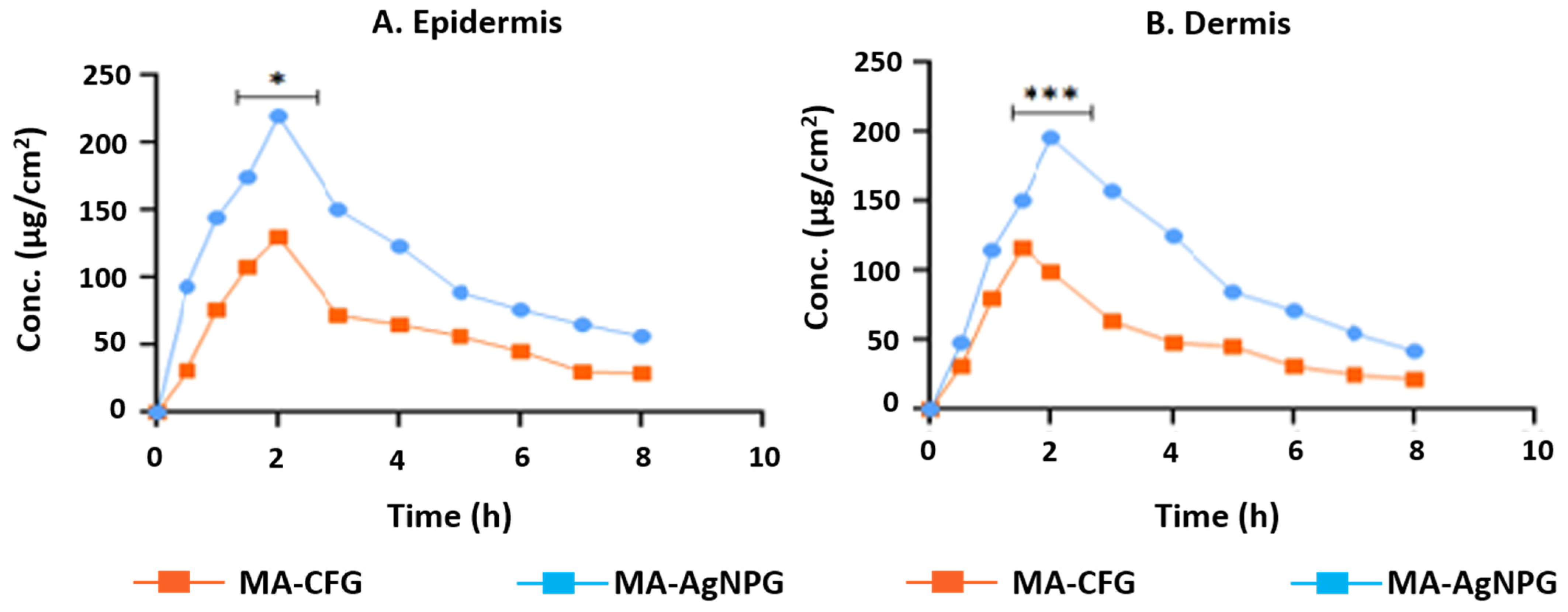
3.11. Dermatokinetic Study
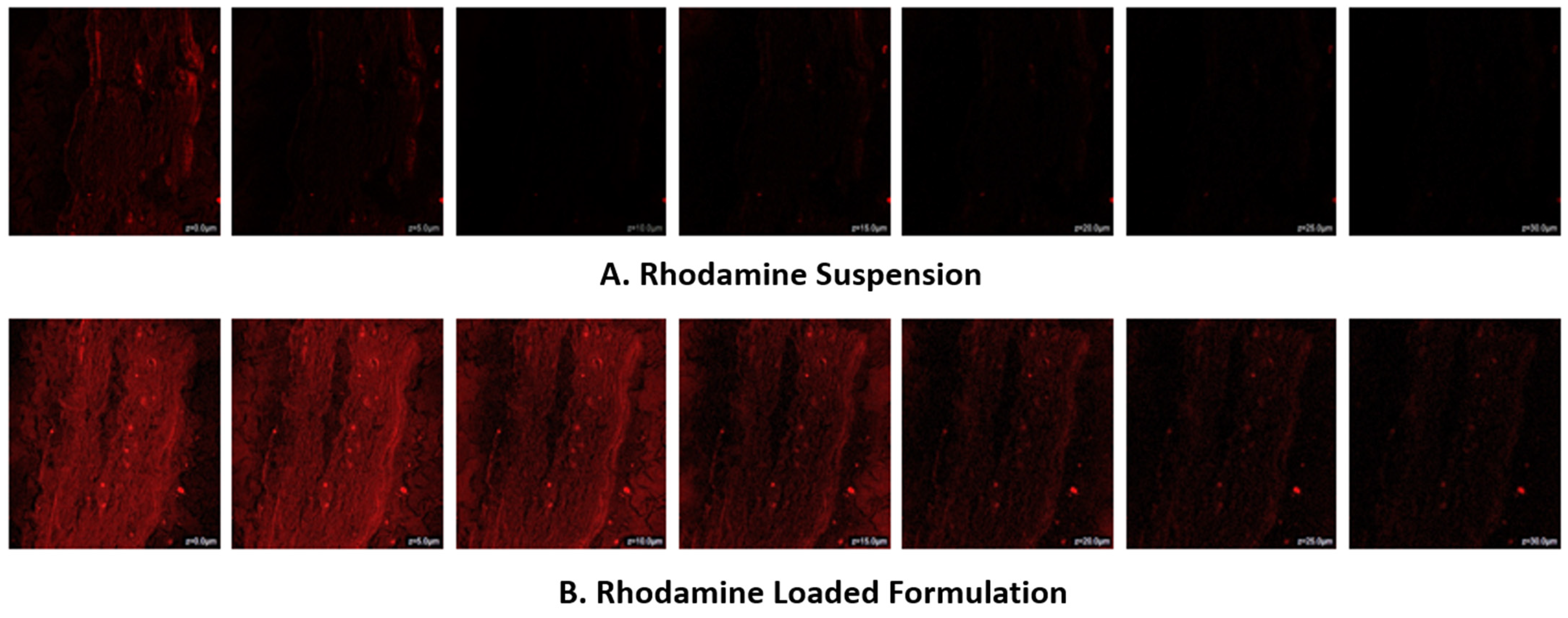
3.12. CLSM
3.13. DPPH Antioxidant Assay
3.14. SPF
3.15. Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.J.F. Sunblock Comparison SPF = 30 and SPF = 100 in Advance of Sunburn. Pajoohandeh 2002, 7, 217. [Google Scholar] [CrossRef]
- Korać, R.R.; Khambholja, K.M. Potential of Herbs in Skin Protection from Ultraviolet Radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Solar UV Index: A Practical Guide A Practical Guide; World Health Organization: Geneva, Switzerland, 2002. Available online: https://apps.who.int/iris/handle/10665/42459 (accessed on 19 February 2023).
- Lucas, R.M.; Mcmichael, A.J.; Armstrong, B.K.; Smith, W.T. Estimating the Global Disease Burden Due to Ultraviolet Radiation Exposure. Int. J. Epidemiol. 2008, 37, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; McLoone, P.; Lesiak, A.; Narbutt, J. The Effect of Chronic Ultraviolet Radiation on the Human Immune System. Photochem. Photobiol. 2008, 84, 19–28. [Google Scholar] [CrossRef]
