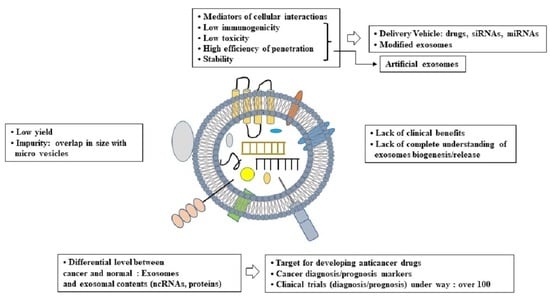
Exosomes: Diagnostic and Therapeutic Implications in Cancer
Abstract
:1. Introduction
2. Biogenesis of Exosomes

3. Contents of Exosomes: Exosomal Proteins
4. Roles of Exosomes and Exosomal Proteins in Cellular Interactions and Antitumor Immune Responses
5. Exosomal Non-Coding RNAs as Regulators of Cancer Progression and Anticancer Drug Resistance
6. Exosomal miRNAs as Diagnostic/Prognostic Markers
7. Inhibitors Targeting Exosomes
8. Exosomes as Carriers of Drug/siRNAs/miRNAs
8.1. Advantages of Exosomes as Carriers of Anticancer Molecules
8.2. Methods of Encapsulating Anticancer Molecules in Exosomes
8.3. Exosomes Loaded with Anticancer Drugs Inhibit Cancer Cell Proliferation

8.4. Exosomes Loaded with miRNAs Inhibit Cancer Cell Proliferation
8.5. Exosomes Loaded with siRNAs Inhibit Cancer Cell Proliferation
9. Clinical Trials Involving Modified Exosomes
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, W.; Du, Y.; Zhang, C.; Pan, F.; Yao, Y.; Zhang, T.; Peng, Q. Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019, 86, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Montgomery, K.C.; Jiang, L.; Lyon, C.J.; Hu, T.Y. Advanced technologies for molecular diagnosis of cancer: State of pre-clinical tumor-derived exosome liquid biopsies. Mater. Today Bio. 2022, 18, 100538. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, V.; Seol, D.; Gomez-Contreras, P.C.; Keen, H.L.; Shin, K.; Martin, J.A. Exosome-Based Cell Homing and Angiogenic Differentiation for Dental Pulp Regeneration. Int. J. Mol. Sci. 2022, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, X.; Wang, K.; Zheng, B.; Lin, Q.; Yu, M.; Xie, L.; Chen, L.; Song, X. Plasma exosomal miR-320d, miR-4479, and miR-6763-5p as diagnostic biomarkers in epithelial ovarian cancer. Front. Oncol. 2022, 12, 986343. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Yashiro, R. Advances in Purification, Modification, and Application of Extracellular Vesicles for Novel Clinical Treatments. Membranes 2022, 12, 1244. [Google Scholar] [CrossRef]
- Carrasco, E.; Soto-Heredero, G.; Mittelbrunn, M. The Role of Extracellular Vesicles in Cutaneous Remodeling and Hair Follicle Dynamics. Int. J. Mol. Sci. 2019, 20, 2758. [Google Scholar] [CrossRef]
- Ding, J.; Li, H.; Liu, W.; Wang, X.; Feng, Y.; Guan, H.; Chen, Z. miR-186-5p Dysregulation in Serum Exosomes from Patients with AMI Aggravates Atherosclerosis via Targeting LOX-1. Int. J. Nanomed. 2022, 17, 6301–6316. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, G.; Zhu, X.; Chen, X.; Ma, X.; Hu, P.; Liu, W.; Yang, W.; Ruan, T.; Zhang, W.; et al. Exosome-Delivered circSTAU2 Inhibits the Progression of Gastric Cancer by Targeting the miR-589/CAPZA1 Axis. Int. J. Nanomed. 2023, 18, 127–142. [Google Scholar] [CrossRef]
- Xiao, Z.; Feng, X.; Zhou, Y.; Li, P.; Luo, J.; Zhang, W.; Zhou, J.; Zhao, J.; Wang, D.; Wang, Y.; et al. Exosomal miR-10527-5p Inhibits Migration, Invasion, Lymphangiogenesis and Lymphatic Metastasis by Affecting Wnt/β-Catenin Signaling via Rab10 in Esophageal Squamous Cell Carcinoma. Int. J. Nanomed. 2023, 18, 95–114. [Google Scholar] [CrossRef]
- Zhou, X.; Lian, H.; Li, H.; Fan, M.; Xu, W.; Jin, Y. Nanotechnology in cervical cancer immunotherapy: Therapeutic vaccines and adoptive cell therapy. Front. Pharmacol. 2022, 13, 1065793. [Google Scholar] [CrossRef]
