Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial
Abstract
:1. Introduction
2. Structural Features and Classification
3. Physiological and Therapeutic Effects of Collagens
3.1. Collagen for Treating Skin Injuries
| Diseases | Therapeutic Effects | Refs. | |
|---|---|---|---|
| Treating skin injuries | Wound healing | Wound closure; anti-bacterial activity | [14,15,16,17,18,19] |
| Burn healing | Accelerated healing and skin appendage generation | [20,21,22,23] | |
| Chronic wound | Faster wound healing | [24,25,26] | |
| Treating orthopedic diseases | Osteoporosis | Improved bone mineral density; increased bone hydroxyproline content; enhanced alkaline phosphatase level | [27] |
| Bone defect healing | New bone tissue forming; guided bone regeneration | [28,29,30,31] | |
| Treating ophthalmic diseases | Corneal defects | Filled corneal defects; restored corneal curvature | [29,32] |
| Keratoconus | Increased corneal rigidity; decreased interfibrillar Bragg spacing | [33,34,35,36] | |
| Promoting nerve regeneration | Central nerve injury | Tuning NSCs; improved motor performance; reduced formation of fluid-filled cysts; impeded collapse of musculature and connective tissue | [37,38,39,40,41,42,43] |
| Peripheral nerve injury | Well-organized fibers; unimpaired myelin sheath | [44] | |
| Anti-aging | Skin anti-aging | Reduced trans-epidermal water loss and skin pore number; increased elasticity; enhanced dermal thickness and acoustic density | [45,46,47,48] |
3.2. Collagen for Treating Orthopedic Diseases
3.3. Collagen for Treating Ophthalmic Diseases
3.4. Collagen for Promoting Nerve Regeneration
3.5. Collagen for Anti-Aging
4. Strategies for Synthetic Collagen Production
4.1. Total Synthesis Strategy
4.2. Strategies for Recombinant Collagen Production
4.2.1. Protein Engineering in Animals
4.2.2. Protein Engineering in Escherichia coli
| Expression System | Host for Transfection | Synthetic Conditions | Productivity | Limitations | Ref. |
|---|---|---|---|---|---|
| Transgenic plants | Tobacco, corn, barley, etc. | Transforming collagen gene into plants such as transgenic tobacco and corn for expression | 20 g/L | Its expression quantity and purification steps need to be improved | [79,80,81,82,83] |
| Escherichia coli | E. coli | Designed collagen target genes and vectors in E. coli to induce expression of the target protein | 0.1–0.2 g/L | Affected by pH, temperature, dissolved oxygen, acetic acid concentration, carbon source, nitrogen source, etc. | [77,84] |
| Yeast | Pichia pastoris | Eukaryotic expression system can ensure the post-translational modification of collagen, including glycosylation, etc. | 0.7–1.5 g/L | Affected by pH, temperature, and methanol content | [85,86,87] |
| Transgenic animals | Glands of mice and silkworms | Construction of the vector and animals are transformed with collagen gene fragments | 8–20 g/L | Low level of hydroxyproline and incomplete triple helix structure | [73,74,75,76] |
4.2.3. Protein Engineering in Yeast Expression System
4.2.4. Protein Engineering in Plants
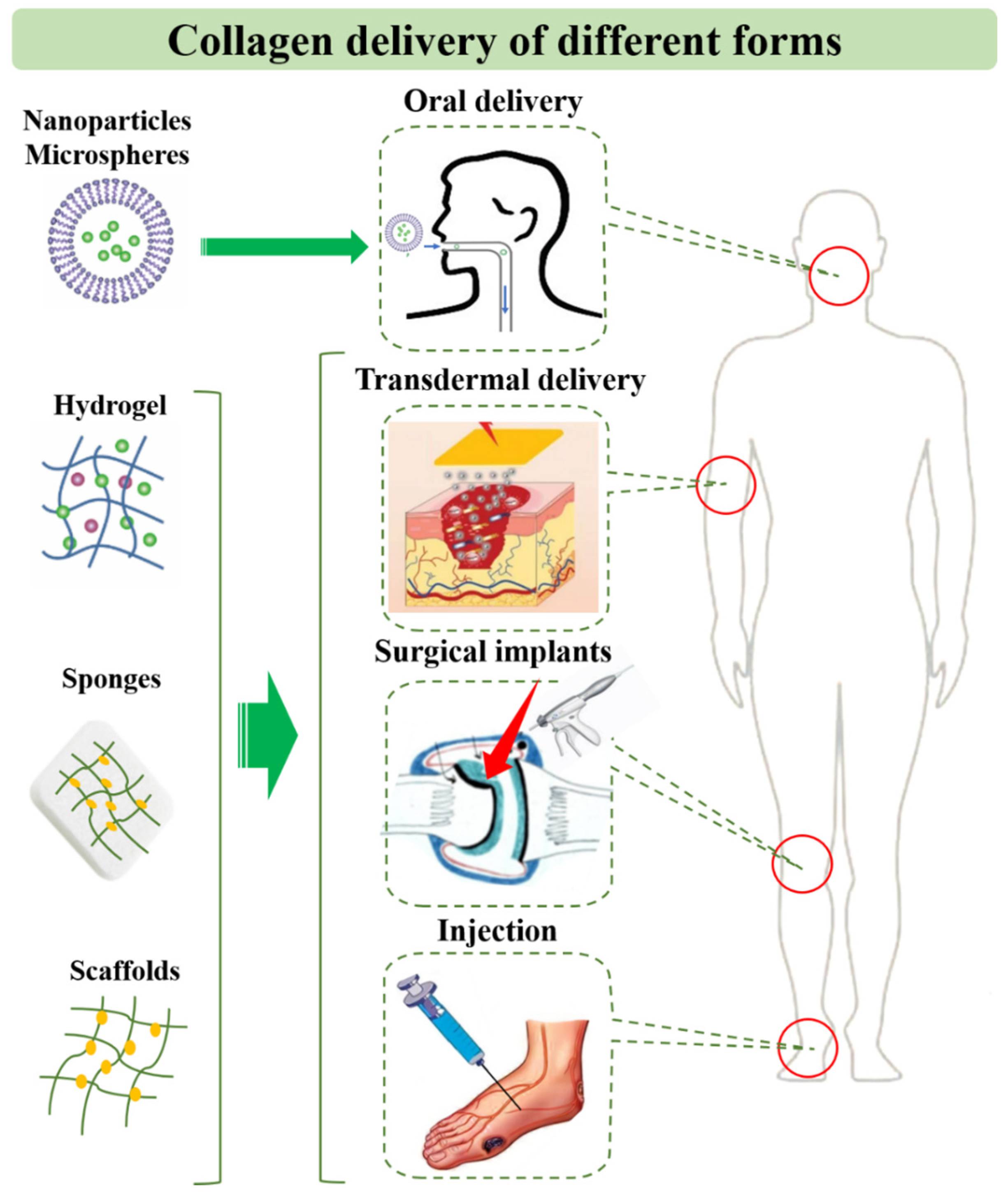
5. Application and Delivery Strategies of Collagen for Diseases
5.1. Collagen Peptide and Solution
5.2. Collagen-Based Drug Delivery Systems
5.3. Collagen-Based Tissue Engineering Systems
6. Discussion and Outlook
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ricard-Blum, S.; Ruggiero, F.; van der Rest, M. The collagen superfarmily. In Collagen: Primer in Structure, Processing and Assembly; Brinckmann, J., Notbohm, H., Muller, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 247, pp. 35–84. [Google Scholar]
