Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers
Abstract
:1. Introduction
2. Plant Derivatives Vehiculated in EXOs and Biomimetic Hybrid Vesicles
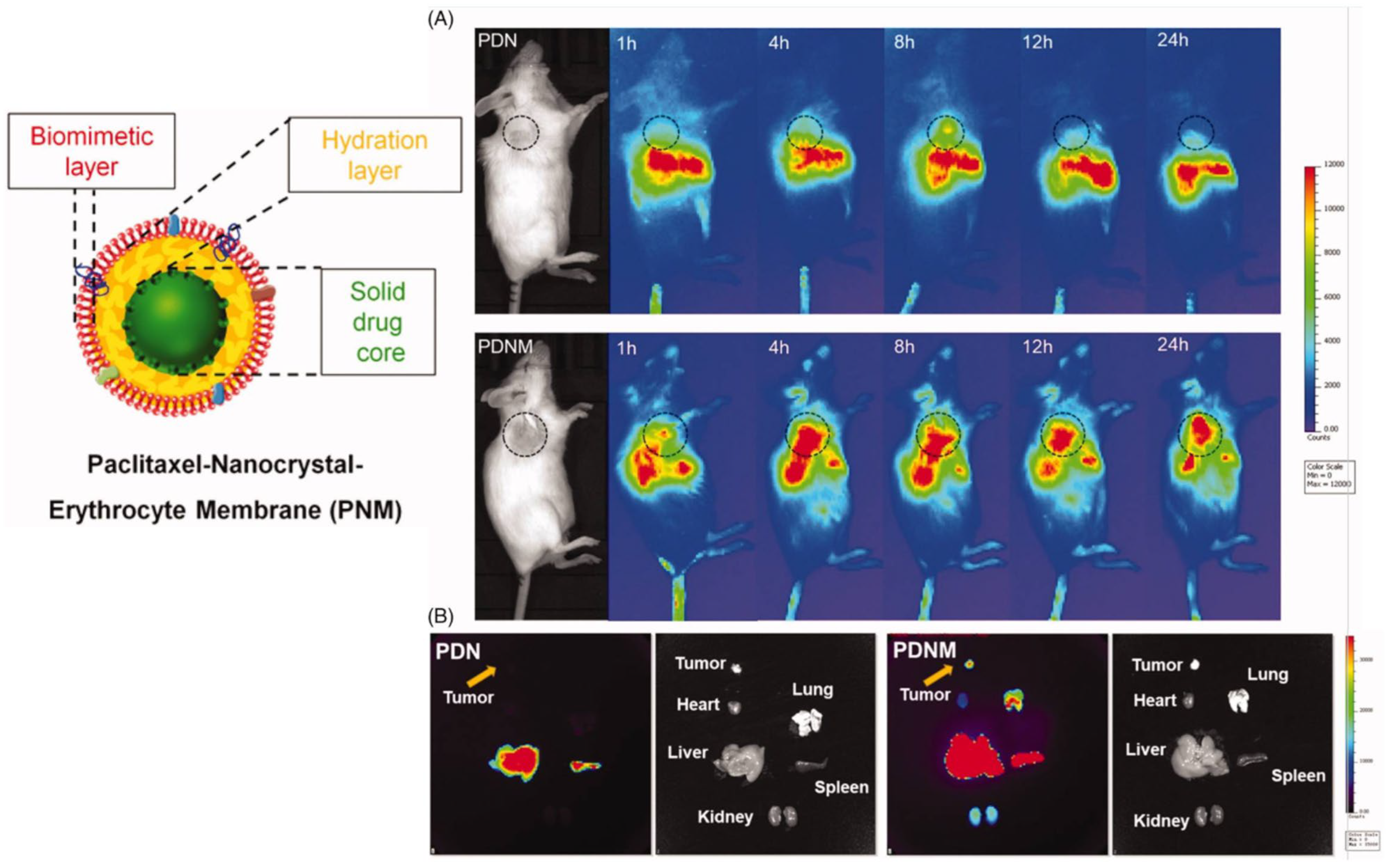
2.1. Paclitaxel
2.2. Camptothecin
2.3. Curcumin
3. EXOs and Biomimetic Vesicles Loaded with Other Phytochemicals
3.1. Vincristine
3.2. Chrysin
3.3. Delphinidin
3.4. Berberine
3.5. Black Bean Extracts
3.6. Anthocyanidins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valent, P.; Bonnet, D.; De Maria, R.; Lapidot, T.; Copland, M.; Melo, J.V.; Chomienne, C.; Ishikawa, F.; Schuringa, J.J.; Stassi, G.; et al. Cancer stem cell definitions and terminology: The devil is in the details. Nat. Rev. Cancer 2012, 12, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Canepa, P.; Canale, C.; Canepa, M.; Cavalleri, O. Morphological and mechanical characterization of DNA SAMs combining nanolithography with AFM and optical methods. Materials 2020, 13, 2888. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Dante, S.; Rotondi, S.M.C.; Canepa, P.; Cavalleri, O.; Canepa, M. Spectroscopic Ellipsometry Investigation of a Sensing Functional Interface: DNA SAMs Hybridization. Adv. Mater. Interfaces 2022, 9, 2200364. [Google Scholar] [CrossRef]
- Qu, J.-H.; Dillen, A.; Saeys, W.; Lammertyn, J.; Spasic, D. Advancements in SPR Biosensing Technology: An Overview of Recent Trends in Smart Layers Design, Multiplexing Concepts, Continuous Monitoring and in Vivo Sensing. Anal. Chim. Acta 2020, 1104, 10–27. [Google Scholar] [CrossRef]
- Pinto, G.; Parisse, P.; Solano, I.; Canepa, P.; Canepa, M.; Casalis, L.; Cavalleri, O. Functionalizing gold with single strand DNA: Novel insight into optical properties via combined spectroscopic ellipsometry and nanolithography measurements. Soft Matter 2019, 15, 2463–2468. [Google Scholar] [CrossRef]
- Hait, W.N. Anticancer drug development: The grand challenges. Nat. Rev. Drug Discov. 2010, 9, 253–254. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asia. Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed]
- Babaei, G.; Aliarab, A.; Abroon, S.; Rasmi, Y.; Aziz, S.G.G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018, 106, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant. Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and Nutritional Supplements on Breast Cancer. Biomed. Res. Int. 2017, 2017, 7207983. [Google Scholar] [CrossRef]
- Jahan, I.; Onay, A. Potentials of plant-based substance to inhabit and probable cure for the COVID-19. Turk. J. Biol. 2020, 44, 228–241. [Google Scholar] [CrossRef]
- Tariq, A.; Sadia, S.; Pan, K.W.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.L.; Song, D.G.; et al. A systematic review on ethnomedicines of anticancer plants. Phytother. Res. 2017, 31, 202–264. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Gao, S.; Basu, S.; Yang, Z.; Deb, A.; Hu, M. Bioavailability Challenges Associated with Development of Saponins as Therapeutic and Chemopreventive Agents. Curr. Drug Targets 2012, 13, 1885–1899. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Schlich, M.; Lai, F.; Pireddu, R.; Pini, E.; Ailuno, G.; Fadda, A.M.; Valenti, D.; Sinico, C. Resveratrol proniosomes as a convenient nanoingredient for functional food. Food Chem. 2020, 310, 125950. [Google Scholar] [CrossRef]
- Shen, Y.X.; Zhang, N.; Tian, J.L.; Xin, G.; Liu, L.; Sun, X.Y.; Li, B. Advanced approaches for improving bioavailability and controlled release of anthocyanins. J. Control. Release 2022, 341, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Valdes, K.; Morales, J.; Rodriguez, L.; Gunther, G. Potential use of nanocarriers with pentacyclic triterpenes in cancer treatments. Nanomedicine 2016, 12, 3139–3156. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Asprea, M.; Risaliti, L.; Vanti, G.; Bergonzi, M.C. Plants Extracts Loaded in Nanocarriers: An Emergent Formulating Approach. Nat. Prod. Commun. 2018, 13, 1157–1160. [Google Scholar] [CrossRef]
- Lai, F.; Schlich, M.; Pireddu, R.; Fadda, A.M.; Sinico, C. Nanocrystals as Effective Delivery Systems of Poorly Water-soluble Natural Molecules. Curr. Med. Chem. 2019, 26, 4657–4680. [Google Scholar] [CrossRef]
- Lin, M.H.; Hung, C.F.; Hsu, C.Y.; Lin, Z.C.; Fang, J.Y. Prodrugs in combination with nanocarriers as a strategy for promoting antitumoral efficiency. Future Med. Chem. 2019, 11, 2131–2150. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef]
- Vanti, G. Recent strategies in nanodelivery systems for natural products: A review. Environ. Chem. Lett. 2021, 19, 4311–4326. [Google Scholar] [CrossRef]