- Alam, A.; Alqarni, M.H.; Foudah, A.I.; Raish, M.; Salkini, M.A. Babchi Oil-Based Nanoemulsion Hydrogel for the Management of Psoriasis: A Novel Energy Economic Approach Employing Biosurfactants. Gels 2022, 8, 761. [Google Scholar] [CrossRef]
- Villalobos-Hernández, J.R.; Müller-Goymann, C.C. In Vitro Erythemal UV-A Protection Factors of Inorganic Sunscreens Distributed in Aqueous Media Using Carnauba Wax–Decyl Oleate Nanoparticles. Eur. J. Pharm. Biopharm. 2007, 65, 122–125. [Google Scholar] [CrossRef]
- Baron, E.D.; Kirkland, E.B.; Domingo, D.S. Advances in photoprotection. Derm. Nurs 2008, 20, 265–272. [Google Scholar]
- Mansuri, R.; Diwan, A.; Kumar, H.; Dangwal, K.; Yadav, D. Potential of natural compounds as sunscreen agents. Pharmacogn. Rev. 2021, 15, 47–56. [Google Scholar] [CrossRef]
- Al-Abdallah, B.; Al-Faiyz, Y.S.; Shaaban, S. Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates. Biomolecules 2022, 12, 1765. [Google Scholar] [CrossRef]
- Available online: https://www.sciencedirect.com/topics/chemistry/anthranilate (accessed on 20 January 2023).