- El Blidi, O.; El Omari, N.; Balahbib, A.; Ghchime, R.; El Menyiy, N.; Ibrahimi, A.; Ben Kaddour, K.; Bouyahya, A.; Chokairi, O.; Barkiyou, M. Extraction Methods, Characterization and Biomedical Applications of Collagen: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13587–13613. [Google Scholar] [CrossRef]
- Fu, R.; Fan, D.; Yang, W.; Chen, L.; Qu, C.; Yang, S.; Xu, L. Industrial development and biomedical application prospect of recombinant collagen. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2022, 38, 3228–3242. [Google Scholar] [CrossRef]
- Yang, C.L.; Hillas, P.J.; Baez, J.A.; Nokelainen, M.; Balan, J.; Tang, J.; Spiro, R.; Polarek, J.W. The application of recombinant human collagen in tissue engineering. Biodrugs 2004, 18, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Hsiuying, W. A review of the effects of collagen treatment in clinical studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Rodriguez, M.I.A.; Barroso, L.G.R.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis, and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, e2000017. [Google Scholar] [CrossRef]
- Israelowitz, M.; Rizvi, S.W.H.; Kramer, J.; von Schroeder, H.P. Computational modeling of type I collagen fibers to determine the extracellular matrix structure of connective tissues. Protein Eng. Des. Sel. 2005, 18, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Darvish, D.M. Collagen fibril formation in vitro: From origin to opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef]
- Nishimoto, M.; Sakamoto, R.; Mizuta, S.; Yoshinaka, R. Identification and characterization of molecular species of collagen in ordinary muscle and skin of the Japanese flounder Paralichthys olivaceus. Food Chem. 2005, 90, 151–156. [Google Scholar] [CrossRef]
- Minor, R.R. Collagen-metabolism—Comparison of diseases of collagen and diseases affecting collagen. Am. J. Pathol. 1980, 98, 225–280. [Google Scholar] [PubMed]
- Asamura, K.; Abe, S.; Imamura, Y.; Aszodi, A.; Suzuki, N.; Hashimoto, S.; Takumi, Y.; Hayashi, T.; Fassler, R.; Nakamura, Y.; et al. Type IX collagen is crucial for normal hearing. Neuroscience 2005, 132, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Goldbloom-Helzner, L.; Hao, D.; Wang, A. Developing Regenerative Treatments for Developmental Defects, Injuries, and Diseases Using Extracellular Matrix Collagen-Targeting Peptides. Int. J. Mol. Sci. 2019, 20, 4072. [Google Scholar] [CrossRef]
- Liu, T.; Dan, W.H.; Dan, N.H.; Liu, X.H.; Liu, X.X.; Peng, X. A novel grapheme oxide-modified collagen-chitosan bio-film for controlled growth factor release in wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Xu, B.; Wang, Y.H.; Li, Y.; Si, H.; Zheng, X.Y.; Chen, Z.Y.; Chen, F.L.; Fan, D.D. Dramatic promotion of wound healing using a recombinant human-like collagen and bFGF cross-linked hydrogel by transglutaminase. J. Biomater. Sci. Polym. Ed. 2019, 30, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Zhou, C.C.; Luo, C.; Qian, B.; Liu, S.K.; Zeng, Y.Y.; Hou, J.F.; Deng, B.; Sun, Y.; Yang, J.; et al. N-acetyl cysteine-loaded graphene oxide-collagen hybrid membrane for scarless wound healing. Theranostics 2019, 9, 5839–5853. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Han, M.; Sun, Y.S.; Hua, Y.Y.; Chen, G.F.; Zhang, L.F. Oligoarginine mediated collagen/chitosan gel composite for cutaneous wound healing. Int. J. Biol. Macromol. 2019, 122, 1120–1127. [Google Scholar] [CrossRef]
- Masci, V.L.; Taddei, A.R.; Courant, T.; Tezgel, O.; Navarro, F.; Giorgi, F.; Mariolle, D.; Fausto, A.M.; Texier, I. Characterization of Collagen/Lipid Nanoparticle-Curcumin Cryostructurates for Wound Healing Applications. Macromol. Biosci. 2019, 19, e1800446. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, M.; Liang, R.; Zhao, M.; Zhang, Z.; Li, Y. Oral administration of marine collagen peptides prepared from chum salmon (Oncorhynchus keta) improves wound healing following cesarean section in rats. Food Nutr. Res. 2015, 59, 26411. [Google Scholar] [CrossRef]