- Truzzi, E.; Rustichelli, C.; de Oliveira, E.R.; Ferraro, L.; Maretti, E.; Graziani, D.; Botti, G.; Beggiato, S.; Iannuccelli, V.; Lima, E.M.; et al. Nasal biocompatible powder of Geraniol oil complexed with cyclodextrins for neurodegenerative diseases: Physicochemical characterization and in vivo evidences of nose to brain delivery. J. Control. Release 2021, 335, 191–202. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte, C.A. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Goniotaki, M.; Hatziantoniou, S.; Dimas, K.; Wagner, M.; Demetzos, C. Encapsulation of naturally occurring flavonoids into liposomes: Physicochemical properties and biological activity against human cancer cell lines. J. Pharm. Pharmacol. 2004, 56, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Mignet, N.; Seguin, J.; Romano, M.R.; Brulle, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a liposomal formulation of the natural flavonoid fisetin. Int. J. Pharm. 2012, 423, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.M.M.; Gowayed, M.A.; El-Ganainy, S.O.; Sheta, E.; Elnaggar, Y.S.R.; Abdallah, O.Y. Pectin coated nanostructured lipid carriers for targeted piperine delivery to hepatocellular carcinoma. Int. J. Pharm. 2022, 619, 121712. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Anticancer Applications of Essential Oils Formulated into Lipid-Based Delivery Nanosystems. Pharmaceutics 2022, 14, 2681. [Google Scholar] [CrossRef]
- Jing, D.D.; Wu, W.; Chen, X.Z.; Xiao, H.W.; Zhang, Z.H.; Chen, F.X.; Zhang, Z.C.; Liu, J.X.; Shao, Z.W.; Pu, F.F. Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 Via the JAK2-STAT3-PDL1. Pharmacol. Res. 2022, 182, 106287. [Google Scholar] [CrossRef]
- Demirbolat, G.M.; Erdogan, O.; Coskun, G.P.; Cevik, O. PEG4000 modified liposomes enhance the solubility of quercetin and improve the liposome functionality: In vitro characterization and the cellular efficacy. Turk. J. Chem. 2022, 46, 1011–1023. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.L.; Peng, J.R.; Zhang, L.; Qu, Y.; Chu, B.Y.; Dong, M.L.; Tan, L.W.; Qian, Z.Y. Biodegradable Self-Assembled Micelles Based on MPEG-PTMC Copolymers: An Ideal Drug Delivery System for Vincristine. J. Biomed. Nanotechnol. 2017, 13, 427–436. [Google Scholar] [CrossRef]
- El-Far, S.W.; Helmy, M.W.; Khattab, S.N.; Bekhit, A.A.; Hussein, A.A.; Elzoghby, A.O. Phytosomal bilayer-enveloped casein micelles for codelivery of monascus yellow pigments and resveratrol to breast cancer. Nanomedicine 2018, 13, 481–499. [Google Scholar] [CrossRef]
- Ghalehkhondabi, V.; Soleymani, M.; Fazlali, A. Folate-targeted nanomicelles containing silibinin as an active drug delivery system for liver cancer therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102157. [Google Scholar] [CrossRef]
- Chen, C.; Du, S.Y.; Zhong, W.; Liu, K.G.; Qu, L.H.; Chu, F.Y.; Yang, J.J.; Han, X. Accurate delivery of pristimerin and paclitaxel by folic acid-linked nano-micelles for enhancing chemosensitivity in cancer therapy. Nano Converg. 2022, 9, 52. [Google Scholar] [CrossRef]
- Tan, X.R.; Feng, K.K.; Le, J.Q.; Shen, J.W.; Shao, J.W. Self-assembled micelles of the natural medicine ginsenosides for cancer metastasis therapy. J. Ind. Eng. Chem. 2022, 116, 303–309. [Google Scholar] [CrossRef]
- Abdifetah, O.; Na-Bangchang, K. Pharmacokinetic studies of nanoparticles as a delivery system for conventional drugs and herb-derived compounds for cancer therapy: A systematic review. Int. J. Nanomed. 2019, 14, 5659–5677. [Google Scholar] [CrossRef] [PubMed]
- Mughees, M.; Wajid, S.; Samim, M. Cytotoxic potential of Artemisia absinthium extract loaded polymeric nanoparticles against breast cancer cells: Insight into the protein targets. Int. J. Pharm. 2020, 586, 119583. [Google Scholar] [CrossRef] [PubMed]
- Mughees, M.; Wajid, S. Herbal Based Polymeric Nanoparticles as a Therapeutic Remedy for Breast Cancer. Anti-Cancer Agents Med. Chem. 2021, 21, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Alemi, A.; Farrokhifar, M.; Zare-Zardini, H.; Karamallah, M.H. A Comparison between the Anticancer Activities of Free Paclitaxel and Paclitaxel-Loaded Niosome Nanoparticles on Human Acute Lymphoblastic Leukemia Cell Line Nalm-6. Iran. J. Pediatr. Hematol. Oncol. 2018, 8, 153–160. [Google Scholar]
- Pourmoghadasiyan, B.; Tavakkoli, F.; Beram, F.M.; Badmasti, F.; Mirzaie, A.; Kazempour, R.; Rahimi, S.; Larijani, S.F.; Hejabi, F.; Sedaghatnia, K. Nanosized paclitaxel-loaded niosomes: Formulation, in vitro cytotoxicity, and apoptosis gene expression in breast cancer cell lines. Mol. Biol. Rep. 2022, 49, 3597–3608. [Google Scholar] [CrossRef]
- Mehrabi, M.R.; Shokrgozar, M.A.; Toliyat, T.; Shirzad, M.; Izadyari, A.; Mofrad, L.Z.; Chiani, M.; Akbarzadeh, A. Enhanced Therapeutic Efficacy of Vincristine Sulfate for Lymphoma Using Niosome-Based Drug Delivery. Jundish. J. Nat. Pharm. Prod. 2020, 15, e82793. [Google Scholar] [CrossRef]
- Sundararajan, M.; Thomas, P.A.; Venkadeswaran, K.; Jeganathan, K.; Geraldine, P. Synthesis and Characterization of Chrysin-Loaded beta-Cyclodextrin-Based Nanosponges to Enhance In-Vitro Solubility, Photostability, Drug Release, Antioxidant Effects and Antitumorous Efficacy. J. Nanosci. Nanotechnol. 2017, 17, 8742–8751. [Google Scholar] [CrossRef]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef]