- Beeby, A.; Jones, A.E. The Photophysical Properties of Menthyl Anthranilate: A UV-A Sunscreen. Photochem. Photobiol. 2000, 72, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Ruan, Y.; Rizvi, S.S. Solubility measurement of methyl anthranilate in supercritical carbon dioxide using dynamic and static equilibrium systems. J. Sci. Food Agric. 2006, 86, 2083–2091. [Google Scholar] [CrossRef]
- Lee, C.M. Fifty years of research and development of cosmeceuticals: A contemporary review. J. Cosmet. Dermatol. 2016, 15, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J.; Shaath, N.A.; Pathak, M.A. Sunscreens: Development, Evaluation, and Regulatory Aspects; Marcel Dekker Inc.: New York, NY, USA, 1997. [Google Scholar]
- Lautenschlager, S.; Wulf, H.C.; Pittelkow, M.R. Photoprotection. Lancet 2007, 370, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.M. Sunblocks: Mechanisms of Action. Photodermatol. Photoimmunol. Photomed. 1999, 15, 34–36. [Google Scholar] [CrossRef]
- Rodrigues, N.D.N.; Cole-Filipiak, N.C.; Horbury, M.D.; Staniforth, M.; Karsili, T.N.V.; Peperstraete, Y.; Stavros, V.G. Photophysics of the Sunscreen Ingredient Menthyl Anthranilate and Its Precursor Methyl Anthranilate: A Bottom-up Approach to Photoprotection. J. Photochem. Photobiol. A Chem. 2018, 353, 376–384. [Google Scholar] [CrossRef]
- Santos, A.C.; Marto, J.; Cha-Cha, R.; Martins, A.M.; Pereira-Silva, M.; Ribeiro, H.M.; Veiga, F. Nanotechnology-based sunscreens—A review. Mater. Today Chem. 2022, 23, 100709. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef]
- Ferreira, L.; Pires, P.C.; Fonseca, M.; Costa, G.; Giram, P.S.; Mazzola, P.G.; Bell, V.; Mascarenhas-Melo, F.; Veiga, F.; Paiva-Santos, A.C. Nanomaterials in Cosmetics: An Outlook for European Regulatory Requirements and a Step Forward in Sustainability. Cosmetics 2023, 10, 53. [Google Scholar] [CrossRef]
- Alsabeelah, N.; Arshad, M.F.; Hashmi, S.; Khan, R.A.; Khan, S. Nanocosmeceuticals for the Management of Ageing: Rigors and Vigors. J. Drug Deliv. Sci. Technol. 2021, 63, 102448. [Google Scholar] [CrossRef]
- Cao, M.; Li, J.; Tang, J.; Chen, C.; Zhao, Y. Gold Nanomaterials in Consumer Cosmetics Nanoproducts: Analyses, Characterization, and Dermal Safety Assessment. Small 2016, 12, 5488–5496. [Google Scholar] [CrossRef]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for skin delivery of cosmeceuticals and pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Wang, S.Q.; Tooley, I.R. Photoprotection in the era of nanotechnology. Semin. Cutan. Med. Surg. 2011, 30, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Singh, I.; Gupta, M.; Singh, L.P.; Tiwari, G. Formulation and Evaluation of Herbal Sunscreens: An Assessment Towards Skin Protection from Ultraviolet Radiation. Pharmacophore 2022, 13, 41–49. [Google Scholar] [CrossRef]
- Burnett, M.E.; Hu, J.Y.; Wang, S.Q. Sunscreens: Obtaining Adequate Photoprotection. Dermatol. Ther. 2012, 25, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Yin, J.J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of Nano Titanium Dioxides in HaCaT Keratinocytes—Generation of Reactive Oxygen Species and Cell Damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef]
- Silver, S.; Phung, L.T.; Silver, G. Silver as Biocides in Burn and Wound Dressings and Bacterial Resistance to Silver Compounds. J. Ind. Microbiol. Biotechnol. 2006, 33, 627–634. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Dunn, K.; Edwards-Jones, V. The Role of ActicoatTM with Nanocrystalline Silver in the Management of Burns. Burns 2004, 30, S1–S9. [Google Scholar] [CrossRef]