- Peng, J.; Li, S.; Li, B.; Hu, W.; Ding, C. Exosomes derived from M1 macrophages inhibit the proliferation of the A549 and H1299 lung cancer cell lines via the miRNA-let-7b-5p-GNG5 axis. PeerJ. 2023, 11, e14608. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.M.; Fu, Y.; Zeng, J.; Zhu, X.Y.; Gao, Y. Cancer-derived exosomal miR-197-3p confers angiogenesis via targeting TIMP2/3 in lung adenocarcinoma metastasis. Cell Death Dis. 2022, 13, 1032. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Cui, D.; Zhu, Y.; Chan, C.K.W.; Choi, C.H.J.; Liu, T.; Lee, N.P.Y.; Law, S.; Tsao, S.W.; Ma, S.; et al. M6PR- and EphB4-Rich Exosomes Secreted by Serglycin-Overexpressing Esophageal Cancer Cells Promote Cancer Progression. Int. J. Biol. Sci. 2023, 19, 625–640. [Google Scholar] [CrossRef]
- Jiang, T.; Zhu, Z.; Zhang, J.; Chen, M.; Chen, S. Role of tumor-derived exosomes in metastasis, drug resistance and diagnosis of clear cell renal cell carcinoma. Front. Oncol. 2022, 12, 1066288. [Google Scholar] [CrossRef]
- Mo, Y.; Leung, L.L.; Mak, C.S.L.; Wang, X.; Chan, W.S.; Hui, L.M.N.; Tang, H.W.M.; Siu, M.K.Y.; Sharma, R.; Xu, D.; et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol. Cancer 2023, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tan, J.; Guo, J.; Wu, Z.; Zhan, Q. Exosome-mediated transfer of circ_0063526 enhances cisplatin resistance in gastric cancer cells via regulating miR-449a/SHMT2 axis. Anticancer. Drugs 2022, 33, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, C.; Li, Z.; Ai, M.; Wang, B.; Du, K.; Liu, W.; Wang, H.; Yu, P.; Chen, C.; et al. Exosomal B7-H4 from irradiated glioblastoma cells contributes to increase FoxP3 expression of differentiating Th1 cells and promotes tumor growth. Redox Biol. 2022, 56, 102454. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, X.; Lu, Y.; Zhang, T.; Du, Z.; Xu, J.; You, J.; Chen, N.; Deng, X.; Wu, J. Edible Pueraria lobata-Derived Exosomes Promote M2 Macrophage Polarization. Molecules 2022, 27, 8184. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Fang, P.; Ma, Z.; Wang, X.; Chen, H.; Wang, S.; Meng, F.; Wang, C.; Zhang, E.; et al. Cancer-associated fibroblast-specific lncRNA LINC01614 enhances glutamine uptake in lung adenocarcinoma. J. Hematol. Oncol. 2022, 15, 141. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Q.; Shi, X.; Jiang, M. Targeting Endocytosis and Cell Communications in the Tumor Immune Microenvironment. Cell Commun. Signal 2022, 20, 161. [Google Scholar] [CrossRef]
- Ivanova, D.; Imig, C.; Camacho, M.; Reinhold, A.; Guhathakurta, D.; Montenegro-Venegas, C.; Cousin, M.A.; Gundelfinger, E.D.; Rosenmund, C.; Cooper, B.; et al. CtBP1-Mediated Membrane Fission Contributes to Effective Recycling of Synaptic Vesicles. Cell Rep. 2020, 30, 2444–2459. [Google Scholar] [CrossRef]
- Qi, L.; Ge, W.; Pan, C.; Jiang, W.; Lin, D.; Zhang, L. Compromised osteogenic effect of exosomes internalized by senescent bone marrow stem cells via endocytoses involving clathrin, macropinocytosis and caveolae. Front. Bioeng. Biotechnol. 2023, 10, 1090914. [Google Scholar] [CrossRef] [PubMed]
- Desale, S.E.; Chinnathambi, S. α- Linolenic acid modulates phagocytosis and endosomal pathways of extracellular Tau in microglia. Cell Adhes. Migr. 2021, 15, 84–100. [Google Scholar] [CrossRef]