- Clemente, N.; Argenziano, M.; Gigliotti, C.L.; Ferrara, B.; Boggio, E.; Chiocchetti, A.; Caldera, F.; Trotta, F.; Benetti, E.; Annaratone, L.; et al. Paclitaxel-Loaded Nanosponges Inhibit Growth and Angiogenesis in Melanoma Cell Models. Front. Pharmacol. 2019, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Hariri, G.; Edwards, A.D.; Merrill, T.B.; Greenbaum, J.M.; van der Ende, A.E.; Harth, E. Sequential Targeted Delivery of Paclitaxel and Camptothecin Using a Cross-Linked “Nanosponge” Network for Lung Cancer Chemotherapy. Mol. Pharm. 2014, 11, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, C.L.; Ferrara, B.; Occhipinti, S.; Boggio, E.; Barrera, G.; Pizzimenti, S.; Giovarelli, M.; Fantozzi, R.; Chiocchetti, A.; Argenziano, M.; et al. Enhanced cytotoxic effect of camptothecin nanosponges in anaplastic thyroid cancer cells in vitro and in vivo on orthotopic xenograft tumors. Drug Deliv. 2017, 24, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Allahyari, S.; Zahednezhad, F.; Khatami, M.; Hashemzadeh, N.; Zakeri-Milani, P.; Trotta, F. Cyclodextrin nanosponges as potential anticancer drug delivery systems to be introduced into the market, compared with liposomes. J. Drug Deliv. Sci. Technol. 2022, 67, 102931. [Google Scholar] [CrossRef]
- Zhang, H.; Hollis, C.P.; Zhang, Q.; Li, T.L. Preparation and antitumor study of camptothecin nanocrystals. Int. J. Pharm. 2011, 415, 293–300. [Google Scholar] [CrossRef]
- Wang, J.H.; Muhammad, N.; Li, T.T.; Wang, H.; Liu, Y.J.; Liu, B.N.; Zhan, H.L. Hyaluronic Acid-Coated Camptothecin Nanocrystals for Targeted Drug Delivery to Enhance Anticancer Efficacy. Mol. Pharm. 2020, 17, 2411–2425. [Google Scholar] [CrossRef]
- Ji, P.; Wang, L.; Chen, Y.W.; Wang, S.Q.; Wu, Z.H.; Qi, X.L. Hyaluronic acid hydrophilic surface rehabilitating curcumin nanocrystals for targeted breast cancer treatment with prolonged biodistribution. Biomater. Sci. 2020, 8, 462–472. [Google Scholar] [CrossRef]
- Manca, M.L.; Lai, F.; Pireddu, R.; Valenti, D.; Schlich, M.; Pini, E.; Ailuno, G.; Fadda, A.M.; Sinico, C. Impact of nanosizing on dermal delivery and antioxidant activity of quercetin nanocrystals. J. Drug Deliv. Sci. Technol. 2020, 55, 101482. [Google Scholar] [CrossRef]
- Han, S.D.; Li, X.P.; Zhou, C.H.; Hu, X.P.; Zhou, Y.H.; Jin, Y.; Liu, Q.; Wang, L.Q.; Li, X.R.; Liu, Y. Further Enhancement in Intestinal Absorption of Paclitaxel by Using Transferrin-Modified Paclitaxel Nanocrystals. ACS Appl. Biomater. 2020, 3, 4684–4695. [Google Scholar] [CrossRef]
- Sreeharsha, N.; Prasanthi, S.; Mahalakshmi, S.; Goudanavar, P.S.; Naveen, N.R.; Gowthami, B.; Fattepur, S.; Meravanige, G.; Asdaq, S.M.B.; Anwer, M.K.; et al. Enhancement of Anti-Tumoral Properties of Paclitaxel Nano-Crystals by Conjugation of Folic Acid to Pluronic F127: Formulation Optimization, In vitro and In vivo Study. Molecules 2022, 27, 7914. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Ailuno, G.; Baldassari, S.; Lai, F.; Florio, T.; Caviglioli, G. Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research. Cells 2020, 9, 2569. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFrvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, U619–U624. [Google Scholar] [CrossRef] [PubMed]
- Ristorcelli, E.; Beraud, E.; Verrando, P.; Villard, C.; Lafitte, D.; Sbarra, V.; Lombardo, D.; Verine, A. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008, 22, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, H.T.; Wang, S.; Huang, S.L.; Zheng, Y.; Wang, C.Q.; Hu, B.Y.; Qin, W.; Zou, T.T.; Fu, Y.; et al. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef]
- Alzhrani, G.N.; Alanazi, S.T.; Alsharif, S.Y.; Albalawi, A.M.; Alsharif, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol. Int. 2021, 45, 1807–1831. [Google Scholar] [CrossRef]
- Lu, M.; Huang, Y.Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119925. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lotvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Pascucci, L.; Cocce, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Vigano, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Melzer, C.; Rehn, V.; Yang, Y.Y.; Bahre, H.; von der Ohe, J.; Hass, R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.L.; Yuan, D.F.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Wang, H.H.; Huang, Q.Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.F.; Wang, L.; Chen, W.D. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics 2019, 9, 1714–1727. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.N.; Chaudhuri, A.; Dehari, D.; Shekher, A.; Gupta, S.C.; Majumdar, S.; Krishnamurthy, S.; Singh, S.; Kumar, D.; Agrawal, A.K. Combination Therapy Comprising Paclitaxel and 5-Fluorouracil by Using Folic Acid Functionalized Bovine Milk Exosomes Improves the Therapeutic Efficacy against Breast Cancer. Life 2022, 12, 1143. [Google Scholar] [CrossRef]