- Ge, B.S.; Wang, H.N.; Li, J.; Liu, H.H.; Yin, Y.H.; Zhang, N.L.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhu, C.H.; Fan, D.D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; Rahman, M.S.; Ullah, M.A.; Siddika, A.; Hossain, M.L.; Akhter, M.S.; Hasan, M.Z.; Asaduzzaman, S.M. Amnion and collagen-based blended hydrogel improves burn healing efficacy on a rat skin wound model in the presence of wound dressing biomembrane. Bio-Med. Mater. Eng. 2020, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Ying, H.Y.; Zhou, J.; Wang, M.Y.; Su, D.D.; Ma, Q.Q.; Lv, G.Z.; Chen, J.H. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 101, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Sisakht, M.M.; Amirkhani, M.A.; Seifalian, A.M.; Banafshe, H.R.; Verdi, J.; Nouradini, M. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin-collagen hydrogel: A clinical study for diabetic wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Song, H.; Li, B. Effect of Collagen Hydrolysates from Silver Carp Skin (Hypophthalmichthys molitrix) on Osteoporosis in Chronologically Aged Mice: Increasing Bone Remodeling. Nutrients 2018, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Zheng, L.; Luo, F.; Luo, J.; Qian, Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef]
- Guo, J.L.; Zhang, Q.; Li, J.; Llu, Y.S.; Hou, Z.Y.; Chen, W.; Jin, L.; Tian, Y.; Ju, L.L.; Liu, B.; et al. Local application of an ibandronate/collagen sponge improves femoral fracture healing in ovariectomized rats. PLoS ONE 2017, 12, e0187683. [Google Scholar] [CrossRef] [PubMed]
- Toosi, S.; Naderi-Meshkin, H.; Kalalinia, F.; HosseinKhani, H.; Heirani-Tabasi, A.; Havakhah, S.; Nekooei, S.; Jafarian, A.H.; Rezaie, F.; Peivandi, M.T.; et al. Bone defect healing is induced by collagen sponge/polyglycolic acid. J. Mater. Sci. Mater. Med. 2019, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Nabavi, M.A.; Semyari, H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2020, 10, 108–121. [Google Scholar] [CrossRef]
- Calderon-Colon, X.; Xia, Z.Y.; Breidenich, J.L.; Mulreany, D.G.; Guo, Q.Y.; Uy, O.M.; Tiffany, J.E.; Freund, D.E.; McCally, R.L.; Schein, O.D.; et al. Structure and properties of collagen vitrigel membranes for ocular repair and regeneration applications. Biomaterials 2012, 33, 8286–8295. [Google Scholar] [CrossRef]
- Connon, C.J.; Meek, K.M.; Newton, R.H.; Kenney, M.C.; Alba, S.A.; Karageozian, H. Hyaluronidase treatment, collagen fibril packing, and normal transparency in rabbit corneas. J. Refract. Surg. 2000, 16, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G. Crosslinking treatment of progressive keratoconus: New hope. Curr. Opin. Ophthalmol. 2006, 17, 356–360. [Google Scholar] [CrossRef]
- Dias, J.; Diakonis, V.F.; Kankariya, V.P.; Yoo, S.H.; Ziebarth, N.M. Anterior and posterior corneal stroma elasticity after corneal collagen crosslinking treatment. Exp. Eye Res. 2013, 116, 58–62. [Google Scholar] [CrossRef]
- Li, N.; Peng, X.J.; Fan, Z.J.; Xia, Y.; Wu, T.F. New techniques to improve classical corneal collagen cross-linking treatment. Chin. Med. J. 2014, 127, 1558–1565. [Google Scholar]
