PPAR-γ Agonist GW1929 Targeted to Macrophages with Dendrimer–Graphene Nanostars Reduces Liver Fibrosis and Inflammation
Abstract
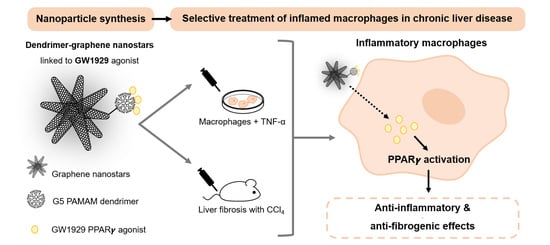
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Dendrimer–Graphene Nanostars Linked to GW1929 (DGNS-GW) or Mannitol (DGNS-Man)
2.2. Physicochemical Characterization of Nanoparticles
2.3. Cell Culture
2.4. Animal Studies
2.5. Gene Expression Assay
2.6. Fibrosis Quantification
2.7. Immunofluorescence and Imaging in Liver Tissues
2.8. Statistical Analysis
3. Results
3.1. Synthesis and Physicochemical Characterization of Dendrimer–Graphene Nanostars Linked to GW1929 PPARγ Agonist
3.2. In Vitro Evaluation of the Activity of DGNS-GW to Stimulate Macrophage Polarization
3.3. Evaluation of the Therapeutic Utility of DGNS-GW in Mice with Liver Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Perramón, M.; Carvajal, S.; Reichenbach, V.; Fernández-Varo, G.; Boix, L.; Macias-Muñoz, L.; Melgar-Lesmes, P.; Bruix, J.; Melmed, S.; Lamas, S.; et al. The pituitary tumour-transforming gene 1/delta-like homologue 1 pathway plays a key role in liver fibrogenesis. Liver Int. 2022, 42, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef]
- Melgar-Lesmes, P.; Luquero, A.; Parra-Robert, M.; Mora, A.; Ribera, J.; Edelman, E.R.; Jiménez, W. Graphene–Dendrimer Nanostars for Targeted Macrophage Overexpression of Metalloproteinase 9 and Hepatic Fibrosis Precision Therapy. Nano Lett. 2018, 18, 5839–5845. [Google Scholar] [CrossRef]
- Carvajal, S.; Perramón, M.; Casals, G.; Oró, D.; Ribera, J.; Morales-Ruiz, M.; Casals, E.; Casado, P.; Melgar-Lesmes, P.; Fernández-Varo, G.; et al. Cerium Oxide Nanoparticles Protect against Oxidant Injury and Interfere with Oxidative Mediated Kinase Signaling in Human-Derived Hepatocytes. Int. J. Mol. Sci. 2019, 20, 5959. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell. Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef]
- Tacke, F.; Zimmermann, H.W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef]
- Melgar-Lesmes, P.; Edelman, E.R. Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse. J. Hepatol. 2015, 63, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Bosch, M.; Moreno-Lanceta, A.; Melgar-Lesmes, P. Nanoparticles to target and treat macrophages: The ockham’s concept? Pharmaceutics 2021, 13, 1340. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Lanceta, A.; Medrano-Bosch, M.; Melgar-Lesmes, P. Single-Walled Carbon Nanohorns as Promising Nanotube-Derived Delivery Systems to Treat Cancer. Pharmaceutics 2020, 12, 850. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, Properties, Functionalization, and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Moore, K.J.; Rosen, E.D.; Fitzgerald, M.L.; Randow, F.; Andersson, L.P.; Altshuler, D.; Milstone, D.S.; Mortensen, R.M.; Spiegelman, B.M.; Freeman, M.W. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nat. Med. 2001, 7, 41–47. [Google Scholar] [CrossRef]
- Willson, T.M.; Brown, P.J.; Sternbach, D.D.; Henke, B.R. The PPARs: From Orphan Receptors to Drug Discovery. J. Med. Chem. 2000, 43, 527–550. [Google Scholar] [CrossRef]
- Chandra, V.; Huang, P.; Hamuro, Y.; Raghuram, S.; Wang, Y.; Burris, T.P.; Rastinejad, F. Structure of the intact PPAR-γ–RXR-α nuclear receptor complex on DNA. Nature 2009, 456, 350–356. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, C.; Wu, J. The agonists of peroxisome proliferator-activated receptor-γ for liver fibrosis. Drug Des Devel Ther. 2021, 15, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Ha, Y.M.; Kim, Y.M.; Lee, Y.S.; Kim, H.J.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Activation of PPAR-γ by Carbon Monoxide from CORM-2 Leads to the Inhibition of iNOS but not COX-2 Expression in LPS-Stimulated Macrophages. Inflammation 2009, 32, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kaundal, R.K.; Sharma, S.S. GW1929: A nonthiazolidinedione PPARγ agonist, ameliorates neurological damage in global cerebral ischemic-reperfusion injury through reduction in inflammation and DNA fragmentation. Behav. Brain Res. 2011, 216, 606–612. [Google Scholar] [CrossRef]