- Salarpour, S.; Forootanfar, H.; Pournamdari, M.; Ahmadi-Zeidabadi, M.; Esmaeeli, M.; Pardakhty, A. Paclitaxel incorporated exosomes derived from glioblastoma cells: Comparative study of two loading techniques. Daru 2019, 27, 533–539. [Google Scholar] [CrossRef]
- Su, J.H.; Sun, H.P.; Meng, Q.S.; Yin, Q.; Tang, S.; Zhang, P.C.; Chen, Y.; Zhang, Z.W.; Yu, H.J.; Li, Y.P. Long Circulation Red-Blood-Cell-Mimetic Nanoparticles with Peptide-Enhanced Tumor Penetration for Simultaneously Inhibiting Growth and Lung Metastasis of Breast Cancer. Adv. Funct. Mater. 2016, 26, 1243–1252. [Google Scholar] [CrossRef]
- Su, J.H.; Sun, H.P.; Meng, Q.S.; Yin, Q.; Zhang, P.C.; Zhang, Z.W.; Yu, H.J.; Li, Y.P. Bioinspired Nanoparticles with NIR-Controlled Drug Release for Synergetic Chemophotothermal Therapy of Metastatic Breast Cancer. Adv. Funct. Mater. 2016, 26, 7495–7506. [Google Scholar] [CrossRef]
- Zhai, Z.; Xu, P.C.; Yao, J.; Li, R.D.; Gong, L.D.; Yin, Y.X.; Lin, Z.Q. Erythrocyte-mimicking paclitaxel nanoparticles for improving biodistributions of hydrophobic drugs to enhance antitumor efficacy. Drug Deliv. 2020, 27, 387–399. [Google Scholar] [CrossRef]
- Song, M.; Dong, S.; An, X.; Zhang, W.; Shen, N.; Li, Y.; Guo, C.; Liu, C.; Li, X.; Chen, S. Erythrocyte-biomimetic nanosystems to improve antitumor effects of paclitaxel on epithelial cancers. J. Control. Release 2022, 345, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tan, T.F.; Zhu, D.C.; Yu, H.X.; Liu, Y.R.; Zhou, H.Y.; Jin, Y.; Xia, Q. Paclitaxel-Loaded Macrophage Membrane Camouflaged Albumin Nanoparticles for Targeted Cancer Therapy. Int. J. Nanomed. 2020, 15, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, W.; Hu, Y.; Li, W.P.; Di, W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics 2020, 10, 3325–3339. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.X.; Liu, J.H.; Wu, J.Y.; Li, Y.J.; Qiu, X.H.; Xu, W.J.; Xu, P.; Xiang, D.X. Hybrid Cell Membrane-Functionalized Biomimetic Nanoparticles for Targeted Therapy of Osteosarcoma. Int. J. Nanomed. 2022, 17, 837–854. [Google Scholar] [CrossRef]
- Yang, Y.L.; Ren, S.Q.; Huang, W.P.; Dong, J.H.; Guo, J.C.; Zhao, J.; Zhang, Y.G. Camptothecin Delivery via Tumor-Derived Exosome for Radiosensitization by Cell Cycle Regulation on Patient-Derived Xenograft Mice. Front. Bioeng. Biotechnol. 2022, 10, 876641. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Kang, S.T.; Lin, Y.H.; Ho, Y.J.; Wang, C.H.; Yeh, C.K.; Chang, C.W. Biomimetic Acoustically-Responsive Vesicles for Theranostic Applications. Theranostics 2015, 5, 1264–1274. [Google Scholar] [CrossRef]
- Ghasemzadeh, T.; Hasannia, M.; Abnous, K.; Taghdisi, S.M.; Nekooei, S.; Nekooei, N.; Ramezani, M.; Alibolandi, M. Preparation of targeted theranostic red blood cell membranes-based nanobubbles for treatment of colon adenocarcinoma. Expert Opin. Drug Del. 2023, 20, 131–143. [Google Scholar] [CrossRef]
- Malhotra, S.; Dumoga, S.; Sirohi, P.; Singh, N. Red Blood Cells-Derived Vesicles for Delivery of Lipophilic Drug Camptothecin. ACS Appl. Mater. Interfaces 2019, 11, 22141–22151. [Google Scholar] [CrossRef]
- Malhotra, S.; Dumoga, S.; Mehta, S.; Rao, E.P.; Mohanty, S.; Singh, N. Engineering Cellular Membrane for Dual Mode Therapy Using NIR Responsive Photosensitizer and Reversible Topoisomerase Inhibition Activity. ACS Appl. Biomater. 2022, 5, 570–582. [Google Scholar] [CrossRef]
- Malhotra, S.; Dumoga, S.; Joshi, A.; Mohanty, S.; Singh, N. Polymeric micelles coated with hybrid nanovesicles enhance the therapeutic potential of the reversible topoisomerase inhibitor camptothecin in a mouse model. Acta Biomater. 2021, 121, 579–591. [Google Scholar] [CrossRef]
- Ying, K.K.; Zhu, Y.F.; Wan, J.Q.; Zhan, C.Y.; Wang, Y.C.; Xie, B.B.; Xu, P.R.; Pan, H.M.; Wang, H.X. Macrophage membrane-biomimetic adhesive polycaprolactone nanocamptothecin for improving cancer-targeting efficiency and impairing metastasis. Bioact. Mater. 2023, 20, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Gupta, R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sarrias, A.; Iglesias-Aguirre, C.E.; Cortes-Martin, A.; Vallejo, F.; Cattivelli, A.; del Pozo-Acebo, L.; Del Saz, A.; de las Hazas, M.C.L.; Davalos, A.; Espin, J.C. Milk-Derived Exosomes as Nanocarriers to Deliver Curcumin and Resveratrol in Breast Tissue and Enhance Their Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 2860. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.L.; Ding, Y.A.; He, C.; Wang, X.H.; Tang, Q.S. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Li, Y.; Huang, C.Y.; Xu, Y.H. Colon cancer exosome-derived biomimetic nanoplatform for curcumin-mediated sonodynamic therapy and calcium overload. Front. Bioeng. Biotechnol. 2022, 10, 1069676. [Google Scholar] [CrossRef]