- Han, Q.; Sun, W.; Lin, H.; Zhao, W.; Gao, Y.; Zhao, Y.; Chen, B.; Xiao, Z.; Hu, W.; Li, Y.; et al. Linear Ordered Collagen Scaffolds Loaded with Collagen-Binding Brain-Derived Neurotrophic Factor Improve the Recovery of Spinal Cord Injury in Rats. Tissue Eng. Part A 2009, 15, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xiao, Z.; Zhang, H.; Chen, B.; Tang, G.; Hou, X.; Ding, W.; Wang, B.; Zhang, P.; Dai, J.; et al. Linear Ordered Collagen Scaffolds Loaded with Collagen-Binding Neurotrophin-3 Promote Axonal Regeneration and Partial Functional Recovery after Complete Spinal Cord Transection. J. Neurotrauma 2010, 27, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Cholas, R.H.; Hsu, H.-P.; Spector, M. The reparative response to cross-linked collagen-based scaffolds in a rat spinal cord gap model. Biomaterials 2012, 33, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.-Y.; Knowles, J.C.; Lee, G.-S.; Kim, J.-W.; Kim, H.-W.; Son, Y.-J.; Hyun, J.K. Effects of phosphate glass fiber-collagen scaffolds on functional recovery of completely transected rat spinal cords. Acta Biomater. 2012, 8, 1802–1812. [Google Scholar] [CrossRef]
- Altinova, H.; Moellers, S.; Fuehrmann, T.; Deumens, R.; Bozkurt, A.; Heschel, I.; Damink, L.H.H.O.; Schuegner, F.; Weis, J.; Brook, G.A. Functional improvement following implantation of a microstructured, type-I collagen scaffold into experimental injuries of the adult rat spinal cord. Brain Res. 2014, 1585, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, J. Bridging the gap with functional collagen scaffolds: Tuning endogenous neural stem cells for severe spinal cord injury repair. Biomater. Sci. 2018, 6, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef]
- Samadian, H.; Vaez, A.; Ehterami, A.; Salehi, M.; Farzamfar, S.; Sahrapeyma, H.; Norouzi, P. Sciatic nerve regeneration by using collagen type I hydrogel containing naringin. J. Mater. Sci. Mater. Med. 2019, 30, 107. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxidative Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Seki, S.; Ueda, F. Effects of Composite Supplement Containing Collagen Peptide and Ornithine on Skin Conditions and Plasma IGF-1 LevelsA Randomized, Double-Blind, Placebo-Controlled Trial. Mar. Drugs 2018, 16, 482. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2021, 20, 825–834. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Fleck, C.A.; Simman, R. Modern collagen wound dressings: Function and purpose. J. Am. Coll. Certif. Wound Spec. 2010, 2, 50–54. [Google Scholar] [CrossRef]
- Felician, F.F.; Yu, R.H.; Li, M.Z.; Li, C.J.; Chen, H.Q.; Jiang, Y.; Tang, T.; Qi, W.Y.; Xu, H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. Zhonghua Chuang Shang Za Zhi 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Liang, C.; Luo, P.; Huang, J.; He, J.; Wang, Z.; Cao, X.; Peng, C.; Wu, S. Pericellular collagen I coating for enhanced homing and chondrogenic differentiation of mesenchymal stem cells in direct intra-articular injection. Stem Cell Res. Ther. 2018, 9, 174. [Google Scholar] [CrossRef]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef]
- Hoemann, C.; Kandel, R.; Roberts, S.; Saris, D.B.; Creemers, L.; Mainil-Varlet, P.; Méthot, S.; Hollander, A.P.; Buschmann, M.D. International Cartilage Repair Society (ICRS) Recommended Guidelines for Histological Endpoints for Cartilage Repair Studies in Animal Models and Clinical Trials. Cartilage 2011, 2, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Lindert, U.; Gnoli, M.; Maioli, M.; Bedeschi, M.F.; Sangiorgi, L.; Rohrbach, M.; Giunta, C. Insight into the Pathology of a COL1A1 Signal Peptide Heterozygous Mutation Leading to Severe Osteogenesis Imperfecta. Calcif. Tissue Int. 2018, 102, 373–379. [Google Scholar] [CrossRef]
- Tabeta, K.; Du, X.; Arimatsu, K.; Yokoji, M.; Takahashi, N.; Amizuka, N.; Hasegawa, T.; Crozat, K.; Maekawa, T.; Miyauchi, S.; et al. An ENU-induced splice site mutation of mouse Col1a1 causing recessive osteogenesis imperfecta and revealing a novel splicing rescue. Sci. Rep. 2017, 7, 11717. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Valle, D. Cornea, Fundamentals, Diagnosis and Management. Arch. De La Soc. Española De Oftalmol. 2005, 80, 373–374. [Google Scholar]