- Silver, S. Bacterial Silver Resistance: Molecular Biology and Uses and Misuses of Silver Compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A Nanoproduct in Medical Application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Foudah, A.I.; Alqarni, M.H.; Yusufoglu, H.S. Microwave-assisted and chemically tailored chlorogenic acid-functionalized silver nanoparticles of Citrus sinensis in gel matrix aiding QbD design for the treatment of acne. J. Cosmet. Dermatol. 2023, 22, 1613–1627. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Mankad, V.; Gupta, S.K.; Jha, P.K. Size Distribution of Silver Nanoparticles: UV-Visible Spectroscopic Assessment. Nanosci. Nanotechnol. Lett. 2012, 4, 30–34. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Wang, Z.; Liu, P.; Liu, X.; Ding, J.; Zhou, W. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and Re-polarization. Biomaterials 2021, 264, 120390. [Google Scholar] [CrossRef]
- Ho, Y.Y.; Sun, D.S.; Chang, H.H. Silver nanoparticles protect skin from ultraviolet b-induced damage in mice. Int. J. Mol. Sci. 2020, 21, 7082. [Google Scholar] [CrossRef] [PubMed]
- Palanki, R.; Arora, S.; Tyagi, N.; Rusu, L.; Singh, A.P.; Palanki, S.; Carter, J.E.; Singh, S. Size Is an Essential Parameter in Governing the UVB-Protective Efficacy of Silver Nanoparticles in Human Keratinocytes. BMC Cancer 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Rudrappa, M.; Kumar, R.S.; Nagaraja, S.K.; Hiremath, H.; Gunagambhire, P.V.; Almansour, A.I.; Perumal, K.; Nayaka, S. Myco-Nanofabrication of Silver Nanoparticles by Penicillium brasilianum NP5 and Their Antimicrobial, Photoprotective and Anticancer Effect on MDA-MB-231 Breast Cancer Cell Line. Antibiotics 2023, 12, 567. [Google Scholar] [CrossRef]
- Nakamura, S.; Ando, N.; Sato, M.; Ishihara, M. Ultraviolet irradiation enhances the microbicidal activity of silver nanoparticles by hydroxyl radicals. Int. J. Mol. Sci. 2020, 21, 3204. [Google Scholar] [CrossRef]
- Jahan, S.; Aqil, M.; Ahad, A.; Imam, S.S.; Waheed, A.; Qadir, A.; Ali, A. Nanostructured Lipid Carrier for Transdermal Gliclazide Delivery: Development and Optimization by Box-Behnken Design. Inorg. Nano-Met. Chem. 2022, 1–14. [Google Scholar] [CrossRef]
- Imam, S.S.; Ahad, A.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by Design Based Risperidone Nano Soft Lipid Vesicle as a New Strategy for Enhanced Transdermal Drug Delivery: In-Vitro Characterization, and in-Vivo Appraisal. Mater. Sci. Eng. C 2017, 75, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C.; Mulfinger, L. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Ataide, J.A.; da Fonseca, J.H.L.; Silvério, L.A.L.; Lancellotti, M.; Paiva-Santos, A.C.; d’Ávila, M.A.; Kohane, D.S.; Mazzola, P.G. Terbinafine Nanohybrid: Proposing a Hydrogel Carrying Nanoparticles for Topical Release. Pharmaceutics 2023, 15, 841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bu, H.; Gao, Z.; Huang, Y.; Gao, F.; Li, Y. The Characteristics and Mechanism of Simvastatin Loaded Lipid Nanoparticles to Increase Oral Bioavailability in Rats. Int. J. Pharm. 2010, 394, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Chaudhari, B. Optimization of Microemulsion Based Transdermal Gel of Triamcinolone. Recent Pat. Antiinfect. Drug Discov. 2017, 12, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Tian, C.; Liu, L.; Hu, R.; Gui, S.; Chu, X. Transdermal Administration of Ibuprofen-Loaded Gel: Preparation, Pharmacokinetic Profile, and Tissue Distribution. AAPS Pharm. Sci. Tech. 2020, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Da Silva Almeida, J.R.G. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef] [PubMed]