- Del Fattore, A.; Luciano, R.; Saracino, R.; Battafarano, G.; Rizzo, C.; Pascucci, L.; Alessandri, G.; Pessina, A.; Perrotta, A.; Fierabracci, A.; et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin. Biol. Ther. 2015, 15, 495–504. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, D.; Zhou, H.Z.; Tao, B.Q.; Chang, L.; Liu, H.M.; Luo, H.M.; Wang, D.X.; Liu, W.W. A New Nanomaterial Based on Extracellular Vesicles Containing Chrysin-Induced Cell Apoptosis Through Let-7a in Tongue Squamous Cell Carcinoma. Front. Bioeng. Biotechnol. 2021, 9, 766380. [Google Scholar] [CrossRef]
- Barkallah, M.; Nzoughet-Kouassi, J.; Simard, G.; Thoulouze, L.; Marze, S.; Ropers, M.H.; Andriantsitohaina, R. Enhancement of the Anti-Angiogenic Effects of Delphinidin When Encapsulated within Small Extracellular Vesicles. Nutrients 2021, 13, 4378. [Google Scholar] [CrossRef]
- Salek, A.; Selmi, M.; Barboura, M.; Martinez, M.C.; Chekir-Ghedira, L.; Andriantsitohaina, R. Enhancement of the In vitro Antitumor Effects of Berberine Chloride When Encapsulated within Small Extracellular Vesicles. Pharmaceutics 2022, 14, 1913. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Guajardo-Flores, D.; Gonzalez-Valdez, J. Enhanced exosome-mediated delivery of black bean phytochemicals (Phaseolus vulgaris L.) for cancer treatment applications. Biomed. Pharmacother. 2020, 131, 110771. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Mudd, A.M.; Kyakulaga, A.H.; Singh, I.P.; Vadhanam, M.V.; Gupta, R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017, 393, 94–102. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.H.; Munagala, R.; Parker, L.; Gupta, R.C. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017, 8, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Conner, C.F. Taxanes and taxoids of the genus Taxus-A comprehensive inventory of chemical diversity. Phytochemistry 2021, 190, 112829. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updates 2021, 54, 100742. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Antonarakis, E.S. Cabazitaxel: A novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des. Dev. Ther. 2011, 5, 117–124. [Google Scholar] [CrossRef]
- Yang, C.P.H.; Horwitz, S.B. Taxol((R)): The First Microtubule Stabilizing Agent. Int. J. Mol. Sci. 2017, 18, 1733. [Google Scholar] [CrossRef]
- Wang, S.P.; Qiu, J.G.; Shi, Z.; Wang, Y.T.; Chen, M.W. Nanoscale drug delivery for taxanes based on the mechanism of multidrug resistance of cancer. Biotechnol. Adv. 2015, 33, 224–241. [Google Scholar] [CrossRef]
- Reagan, M.R.; Kaplan, D.L. Concise Review: Mesenchymal Stem Cell Tumor-Homing: Detection Methods in Disease Model Systems. Stem Cells 2011, 29, 920–927. [Google Scholar] [CrossRef]
- Xie, M.Y.; Tao, L.; Zhang, Z.Q.; Wang, W. Mesenchymal Stem Cells Mediated Drug Delivery in Tumor-Targeted Therapy. Curr. Drug Del. 2021, 18, 864–879. [Google Scholar] [CrossRef]
- Pessina, A.; Bonomi, A.; Cocce, V.; Invernici, G.; Navone, S.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Vigano, L.; Locatelli, A.; et al. Mesenchymal Stromal Cells Primed with Paclitaxel Provide a New Approach for Cancer Therapy. PLoS ONE 2011, 6, e28321. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; van der Meel, R.; Fens, M.; Heijnen, H.F.G.; Henegouwen, P.; Vader, P.; Schiffelers, R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 2016, 224, 77–85. [Google Scholar] [CrossRef]
- van Waarde, A.; Rybczynska, A.A.; Ramakrishnan, N.K.; Ishiwata, K.; Elsinga, P.H.; Dierckx, R. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. BBA Biomembr. 2015, 1848, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.L.; Tang, C.Y.; Sun, C.; Ying, X.J.; Shen, R.L. M1 macrophage-derived exosomes synergistically enhance the anti-bladder cancer effect of gemcitabine. Aging 2022, 14, 7364–7377. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Updates 2014, 17, 89–95. [Google Scholar] [CrossRef]
- Luo, S.L.; Zhang, E.L.; Su, Y.P.; Cheng, T.M.; Shi, C.M. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Khatoon, N.; Zhang, Z.F.; Zhou, C.H.; Chu, M.Q. Macrophage membrane coated nanoparticles: A biomimetic approach for enhanced and targeted delivery. Biomater. Sci. 2022, 10, 1193–1208. [Google Scholar] [CrossRef]
- Zeng, Y.P.; Li, S.F.; Zhang, S.F.; Wang, L.; Yuan, H.; Hu, F.Q. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 3233–3254. [Google Scholar] [CrossRef]
- Suski, J.M.; Lebiedzinska, M.; Wojtala, A.; Duszynski, J.; Giorgi, C.; Pinton, P.; Wieckowski, M.R. Isolation of plasma membrane-associated membranes from rat liver. Nat. Protoc. 2014, 9, 312–322. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Amin, S.A.; Adhikari, N.; Jha, T.; Gayen, S. A Review on Camptothecin Analogs with Promising Cytotoxic Profile. Anti-Cancer Agent Med. Chem. 2018, 18, 1796–1814. [Google Scholar] [CrossRef]
- Wall, M.E. Camptothecin and taxol: Discovery to clinic. Med. Res. Rev. 1998, 18, 299–314. [Google Scholar] [CrossRef]
- Li, F.Z.; Jiang, T.; Li, Q.Y.; Ling, X. Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: Did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am. J. Cancer Res. 2017, 7, 2350–2394. [Google Scholar] [PubMed]