- Kabosova, A.; Azar, D.T.; Bannikov, G.A.; Campbell, K.P.; Durbeej, M.; Ghohestani, R.F.; Jones, J.C.; Kenney, M.C.; Koch, M.; Ninomiya, Y.; et al. Compositional differences between infant and adult human corneal basement membranes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4989–4999. [Google Scholar] [CrossRef] [PubMed]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef]
- Danysh, B.P.; Duncan, M.K. The lens capsule. Exp. Eye Res. 2009, 88, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Alavi, M.V.; Labelle-Dumais, C.; Gould, D.B. Type IV Collagens and Basement Membrane Diseases: Cell Biology and Pathogenic Mechanisms. Curr. Top. Membr. 2015, 76, 61–116. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Jerdon, J.A.; Glaser, B.M. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1615–1621. [Google Scholar]
- Sun, J.H.; Huang, M.; Fang, Z.; Li, T.X.; Wu, T.T.; Chen, Y.; Quan, D.P.; Xu, Y.Y.; Wang, Y.M.; Yang, Y.; et al. Nerve bundle formation during the promotion of peripheral nerve regeneration: Collagen VI-neural cell adhesion molecule 1 interaction. Neural Regen. Res. 2022, 17, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cescon, M.; Zuccolotto, G.; Nobbio, L.; Colombelli, C.; Filaferro, M.; Vitale, G.; Feltri, M.L.; Bonaldo, P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015, 129, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhou, L.; Zheng, X.; Hu, Y. Sustained release of collagen VI potentiates sciatic nerve regeneration by modulating macrophage phenotype. Eur. J. Neurosci. 2017, 45, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Roberton, V.; Costa, E.; Phillips, J.B. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021, 126, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Song, H.; He, J.; Li, G.; Zheng, Y.; Li, B. Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food Funct. 2019, 10, 6121–6134. [Google Scholar] [CrossRef]
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, H.; Wang, S.; Zhao, X.; Li, B. Effects of early enteral nutrition supplemented with collagen peptides on post-burn inflammatory responses in a mouse model. Food Funct. 2017, 8, 1933–1941. [Google Scholar] [CrossRef]
- Kim, B.S.; Choi, J.S.; Kim, J.D.; Yoon, H.I.; Choi, Y.C.; Cho, Y.W. Human collagen isolated from adipose tissue. Biotechnol. Prog. 2012, 28, 973–980. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Olsen, D.; Yang, C.; Bodo, M.; Chang, R.; Leigh, S.; Baez, J.; Carmichael, D.; Perälä, M.; Hämäläinen, E.R.; Jarvinen, M.; et al. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev. 2003, 55, 1547–1567. [Google Scholar] [CrossRef] [PubMed]
- John, D.C.; Watson, R.; Kind, A.J.; Scott, A.R.; Kadler, K.E.; Bulleid, N.J. Expression of an engineered form of recombinant procollagen in mouse milk. Nat. Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Toman, P.D.; Pieper, F.; Sakai, N.; Karatzas, C.; Platenburg, E.; de Wit, I.; Samuel, C.; Dekker, A.; Daniels, G.A.; Berg, R.A.; et al. Production of recombinant human type I procollagen homotrimer in the mammary gland of transgenic mice. Transgenic Res. 1999, 8, 415–427. [Google Scholar] [CrossRef]
- Tomita, M.; Munetsuna, H.; Sato, T.; Adachi, T.; Hino, R.; Hayashi, M.; Shimizu, K.; Nakamura, N.; Tamura, T.; Yoshizato, K. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat. Biotechnol. 2003, 21, 52–56. [Google Scholar] [CrossRef]
- Adachi, T.; Tomita, M.; Shimizu, K.; Ogawa, S.; Yoshizato, K. Generation of hybrid transgenic silkworms that express Bombyx mori prolyl-hydroxylase alpha-subunits and human collagens in posterior silk glands: Production of cocoons that contained collagens with hydroxylated proline residues. J. Biotechnol. 2006, 126, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Rutschmann, C.; Baumann, S.; Cabalzar, J.; Luther, K.B.; Hennet, T. Recombinant expression of hydroxylated human collagen in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 4445–4455. [Google Scholar] [CrossRef]