- Paukkeri, E.-L.; Leppänen, T.; Lindholm, M.; Yam, M.F.; Asmawi, M.Z.; Kolmonen, A.; Aulaskari, P.H.; Moilanen, E. Anti-inflammatory properties of a dual PPARgamma/alpha agonist muraglitazar in in vitro and in vivo models. Arthritis Res. Ther. 2013, 15, R51. [Google Scholar] [CrossRef]
- Wright, M.B.; Bortolini, M.; Tadayyon, M.; Bopst, M. Minireview: Challenges and Opportunities in Development of PPAR Agonists. Mol. Endocrinol. 2014, 28, 1756–1768. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells 2020, 9, 352. [Google Scholar] [CrossRef]
- Bortolini, M.; Wright, M.B.; Bopst, M.; Balas, B. Examining the safety of PPAR agonists—Current trends and future prospects. Expert Opin. Drug Saf. 2013, 12, 65–79. [Google Scholar] [CrossRef]
- Torres-Castro, I.; Arroyo-Camarena, D.; Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Dueñas-Andrade, Y.; Hernández-Ruiz, J.; Béjar, Y.L.; Zaga-Clavellina, V.; Morales-Montor, J.; Terrazas, L.I.; et al. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol. Lett. 2016, 176, 81–89. [Google Scholar] [CrossRef]
- Grosick, R.; Alvarado-Vazquez, P.A.; Messersmith, A.R.; Romero-Sandoval, E.A. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J. Pain Res. 2018, 11, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, S.; Lindsay, J.; Ingram, R.; Xu, H.; Chen, M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Moore-Carrasco, R.; Figueras, M.; Ametller, E.; López-Soriano, F.J.; Argiles, J.M.; Busquets, S. Effects of the PPARγ agonist GW1929 on muscle wasting in tumour-bearing mice. Oncol. Rep. 2008, 19, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Tickner, J.; Fan, L.M.; Du, J.; Meijles, D.; Li, J.-M. Nox2-derived ROS in PPARγ signaling and cell-cycle progression of lung alveolar epithelial cells. Free. Radic. Biol. Med. 2011, 51, 763–772. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Feng, Y.; Tong, Y.; Jia, Y.; Wang, C.; Cui, R.; Qu, K.; Liu, C.; Zhang, J. PPAR γ Alleviates Sepsis-Induced Liver Injury by Inhibiting Hepatocyte Pyroptosis via Inhibition of the ROS/TXNIP/NLRP3 Signaling Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 1269747. [Google Scholar] [CrossRef]
- Alatas, F.S.; Matsuura, T.; Pudjiadi, A.H.; Wijaya, S.; Taguchi, T. Peroxisome Proliferator-Activated Receptor Gamma Agonist Attenuates Liver Fibrosis by Several Fibrogenic Pathways in an Animal Model of Cholestatic Fibrosis. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 346–355. [Google Scholar] [CrossRef]
- Yang, L.; Stimpson, S.A.; Chen, L.; Harrington, W.W.; Rockey, D.C. Effectiveness of the PPARγ agonist, GW570, in liver fibrosis. Inflamm. Res. 2010, 59, 1061–1071. [Google Scholar] [CrossRef]
- Chiarelli, F.; Di Marzio, D. Peroxisome proliferator-activated receptor-γ agonists and diabetes: Current evidence and future perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [CrossRef]
- Laddha, U.D.; Kshirsagar, S.J. Formulation of PPAR-gamma agonist as surface modified PLGA nanoparticles for non-invasive treatment of diabetic retinopathy: In vitro and in vivo evidences. Heliyon 2020, 6, e04589. [Google Scholar] [CrossRef]
- Wei, S.; Xu, C.; Zhang, Y.; Shi, Z.; Wu, M.; Yang, B. Ultrasound Assisted a Peroxisome Proliferator-Activated Receptor (PPAR)γ Agonist-Loaded Nanoparticle-Microbubble Complex to Attenuate Renal Interstitial Fibrosis. Int. J. Nanomed. 2020, 15, 7315–7327. [Google Scholar] [CrossRef]