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.B.; Huang, Q.Q.; Luo, Y.; Lu, W. Total Synthesis of Camptothecin and SN-38. J. Org. Chem. 2012, 77, 713–717. [Google Scholar] [CrossRef]
- Fulzele, D.P.; Satdive, R.K.; Pol, B.B. Growth and production of camptothecin by cell suspension cultures of Nothapodytes foetida. Planta Med. 2001, 67, 150–152. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Oosterhoff, D.; Pinedo, H.M.; van der Meulen, I.H.; de Graaf, M.; Sone, T.; Kruyt, F.A.; van Beusechem, V.W.; Haisma, H.J.; Gerritsen, W.R. Secreted and tumour targeted human carboxylesterase for activation of irinotecan. Br. J. Cancer 2002, 87, 659–664. [Google Scholar] [CrossRef]
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R.; Hamblin, M.R.; et al. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytother. Res. 2022, 36, 189–213. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharm. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. Biofactors 2013, 39, 69–77. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes—A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Dis. 2019, 5, 150. [Google Scholar] [CrossRef]
- Yu, S.W.; Shen, G.X.; Khor, T.O.; Kim, J.H.; Kong, A.N.T. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2609–2620. [Google Scholar] [CrossRef]
- Paciello, F.; Fetoni, A.R.; Mezzogori, D.; Rolesi, R.; Di Pino, A.; Paludetti, G.; Grassi, C.; Troiani, D. The dual role of curcumin and ferulic acid in counteracting chemoresistance and cisplatin-induced ototoxicity. Sci. Rep. 2020, 10, 1063. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B.; Newman, M.; Greenham, J.; Eagles, J. Chrysin and other leaf exudate flavonoids in the genus Pelargonium. Phytochemistry 1997, 46, 1349–1353. [Google Scholar] [CrossRef]
- Salari, N.; Faraji, F.; Jafarpour, S.; Rasoulpoor, S.; Dokaneheifard, S.; Mohammadi, M. Anti-cancer Activity of Chrysin in Cancer Therapy: A Systematic Review. Indian J. Surg. Oncol. 2022, 13, 681–690. [Google Scholar] [CrossRef]
- Salimi, A.; Pourahmad, J. Role of Natural Compounds in Prevention and Treatment of Chronic Lymphocytic Leukemia. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 195–203. [Google Scholar]
- Stompor-Goracy, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Chen, Z.X.; Zhang, R.; Shi, W.M.; Li, L.F.; Liu, H.; Liu, Z.P.; Wu, L.H. The Multifunctional Benefits of Naturally Occurring Delphinidin and Its Glycosides. J. Agric. Food Chem. 2019, 67, 11288–11306. [Google Scholar] [CrossRef]
- Favot, L.; Martin, S.; Keravis, T.; Andriantsitohaina, R.; Lugnier, C. Involvement of cyclin-dependent pathway in the inhibitory effect of delphinidin on angiogenesis. Cardiovasc. Res. 2003, 59, 479–487. [Google Scholar] [CrossRef]
- Keravis, T.; Favot, L.; Abusnina, A.A.; Anton, A.; Justiniano, H.; Soleti, R.; Alibrahim, E.A.; Simard, G.; Andriantsitohaina, R.; Lugnier, C. Delphinidin Inhibits Tumor Growth by Acting on VEGF Signalling in Endothelial Cells. PLoS ONE 2015, 10, e0145291. [Google Scholar] [CrossRef]
- Sharma, A.; Choi, H.K.; Kim, Y.K.; Lee, H.J. Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11500. [Google Scholar] [CrossRef]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Zhu, T.; Li, L.L.; Xiao, G.F.; Luo, Q.Z.; Liu, Q.Z.; Yao, K.T.; Xiao, G.H. Berberine Increases Doxorubicin Sensitivity by Suppressing STAT3 in Lung Cancer. Am. J. Chin. Med. 2015, 43, 1487–1502. [Google Scholar] [CrossRef]
- Pan, G.Y.; Wang, G.J.; Liu, X.D.; Fawcett, J.P.; Xie, Y.Y. The involvement of P-glycoprotein in berberine absorption. Pharmacol. Toxicol. 2002, 91, 193–197. [Google Scholar] [CrossRef]
- Liu, W.K.; Xu, S.X.; Che, C.T. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000, 67, 1297–1306. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W.Q. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Adams, L.S.; Phung, S.; Yee, N.; Seeram, N.P.; Li, L.Y.; Chen, S.A. Blueberry Phytochemicals Inhibit Growth and Metastatic Potential of MDA-MB-231 Breast Cancer Cells through Modulation of the Phosphatidylinositol 3-Kinase Pathway. Cancer Res. 2010, 70, 3594–3605. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.M.; Tang, X.; Liu, Y.L.; Yu, X.P.; Wang, Z.; Liu, W.H. Anthocyanins inhibit trastuzumab-resistant breast cancer in vitro and in vivo. Mol. Med. Rep. 2016, 13, 4007–4013. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Z.; Li, X.; Liu, J.L.; Tian, L.; Chen, J.Q. Exosome-mediated transfer of CLIC1 contributes to the vincristine-resistance in gastric cancer. Mol. Cell. Biochem. 2019, 462, 97–105. [Google Scholar] [CrossRef]