- Uprit, S.; Kumar Sahu, R.; Roy, A.; Pare, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carrier Gel for Effective Treatment of Alopecia. Saudi Pharm. J. 2013, 21, 379–385. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Palanisamy, S.; Boomi, P.; Subaskumar, R.; Ravikumar, S.; Thayumanavan, T. An Eco-Friendly Gnaphalium Polycaulon Mediated Silver Nanoparticles: Synthesis, Characterization, Antimicrobial, Wound Healing and Drug Release Studies. J. Drug Deliv. Sci. Technol. 2021, 61, 102202. [Google Scholar] [CrossRef]
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Katare, O.P. Biocompatible Lidocaine and Prilocaine Loaded-Nanoemulsion System for Enhanced Percutaneous Absorption: QbD-Based Optimisation, Dermatokinetics and in Vivo Evaluation. J. Microencapsul 2015, 32, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.; Mashru, R. Development and validation of analytical method of berberine and its quantification in multicomponent homeopathic formulations. World J. Pharm. Res. 2017, 6, 793–802. [Google Scholar] [CrossRef]
- Albrecht, S.; Jung, S.; Müller, R.; Lademann, J.; Zuberbier, T.; Zastrow, L.; Reble, C.; Beckers, I.; Meinke, M.C. Skin type differences in sun-induced oxidative stress. Br. J. Dermatol. 2019, 180, e71. [Google Scholar] [CrossRef]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Ultrav. Light Hum. Health Dis. Environ. 2017, 996, 15–23. [Google Scholar]
- Gambetta, C.; Natera, J.; Massad, W.A.; García, N.A. Methyl anthranilate as generator and quencher of reactive oxygen species: A photochemical study. J. Photochem. Photobiol. A Chem. 2013, 269, 27–33. [Google Scholar] [CrossRef]
- Ali, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Parveen, A.; Qadir, A.; Ali, M.H.; Akhtar, M. Formulation and Evaluation of Embelin Loaded Nanoliposomes: Optimization, in Vitro and Ex Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022, 72, 103414. [Google Scholar] [CrossRef]
- Santos, E.P.; Freitas, Z.M.; Souza, K.R.; Garcia, S.; Vergnanini, A. In Vitro and In Vivo Determinations of Sun Protection Factors of Sunscreen Lotions with Octylmethoxycinnamate. Int. J. Cosmet. Sci. 1999, 21, 1–5. [Google Scholar] [CrossRef]
- Barbosa, T.C.; Nascimento, L.É.D.; Bani, C.; Almeida, T.; Nery, M.; Santos, R.S.; de Oliveira Menezes, L.R.; Zielinska, A.; Fernandes, A.R.; Cardoso, J.C.; et al. Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-Poly(ε-Caprolactone) Nanocapsules. Toxics 2019, 7, 51. [Google Scholar] [CrossRef]
- Loo, C.H.; Basri, M.; Ismail, R.; Lau, H.L.N.; Tejo, B.A.; Kanthimathi, M.S.; Hassan, H.A.; Choo, Y.M. Effect of Compositions in Nanostructured Lipid Carriers (NLC) on Skin Hydration and Occlusion. Int. J. Nanomed. 2013, 8, 13–22. [Google Scholar] [CrossRef]
- Bamsaoud, S.F.; Basuliman, M.M.; Bin-Hameed, E.A.; Balakhm, S.M.; Alkalali, A.S. May. The effect of volume and concentration of AgNO3 aqueous solutions on silver nanoparticles synthesized using Ziziphus Spina–Christi leaf extract and their antibacterial activity. J. Phys. Conf. Ser. 2021, 1900, 12005. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Mujeeb, M.; Ahad, A.; Moolakkadath, T.; Aqil, M.; Ahmad, A.; Akhter, M.H. Optimization of ethosomes for topical thymoquinone delivery for the treatment of skin acne. J. Drug Deliv. Sci. Technol. 2019, 49, 177–187. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, X.; Wei, J. Localized surface plasmon resonance of silver nanotriangles synthesized by a versatile solution reaction. Nanoscale Res. Lett. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Sakharwade, S. Silver nanoparticles in cosmetics. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 48–53. [Google Scholar] [CrossRef]

| Design Variables | Low (−1) | Medium (0) | High (+1) |
|---|---|---|---|
| Independent variables | |||
| X1 = AgNO3 concentration in mM | 2.5 | 5 | 7.5 |