- Alves, C.; de Melo, N.; Fraceto, L.; Araújo, D.; Napimoga, M. Effects of 15d-PGJ2-loaded poly(D,L-lactide-co-glycolide) nanocapsules on inflammation. Br. J. Pharmacol. 2011, 162, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Fan, J.; Claudel, M.; Sonntag, T.; Didier, P.; Ronzani, C.; Lebeau, L.; Pons, F. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotechnology 2021, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Seki, E. TNFα in liver fibrosis. Curr. Pathobiol. Rep. 2015, 3, 253–261. [Google Scholar] [CrossRef]
- Connolly, M.K.; Bedrosian, A.S.; Clair, J.M.-S.; Mitchell, A.P.; Ibrahim, J.; Stroud, A.; Pachter, H.L.; Bar-Sagi, D.; Frey, A.B.; Miller, G. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J. Clin. Investig. 2009, 119, 3213–3225. [Google Scholar] [CrossRef]
- Ye, J. Regulation of PPARγ function by TNF-α. Biochem Biophys Res Commun. 2008, 374, 405–408. [Google Scholar] [CrossRef]
- Heming, M.; Gran, S.; Jauch, S.-L.; Fischer-Riepe, L.; Russo, A.; Klotz, L.; Hermann, S.; Schäfers, M.; Roth, J.; Barczyk-Kahlert, K. Peroxisome Proliferator-Activated Receptor-γ Modulates the Response of Macrophages to Lipopolysaccharide and Glucocorticoids. Front. Immunol. 2018, 9, 893. [Google Scholar] [CrossRef]
- Muñoz-Luque, J.; Ros, J.; Fern´ández-Varo, G.; Tugues, S.; Morales-Ruiz, M.; Alvarez, C.E.; Friedman, S.L.; Arroyo, V.; Jiménez, W. Regression of Fibrosis after Chronic Stimulation of Cannabinoid CB2 Receptor in Cirrhotic Rats. J. Pharmacol. Exp. Ther. 2008, 324, 475–483. [Google Scholar] [CrossRef]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirili, C.; Strazzabosco, M. Stimulation of nuclear receptor PPAR-γ limits NF-kB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology. 2015, 62, 1551–1562. [Google Scholar] [CrossRef]
- Chawla, A. Control of Macrophage Activation and Function by PPARs. Circ. Res. 2010, 106, 1559–1569. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qu, X.; Zhang, C.; Yu, Y. Interleukin-10 promotes proliferation of vascular smooth muscle cells by inhibiting inflammation in rabbit abdominal aortic aneurysm. Int. J. Clin. Exp. Pathol. 2019, 12, 1260–1271. [Google Scholar] [PubMed]
- Jozkowicz, A.; Dulak, J.; Piatkowska, E.; Placha, W.; Dembinska-Kiec, A. Ligands of peroxisome proliferator-activated receptor- g increase the generation of vascular endothelial growth factor in vascular smooth muscle cells and in macrophages. Acta Biochim Pol. 2000, 47, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kwon, J.; Popov, Y.V.; Gajdos, G.B.; Ordog, T.; Brekken, R.A.; Mukhopadhyay, D.; Schuppan, D.; Bi, Y.; Simonetto, D.; et al. Vascular Endothelial Growth Factor Promotes Fibrosis Resolution and Repair in Mice. Gastroenterology 2014, 146, 1339–1350e1. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Lanceta, A.; Medrano-Bosch, M.; Simón-Codina, B.; Barber-González, M.; Jiménez, W.; Melgar-Lesmes, P. PPAR-γ Agonist GW1929 Targeted to Macrophages with Dendrimer–Graphene Nanostars Reduces Liver Fibrosis and Inflammation. Pharmaceutics 2023, 15, 1452. https://doi.org/10.3390/pharmaceutics15051452
Moreno-Lanceta A, Medrano-Bosch M, Simón-Codina B, Barber-González M, Jiménez W, Melgar-Lesmes P. PPAR-γ Agonist GW1929 Targeted to Macrophages with Dendrimer–Graphene Nanostars Reduces Liver Fibrosis and Inflammation. Pharmaceutics. 2023; 15(5):1452. https://doi.org/10.3390/pharmaceutics15051452
Chicago/Turabian StyleMoreno-Lanceta, Alazne, Mireia Medrano-Bosch, Blanca Simón-Codina, Montserrat Barber-González, Wladimiro Jiménez, and Pedro Melgar-Lesmes. 2023. "PPAR-γ Agonist GW1929 Targeted to Macrophages with Dendrimer–Graphene Nanostars Reduces Liver Fibrosis and Inflammation" Pharmaceutics 15, no. 5: 1452. https://doi.org/10.3390/pharmaceutics15051452
APA StyleMoreno-Lanceta, A., Medrano-Bosch, M., Simón-Codina, B., Barber-González, M., Jiménez, W., & Melgar-Lesmes, P. (2023). PPAR-γ Agonist GW1929 Targeted to Macrophages with Dendrimer–Graphene Nanostars Reduces Liver Fibrosis and Inflammation. Pharmaceutics, 15(5), 1452. https://doi.org/10.3390/pharmaceutics15051452