- Hanley, T.M.; Vankayala, R.; Mac, J.T.; Lo, D.D.; Anvari, B. Acute Immune Response of Micro- and Nanosized Erythrocyte-Derived Optical Particles in Healthy Mice. Mol. Pharm. 2020, 17, 3900–3914. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Hu, C.M.J.; Fang, R.N.H.; Dehaini, D.; Carpenter, C.; Gao, W.W.; Zhang, L.F. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale 2014, 6, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.W.; Hu, C.M.J.; Fang, R.H.; Luk, B.T.; Su, J.; Zhang, L.F. Surface Functionalization of Gold Nanoparticles with Red Blood Cell Membranes. Adv. Mater. 2013, 25, 3549–3553. [Google Scholar] [CrossRef] [PubMed]



| Ref. | Source | Plant-Derived Active | Carrier | Isolation Technique/Preparation Procedure | Loading Technique | Functionalization/Engineering | Assays |
|---|---|---|---|---|---|---|---|
| [70] | Taxus brevifolia (Taxaceae) | Paclitaxel | EXOs from SR4987 murine mesenchymal stem cells | Differential centrifugations | Priming of the parental cells with the drug | / | In vitro: CFPAC-1 human pancreatic adenocarcinoma cells |
| [71] | EXOs from primary human mesenchymal stem cells from umbilical cord | Sequential centrifugations | Priming of the parental cells with the drug | / | In vitro: A549 lung cancer cells, SK-OV-3 ovarian cancer cells, MDA-hyb1 breast cancer cells; ex vivo: female NOD scid mice injected with MDA-hyb1 cells | ||
| [72,73] | EXOs from RAW 264.7 murine macrophages | ExoQuick-TC™ Kit | Incubation/electroporation/ sonication | Aminoethylanisamide-polyethylene glycol (AA-PEG) targeting the sigma receptor | In vitro: murine Lewis lung carcinoma cell subline (3LL-M27), Madin-Darby canine kidney (MDCK) cells; ex vivo, in vivo: C57BL/6 mice injected with 3LL-M27 cells | ||
| [74] | EXOs from RAW 264.7 murine macrophages | Sequential centrifugations | Sonication | / | In vitro: MDA-MB-231, MCF-7, 4T1, A549, Hep G2 and HeLa cells; in vivo: female BALB/c mice injected with 4T1 cells | ||
| [75] | EXOs from milk | Sequential centrifugations | Incubation | / | In vivo: athymic nude mice bearing subcutaneous lung cancer A549 xenografts | ||
| [76] | EXOs from milk | Sequential centrifugations | Sonication | Folic acid | In vitro: MCF-7 and MDA-MB-231 breast cancer cells | ||
| [77] | EXOs from human U87 glioblastoma cells | Exo-spin™ kit | Incubation/sonication | / | In vitro: U-87 glioblastoma cells | ||
| [78] | Taxus brevifolia (Taxaceae) | Paclitaxel | Hybrid NPs: poly(caprolactone) NPs covered with mouse red blood cell (RBC) membranes | Co-extrusion | The drug was dissolved with poly(caprolactone) during the NPs core preparation | / | In vitro: RAW 264.7 macrophages, 4T1 breast cancer cells; ex vivo: female BALB/c nude mice injected with 4T1 cells; in vivo: SD rats |
| [79] | Hybrid NPs: poly(caprolactone) NPs covered with mouse RBC membranes | Co-extrusion | The drug was dissolved with poly(caprolactone) during the NPs core preparation | DiR | In vitro: 4T1 breast cancer cells; ex vivo, in vivo: female BALB/c nude mice injected with 4T1 cells | ||
| [80] | Hybrid NPs: drug nanocrystals covered with mouse RBC membranes | Sonication | The core of the NPs is composed of nanocrystals of the active compound | / | In vitro: 293 T, HT29, HepG2, MDA-MB-486, MCF-7, 4T1 cancer cells; ex vivo, in vivo: female BALB/c nude mice injected with 4T1 cells | ||
| [81] | Hybrid NPs: carboxymethylcellulose and stearic acid core coated with mouse RBC membranes | Sonication | Incubation | Folic acid and PEG chains | In vitro: RAW 264.7 macrophages, HepG2, A549, and A375 cancer cells; ex vivo, in vivo: mouse models of lung cancer, liver cancer, and melanoma | ||
| [82] | Hybrid NPs: albumin NPs coated with RAW 264.7 murine macrophage membranes | Co-extrusion | The drug was dissolved in the solvent mixture during the NPs core preparation | / | In vitro: 4T1, A549, MCF-7, B16F10 cancer cells; ex vivo, in vivo: B16F10-bearing C57BL/6 mice | ||
| [83] | Hybrid NPs: Poly(lactic-co-glycolic acid) (PLGA) NPs coated with human HeLa cell membranes | Co-extrusion | The drug was dissolved with PLGA during the NPs core preparation | / | In vitro: HeLa, Ect1, LO2, and RAW 264.7 cells; ex vivo, in vivo: HeLa xenografted female BALB/c nude mice | ||
| [84] | Hybrid NPs: PLGA NPs coated with human osteosarcoma and mouse macrophage cell membranes | Sonication | The drug was dissolved with PLGA during the NPs core preparation | / | In vitro: 143B cells; ex vivo, in vivo: male BALB/c nude mice injected with 143B cells | ||
| [85] | Camptotheca acuminata (Nyssaceae) | Camptothecin | EXOs from human cervical cancer cells | Ultracentrifugation | Electroporation | / | In vitro: Ect1/E6E7, End1/E6E7, Vk2/E6E7 and HUCEC cervical epithelial cells; ex vivo, in vivo: BALB/c mice implanted with human cervical cancer tissue |
| [86] | Hybrid NPs: mouse RBC-derived droplets with a liquid core of perfluoro-n-pentane | Sequential centrifugations | The drug was mixed with RBC membranes and perfluoro-n-pentane during the droplet preparation | / | In vitro: primary mouse peritoneal macrophages, HeLa cells, BJAB human lymphoblastoid B cells; in vivo: male C57BL mice | ||