- Albacete-Albacete, L.; Navarro-Lérida, I.; López, J.A.; Martín-Padura, I.; Astudillo, A.M.; Ferrarini, A.; Van-Der-Heyden, M.; Balsinde, J.; Orend, G.; Vázquez, J.; et al. ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting. J. Cell Biol. 2020, 219, e202006178. [Google Scholar] [CrossRef] [PubMed]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, N.; Bagheri, D.; Shamsara, M.; Hamblin, M.R.; Farmany, A.; Xu, M.; Liang, Z.; Razi, F.; Hashemi, E. Extracellular vesicles biogenesis, isolation, manipulation and genetic engineering for potential in vitro and in vivo therapeutics: An overview. Front. Bioeng. Biotechnol. 2022, 10, 1019821. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, X.; Miller-Little, W.; Wang, H.; Willard, B.; Bulek, K.; Zhao, J.; Li, X. The Ras GTPase-activating-like protein IQGAP1 bridges Gasdermin D to the ESCRT system to promote IL-1β release via exosomes. EMBO J. 2023, 42, e110780. [Google Scholar] [CrossRef]
- Mendez, P.L.; Obendorf, L.; Jatzlau, J.; Burdzinski, W.; Reichenbach, M.; Nageswaran, V.; Haghikia, A.; Stangl, V.; Hiepen, C.; Knaus, P. Atheroprone fluid shear stress-regulated ALK1-Endoglin-SMAD signaling originates from early endosomes. BMC Biol. 2022, 20, 210. [Google Scholar] [CrossRef]
- Hainan, L.; Huilin, L.; Khan, M.A.; Xin, Z.; YuJiang, Y.; Hui, Z.; Naiquan, Y. The basic route of the nuclear translocation porcine growth hormone (GH)-growth hormone receptor (GHR) complex (pGH/GHR) in porcine hepatocytes. Gen. Comp. Endocrinol. 2018, 266, 101–109. [Google Scholar] [CrossRef]
- Díaz-Salinas, M.A.; Silva-Ayala, D.; López, S.; Arias, C.F. Rotaviruses reach late endosomes and require the cation-dependent mannose-6-phosphate receptor and the activity of cathepsin proteases to enter the cell. J. Virol. 2014, 88, 4389–4402. [Google Scholar] [CrossRef]
- Windhaber, S.; Xin, Q.; Uckeley, Z.M.; Koch, J.; Obr, M.; Garnier, C.; Luengo-Guyonnot, C.; Duboeuf, M.; Schur, F.K.M.; Lozach, P.Y. The Orthobunyavirus Germiston Enters Host Cells from Late Endosomes. J. Virol. 2022, 96, e0214621. [Google Scholar] [CrossRef]
- Gorji-Bahri, G.; Moghimi, H.R.; Hashemi, A. RAB5A is associated with genes involved in exosome secretion: Integration of bioinformatics analysis and experimental validation. J. Cell. Biochem. 2021, 122, 425–441. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Kemper, S.; Brigstock, D.R. Pathways of production and delivery of hepatocyte exosomes. J. Cell Commun. Signal. 2018, 12, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Larsen, K.P.; Ou, C.; Rose, K.; Hurley, J.H. In vitro reconstitution of calcium-dependent recruitment of the human ESCRT machinery in lysosomal membrane repair. Proc. Natl. Acad. Sci. USA 2022, 119, e2205590119. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Park, D.; Kim, J.; Nam, M.Y.; Kwon, S.; Um, D.E.; Oh, J.E.; Youn, E.; Shim, Y.H.; Wagner, K.U.; et al. Peripubertal requirement of Tsg101 in maintaining the integrity of membranous structures in mouse oocytes. Cell Prolif. 2022, 55, e13288. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.; Zhang, Y.Y.; Zhao, S.Z.; Gu, S. The endosomal sorting complex required for transport repairs the membrane to delay cell death. Front. Oncol. 2022, 12, 1007446. [Google Scholar] [CrossRef]