- Báez, J.; Olsen, D.; Polarek, J.W. Recombinant microbial systems for the production of human collagen and gelatin. Appl. Microbiol. Biotechnol. 2005, 69, 245–252. [Google Scholar] [CrossRef]
- Liao, H.M.; Fang, J.S.; Chen, Y.J.; Wu, K.L.; Lee, K.F.; Chen, C.H. Clinical and molecular characterization of a transmitted reciprocal translocation t(1;12)(p32.1;q21.3) in a family co-segregating with mental retardation, language delay, and microcephaly. BMC Med. Genet. 2011, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Baez, J.; Pappu, K.M.; Glatz, C.E. Purification and characterization of a transgenic corn grain-derived recombinant collagen type I alpha 1. Biotechnol. Prog. 2009, 25, 1660–1668. [Google Scholar] [CrossRef]
- Stein, H.; Wilensky, M.; Tsafrir, Y.; Rosenthal, M.; Amir, R.; Avraham, T.; Ofir, K.; Dgany, O.; Yayon, A.; Shoseyov, O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules 2009, 10, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, K.; Ritala, A.; Suntio, T.; Blumer, S.; Holkeri, H.; Wahlström, E.H.; Baez, J.; Mäkinen, K.; Maria, N.A. Production of a recombinant full-length collagen type I alpha-1 and of a 45-kDa collagen type I alpha-1 fragment in barley seeds. Plant Biotechnol. J. 2009, 7, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.; Perret, S.; Lacour, T.; Jonval, V.; Hudaverdian, S.; Garrone, R.; Ruggiero, F.; Theisen, M. Hydroxylated human homotrimeric collagen I in Agrobacterium tumefaciens-mediated transient expression and in transgenic tobacco plant. FEBS Lett. 2002, 515, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.; Rezaei, N.; Chan, C.K.; Xu, C.; Panwar, P.; Brömme, D.; Merschrod, S.E.F. Development and characterization of a eukaryotic expression system for human type II procollagen. BMC Biotechnol. 2015, 15, 1–17. [Google Scholar] [CrossRef]
- Xi, C.; Liu, N.; Liang, F.; Zhao, X.; Long, J.; Yuan, F.; Yun, S.; Sun, Y.; Xi, Y. Molecular assembly of recombinant chicken type II collagen in the yeast Pichia pastoris. Sci. China Life Sci. 2018, 61, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Levin, R.; Landau, S.; Kaduri, M.; Adir, O.; Ianovici, I.; Krinsky, N.; Doppelt-Flikshtain, O.; Shklover, J.; Shainsky-Roitman, J.; et al. Implanted synthetic cells trigger tissue angiogenesis through de novo production of recombinant growth factors. Proc. Natl. Acad. Sci. USA 2022, 119, e2207525119. [Google Scholar] [CrossRef]
- Jianjun, Y.; Hua, Z. Progress of Domestic Recombinant Human-Like Collagen Research and Application. J. China Deterg. Cosmet. 2022, 7, 58–62. [Google Scholar]
- Prockop, D.J.; Kivirikko, K.I. COLLAGENS: Molecular biology, diseases, and potentials for therapy. In Annual Review of Biochemistry; Richardson, C.C., Ed.; Annual Reviews: San Mateo, CA, USA, 1995; Volume 64, pp. 403–434. [Google Scholar]
- Twyman, R.M.; Stoger, E.; Schillberg, S.; Christou, P.; Fischer, R. Molecular farming in plants: Host systems and expression technology. Trends Biotechnol. 2003, 21, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Gu, T.W.; Xu, Y.; Dad, H.A.; Liu, J.X.; Lian, J.Z.; Huang, L.Q. Gene delivery strategies for therapeutic proteins production in plants: Emerging opportunities and challenges. Biotechnol. Adv. 2022, 54, 107845. [Google Scholar] [CrossRef]
- Choi, F.D.; Sung, C.T.; Juhasz, M.L.; Mesinkovsk, N.A. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J. Drugs Dermatol. JDD 2019, 18, 9–16. [Google Scholar]