| [87] | Hybrid NPs: mouse RBC nanobubbles with a sulfur hexafluoride core | Centrifugation and sonication | Dubious | / | In vitro: C26 mouse colon carcinoma cells, CHO Chinese hamster ovary cells; in vivo: male BALB/c mice injected with C26 cells | ||
| [88] | Human RBC-derived vesicles | Extrusion | Incubation and extrusion | / | In vitro: adenocarcinomic human alveolar basal epithelial cells, A549 lung carcinoma cells and THP-1 human monocytes; in vivo: male BALB/c mice | ||
| [89] | Human RBC-derived vesicles | Extrusion | The drug was incubated with the photosensitizing agent before extrusion | ICG | In vitro: A549 cells; in vivo: female BALB/c mice subcutaneously inoculated with Ehrlich Ascites Carcinoma (EAC) cell suspension | ||
| [90] | Hybrid NPs: human RBC membrane-coated polymeric micelles | Extrusion | The drug was dissolved in the organic phase during the polymeric NPs core preparation | / | In vitro: A549 human adenocarcinomic alveolar basal epitheliel cells; in vivo: EAC mice | ||
| [91] | Hybrid NPs: mouse macrophage membrane-coated solid lipid NPs | Sequential centrifugations and extrusion | The prodrug was dissolved with the lipids during the lipid particles core formation | / | In vitro: B16F10 mouse melanoma and 4T1 mouse breast cancer cells; in vivo: BALB/c mice | ||
| [92] | Curcuma longa (Zingiberaceae) | Curcumin | EXOs from milk | Sequential centrifugations | Incubation | / | In vitro: H1299 and A549 human lung cancer cells, HeLa cells, MDA-MB-231 and T47D breast cancer cells; ex vivo: athymic nude mice injected with CaSki cervical cancer cells |
| [93] | Curcumin (and resveratrol) | EXOs from milk | Ultracentrifugation | Sonication/electroporation/ incubation | / | In vitro: MCF-7 human breast cancer cells; ex vivo: female Sprague–Dawley rats | |
| [94] | Curcumin | EXOs from RAW 264.7 murine macrophages | Ultrafiltration and sequential centrifugations | Electroporation | NPR-1 glycoprotein | In vitro: U251 human malignant glioblastoma multiforme and Bel-7404 human hepatoma cells; in vivo: glioma-xenografted female BALB/c nude mice | |
| [95] | EXOs from CT26 murine colon cancer cells and from erythrocytes | Ultracentrifugation | The drug was included in the mixture during the preparation of calcium carbonate NPs encapsulated in the exosomes | / | In vitro: CT26 mouse colon cancer cells, RAW 264.7 mouse macrophages; ex vivo, in vivo: BALB/c mice, CT26-injected BALB/c mice | ||
| [96] | Catharanthus roseus (Apocynaceae) | Vincristine | EVs from three types of human mesenchymal stem cells | Ultracentrifugation | Incubation | / | In vitro: U87MG human glioblastoma cells |
| [97] | Different sources: honey, propolis, Passiflora caerulea and Passiflora incarnata (Passifloraceae), Oroxylum inolicum (Bignoniaceae) | Chrysin | EVs from SCC9 human tongue squamous carcinoma cells | Sequential centrifugations | Priming of the parental cells with the drug | / | In vitro: SCC9 tongue squamous carcinoma cells; ex vivo, in vivo: female nude mice injected with SCC9 cells |
| [98] | Beans, berries, fruits, flowers (Viola and Delphinium) | Delphinidin | EVs from JAWS II murine dendritic cells | Sequential centrifugations | Incubation | / | In vitro: HAOEC human aortic endothelial cells |
| [99] | Berberis vulgaris, Berberis aquifolium and Berberis aristate (Berberidaceae), Tinospora cordifolia (Menispermaceae) | Berberin | EVs from JAWS II murine dendritic cells | Sequential centrifugations | Impregnation | / | In vitro: MDA-MB-231 human breast cancer cells, HUVECs |
| [100] | Black beans extract | Soyasaponins, flavonoids | EXOs from 4 different human cancer cell lines | Total Exosome Isolation Reagent kit | Incubation/electroporation | / | In vitro: human hormone-dependent mammary (MCF7), prostate (PC3), colon (Caco2) and liver (HepG2) cancer cells |
| [101,102] | Bilberry extract | Anthocyanins | EXOs obtained from milk | Differential centrifugation | Incubation | / | In vitro: human lung (A549 and H1299), breast (MDA-MB-231 and MCF7), pancreatic (PANC1 and Mia PaCa2), prostate (PC3 and DU145), colon (HCT116) and ovarian (A2780, A2780/CP70, OVCA432 and OVCA433) cancer cells; in vivo: wild-type female C57BL/6 mice, female athymic nude mice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldassari, S.; Balboni, A.; Drava, G.; Donghia, D.; Canepa, P.; Ailuno, G.; Caviglioli, G. Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers. Pharmaceutics 2023, 15, 1445. https://doi.org/10.3390/pharmaceutics15051445
Baldassari S, Balboni A, Drava G, Donghia D, Canepa P, Ailuno G, Caviglioli G. Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers. Pharmaceutics. 2023; 15(5):1445. https://doi.org/10.3390/pharmaceutics15051445
Chicago/Turabian StyleBaldassari, Sara, Alice Balboni, Giuliana Drava, Daniela Donghia, Paolo Canepa, Giorgia Ailuno, and Gabriele Caviglioli. 2023. "Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers" Pharmaceutics 15, no. 5: 1445. https://doi.org/10.3390/pharmaceutics15051445