| X2 = methyl anthranilate concentration % | 2 | 4 | 6 |
| X3 = power of microwave in Watt | 400 | 600 | 800 |
| Dependent variables | |||
| Particle size (Y1) | Minimize | ||
| Absorbance (Y2) | Maximize |
| In-Dependent Variables | Responses | ||||
|---|---|---|---|---|---|
| Run | X1 (AgNO3 Concentration in mM) | X2 (Methyl Anthranilate Concentration %) | X3 (Power of Microwave in Watt) | Particle Size (nm) | Absorbance |
| 1 | 5 | 4 | 600 | 200.12 | 0.991 |
| 2 | 2.5 | 6 | 600 | 110.76 | 0.534 |
| 3 | 5 | 6 | 400 | 245.61 | 0.557 |
| 4 | 7.5 | 4 | 800 | 125.43 | 0.459 |
| 5 | 5 | 4 | 600 | 245.76 | 0.912 |
| 6 | 5 | 4 | 600 | 245.89 | 0.893 |
| 7 | 5 | 4 | 600 | 229.81 | 0.821 |
| 8 | 5 | 2 | 400 | 188.77 | 0.432 |
| 9 | 5 | 6 | 800 | 98.65 | 0.342 |
| 10 | 7.5 | 6 | 600 | 221.56 | 0.548 |
| 11 | 2.5 | 4 | 800 | 123.43 | 0.229 |
| 12 | 2.5 | 2 | 600 | 87.54 | 0.328 |
| 13 | 7.5 | 2 | 600 | 121.76 | 0.426 |
| 14 | 5 | 4 | 600 | 226.87 | 0.871 |
| 15 | 5 | 2 | 800 | 75.43 | 0.221 |
| 16 | 7.5 | 4 | 400 | 339.45 | 0.491 |
| 17 | 2.5 | 4 | 400 | 195.51 | 0.547 |
| Responses | Analysis | R2 | Adjusted R2 | Predicted R2 | SD | %CV | Model |
|---|---|---|---|---|---|---|---|
| Particle size (nm) | Polynomial | 0.9800 | 0.9543 | 0.9160 | 15.66 | 8.64 | Quadratic Model F Value = 38.16 Adequate Precision = 22.417 |
| Absorbance | Polynomial | 0.9817 | 0.9582 | 0.9407 | 0.0501 | 8.87 | Quadratic Model F Value = 41.77 Adequate Precision = 17.6278 |
| Homogeneity | Appearance | Washability | Phase separation | Odor |
|---|---|---|---|---|
| Good | Translucent | Washable | NO | NO |
| Color | Active ingredient content (%) | pH | Spreadability (g·cm2/s) | Extrudability (gm) |
| Off-white | 86.25 | 7.09 | 16.20 | 15.19 |
| Cohesiveness | Consistency (gm·s) | Firmness (gm) | Work of cohesion | |
| −121.44 | 2513.16 | 322.10 | −16.43 | |
| Dermatokinetics Parameters | MA-AgNPs-Gel | MACF-Gel | ||
|---|---|---|---|---|
| Epidermis | Dermis | Epidermis | Dermis | |
| Tskin max (h) | 2 | 2 | 2 | 2 |
| Cskin max (μg/cm2) | 219.80 | 195.87 | 130.03 | 116.35 |
| AUC0–8 (μg/cm2h) | 903.28 | 817.35 | 488.41 | 411.04 |
| Ke (h−1) | 0.133 | 0.111 | 0.117 | 0.161 |
| Evaluation Parameters for MA-AgNP Gel | Months | |||
|---|---|---|---|---|
| Initial | 1 | 2 | 3 | |
| Appearance | No change in appearance | |||
| Phase separation | No phase separation was observed | |||
| Homogeneity | No change in homogeneity | |||
| pH | 7.09 | 7.09 | 7.07 | 7.16 |
| Clarity | No change in clarity | |||
| Washability | Washable | |||
| Odor | Good | Good | Good | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazwani, M.; Hani, U.; Alqarni, M.H.; Alam, A. Development and Characterization of Methyl-Anthranilate-Loaded Silver Nanoparticles: A Phytocosmetic Sunscreen Gel for UV Protection. Pharmaceutics 2023, 15, 1434. https://doi.org/10.3390/pharmaceutics15051434
Ghazwani M, Hani U, Alqarni MH, Alam A. Development and Characterization of Methyl-Anthranilate-Loaded Silver Nanoparticles: A Phytocosmetic Sunscreen Gel for UV Protection. Pharmaceutics. 2023; 15(5):1434. https://doi.org/10.3390/pharmaceutics15051434
Chicago/Turabian StyleGhazwani, Mohammed, Umme Hani, Mohammed H. Alqarni, and Aftab Alam. 2023. "Development and Characterization of Methyl-Anthranilate-Loaded Silver Nanoparticles: A Phytocosmetic Sunscreen Gel for UV Protection" Pharmaceutics 15, no. 5: 1434. https://doi.org/10.3390/pharmaceutics15051434
APA StyleGhazwani, M., Hani, U., Alqarni, M. H., & Alam, A. (2023). Development and Characterization of Methyl-Anthranilate-Loaded Silver Nanoparticles: A Phytocosmetic Sunscreen Gel for UV Protection. Pharmaceutics, 15(5), 1434. https://doi.org/10.3390/pharmaceutics15051434