- Gu, L.S.; Shan, T.T.; Ma, Y.X.; Tay, F.R.; Niu, L.N. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Jhawar, N.; Wang, J.V.; Saedi, N. Oral collagen supplementation for skin aging: A fad or the future? J Cosmet Derm. 2020, 19, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Genovese, L.; Corbo, A.; Sibilla, S. An Insight into the Changes in Skin Texture and Properties following Dietary Intervention with a Nutricosmeceutical Containing a Blend of Collagen Bioactive Peptides and Antioxidants. Ski. Pharmacol. Physiol. 2017, 30, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.C.; Arnhold, S. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histochem. Cell Biol. 2010, 134, 545–554. [Google Scholar] [CrossRef]
- Ohnishi, A.; Osaki, T.; Matahira, Y.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Evaluation of the chondroprotective effects of glucosamine and fish collagen peptide on a rabbit ACLT model using serum biomarkers. J. Vet. Med. Sci. 2013, 75, 421–429. [Google Scholar] [CrossRef]
- Li, D.X.; Fan, H.S.; Zhu, X.D.; Tan, Y.F.; Xiao, W.Q.; Lu, J.; Xiao, Y.M.; Chen, J.Y.; Zhang, X.D. Controllable release of salmon-calcitonin in injectable calcium phosphate cement modified by chitosan oligosaccharide and collagen polypeptide. J. Mater. Sci. Mater. Med. 2007, 18, 2225–2231. [Google Scholar] [CrossRef]
- Vardar, E.; Larsson, H.M.; Allazetta, S.; Engelhardt, E.M.; Pinnagoda, K.; Vythilingam, G.; Hubbell, J.A.; Lutolf, M.P.; Frey, P. Microfluidic production of bioactive fibrin micro-beads embedded in crosslinked collagen used as an injectable bulking agent for urinary incontinence treatment. Acta Biomater. 2018, 67, 156–166. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265–266. [Google Scholar] [CrossRef]
- Ahn, S.; Yoon, H.; Kim, G.; Kim, Y.; Lee, S.; Chun, W. Designed three-dimensional collagen scaffolds for skin tissue regeneration. Tissue Eng. Part C Methods 2010, 16, 813–820. [Google Scholar] [CrossRef]
- Vaneerdeweg, W.; Bresseleers, T.; Du Jardin, P.; Lauwers, P.; Pauli, S.; Thyssens, K.; Van Marck, E.; Elseviers, M.; Eyskens, E. Comparison between plain and gentamicin containing collagen sponges in infected peritoneal cavity in rats. Eur. J. Surg. = Acta Chir. 1998, 164, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Stemberger, A.; Grimm, H.; Bader, F.; Rahn, H.D.; Ascherl, R. Local treatment of bone and soft tissue infections with the collagen-gentamicin sponge. Eur. J. Surg. Suppl. Acta Chir. Suppl. 1997, 578, 17–26. [Google Scholar]
- Barrientos, I.J.H.; Paladino, E.; Szabo, P.; Brozio, S.; Hall, P.J.; Oseghale, C.I.; Passarelli, M.K.; Moug, S.J.; Black, R.A.; Wilson, C.G.; et al. Electrospun collagen-based nanofibres: A sustainable material for improved antibiotic utilisation in tissue engineering applications. Int. J. Pharm. 2017, 531, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.; Nash, J.R.; Kingsnorth, A.N. Effect of transforming growth factor beta and basic fibroblast growth factor on steroid-impaired healing intestinal wounds. Br. J. Surg. 1992, 79, 69–72. [Google Scholar] [CrossRef]
- Saltzman, W.M.; Parkhurst, M.R.; Parsons-Wingerter, P.; Zhu, W.H. Three-dimensional cell cultures mimic tissues. Ann. N. Y. Acad. Sci. 1992, 665, 259–273. [Google Scholar] [CrossRef]
- Cascone, M.G.; Sim, B.; Downes, S. Blends of synthetic and natural polymers as drug delivery systems for growth hormone. Biomaterials 1995, 16, 569–574. [Google Scholar] [CrossRef]
- Uchio, Y.; Ochi, M.; Matsusaki, M.; Kurioka, H.; Katsube, K. Human chondrocyte proliferation and matrix synthesis cultured in Atelocollagen gel. J. Biomed. Mater. Res. 2000, 50, 138–143. [Google Scholar] [CrossRef]
- Rubin, A.L.; Stenzel, K.H.; Miyata, T.; White, M.J.; Dunn, M. Collagen as a vehicle for drug delivery. Preliminary report. J. Clin. Pharmacol. 1973, 13, 309–312. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. A Rev. J. 2021, 146, 100641. [Google Scholar] [CrossRef]
- Jin, X.; Liu, W.; Wang, J.; Xiao, Z.; Niu, Y.; Chen, B.; Zhao, Y.; Dai, J. Clinical study of injectable collagen scaffold with autologous fat cells for repair of severe vocal fold injury. Biomed. Mater. 2022, 17, 035004. [Google Scholar] [CrossRef]
- Panayi, A.C.; Haug, V.; Liu, Q.; Wu, M.; Karvar, M.; Aoki, S.; Ma, C.; Hamaguchi, R.; Endo, Y.; Orgill, D.P. Novel application of autologous micrografts in a collagen-glycosaminoglycan scaffold for diabetic wound healing. Biomed. Mater. 2021, 16, 035032. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Kadota, K.; Kajihara, M.; Sano, A.; Fujioka, K. Sustained release of human growth hormone (hGH) from collagen film and evaluation of effect on wound healing in db/db mice. J. Control. Release Off. J. Control. Release Soc. 2001, 77, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.J. Biological dressings in burns--a review. Ann. Plast. Surg. 1980, 4, 133–137. [Google Scholar] [CrossRef]
- Gogia, P.P.; Marquez, R.R. Effects of helium-neon laser on wound healing. Ostomy/Wound Manag. 1992, 38, 33, 36, 38–41. [Google Scholar]
- Boyce, S.T. Skin substitutes from cultured cells and collagen-GAG polymers. Med. Biol. Eng. Comput. 1998, 36, 791–800. [Google Scholar] [CrossRef]
- Mi, S.; Connon, C.J. The formation of a tissue-engineered cornea using plastically compressed collagen scaffolds and limbal stem cells. In Corneal Regenerative Medicine; Humana Press: Totowa, NJ, USA, 2013; Volume 1014, pp. 143–155. [Google Scholar] [CrossRef]
- Takezawa, T.; Ozaki, K.; Nitani, A.; Takabayashi, C.; Shimo-Oka, T. Collagen vitrigel: A novel scaffold that can facilitate a three-dimensional culture for reconstructing organoids. Cell Transplant. 2004, 13, 463–473. [Google Scholar] [CrossRef]
- King, V.R.; Alovskaya, A.; Wei, D.Y.T.; Brown, R.A.; Priestley, J.V. The use of injectable forms of fibrin and fibronectin to support axonal ingrowth after spinal cord injury. Biomaterials 2010, 31, 4447–4456. [Google Scholar] [CrossRef]



| Types | Subunits and Composition | Composition of Molecular Aggregates | Tissue Distribution | Functions |
|---|---|---|---|---|
| Ⅰ | α1(I) × 2, α2(I) | Large-diameter cross strip fiber | Bone, cornea, skin, tendon, ligament, tumor | Support fiber |
| II | α1(II) × 3 | Small-diameter cross strip fiber | Hyaline cartilage, vitreous body, intervertebral disc | Support fiber |
| III | α1(III) × 3 | Small-diameter cross strip fiber | Skin, blood vessels, muscles, internal organs | Support fiber |
| IV | α1(IV) × 3; α2(IV) × 3; α1(IV) × 2, α2(IV) | Non-fibrous reticular structure | Basement membrane | Reticular scaffold, control of multifunctional cells, site binding |
| V | α1(V) × 2, α2(V); α1(V), α2(V), α3(V) | Small-diameter cross fibers, or forming molecules with type VI chains | Smooth muscle, cultured cells, embryonic tissue, peritoneum, placenta, skin, bone | Small fibers around the supporting cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, N.; Liu, Y.-D.; Zhang, Y.; Gu, T.-W.; Peng, L.-H. Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics 2023, 15, 1443. https://doi.org/10.3390/pharmaceutics15051443
Zhou N, Liu Y-D, Zhang Y, Gu T-W, Peng L-H. Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics. 2023; 15(5):1443. https://doi.org/10.3390/pharmaceutics15051443
Chicago/Turabian StyleZhou, Nan, Yu-Da Liu, Yue Zhang, Ting-Wei Gu, and Li-Hua Peng. 2023. "Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial" Pharmaceutics 15, no. 5: 1443. https://doi.org/10.3390/pharmaceutics15051443
APA StyleZhou, N., Liu, Y.-D., Zhang, Y., Gu, T.-W., & Peng, L.-H. (2023). Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics, 15(5), 1443. https://doi.org/10.3390/pharmaceutics15051443