- Lu, P.; Li, H.; Li, N.; Singh, R.N.; Bishop, C.E.; Chen, X.; Lu, B. MEX3C interacts with adaptor-related protein complex 2 and involves in miR-451a exosomal sorting. PLoS ONE 2017, 12, e0185992. [Google Scholar] [CrossRef]
- Strickland, M.; Watanabe, S.; Bonn, S.M.; Camara, C.M.; Starich, M.R.; Fushman, D.; Carter, C.A.; Tjandra, N. Tsg101/ESCRT-I recruitment regulated by the dual binding modes of K63-linked diubiquitin. Structure 2022, 30, 289–299.e6. [Google Scholar] [CrossRef] [PubMed]
- García-León, M.; Rubio, V. Biochemical and Imaging Analysis of ALIX Function in Endosomal Trafficking of Arabidopsis Protein Cargoes. Methods Mol. Biol. 2020, 2177, 49–58. [Google Scholar] [PubMed]
- Wei, T.; Zhu, X. Knockdown of ANXA10 inhibits proliferation and promotes apoptosis of papillary thyroid carcinoma cells by down-regulating TSG101 thereby inactivating the MAPK/ERK signaling pathway. J. Bioenerg. Biomembr. 2021, 53, 429–440. [Google Scholar] [CrossRef]
- Li, Q.; Hu, J.; Shi, Y.; Xiao, M.; Bi, T.; Wang, C.; Yan, L.; Li, X. Exosomal lncAY927529 enhances prostate cancer cell proliferation and invasion through regulating bone microenvironment. Cell Cycle 2021, 20, 2531–2546. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, T.; Feng, H.; Duyvesteyn, H.M.E.; Fusco, W.G.; McKnight, K.L.; Xie, L.; Boyce, M.; Kumar, S.; Barouch-Bentov, R.; González-López, O.; et al. Nonlytic cellular release of hepatitis A virus requires dual capsid recruitment of the ESCRT-associated Bro1 domain proteins HD-PTP and ALIX. PLoS Pathog. 2022, 18, e1010543. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Lui, G.Y.L.; Lai, S.L.; Wilmott, J.S.; Tikoo, S.; Jackett, L.A.; Quek, C.; Brown, D.L.; Sharp, D.M.; Kwan, R.Y.Q.; et al. RAB27A promotes melanoma cell invasion and metastasis via regulation of pro-invasive exosomes. Int. J. Cancer 2019, 144, 3070–3085. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Lee, J.S.; Yang, J.W.; Kim, M.H.; Na, J.M.; Song, D.H. RAB27A and RAB27B Expression May Predict Lymph Node Metastasis and Survival in Patients with Gastric Cancer. Cancer Genom. Proteom. 2022, 19, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Nambara, S.; Masuda, T.; Hirose, K.; Hu, Q.; Tobo, T.; Ozato, Y.; Kurashige, J.; Hiraki, Y.; Hisamatsu, Y.; Iguchi, T.; et al. Rab27b, a Regulator of Exosome Secretion, Is Associated with Peritoneal Metastases in Gastric Cancer. Cancer Genom. Proteom. 2023, 20, 30–39. [Google Scholar] [CrossRef]
- Qiu, X.; Campos, Y.; van de Vlekkert, D.; Gomero, E.; Tanwar, A.C.; Kalathur, R.; Weesner, J.A.; Bongiovanni, A.; Demmers, J.; d’Azzo, A. Distinct functions of dimeric and monomeric scaffold protein Alix in regulating F-actin assembly and loading of exosomal cargo. J. Biol. Chem. 2022, 298, 102425. [Google Scholar] [CrossRef]
- D’Acunzo, P.; Hargash, T.; Pawlik, M.; Goulbourne, C.N.; Pérez-González, R.; Levy, E. Enhanced generation of intraluminal vesicles in neuronal late endosomes in the brain of a Down syndrome mouse model with endosomal dysfunction. Dev. Neurobiol. 2019, 79, 656–663. [Google Scholar] [CrossRef]
- Mańka, R.; Janas, P.; Sapoń, K.; Janas, T.; Janas, T. Role of RNA Motifs in RNA Interaction with Membrane Lipid Rafts: Implications for Therapeutic Applications of Exosomal RNAs. Int. J. Mol. Sci. 2021, 22, 9416. [Google Scholar] [CrossRef]
- Janas, T.; Janas, P.; Sapoń, K.; Janas, T. Binding of RNA Aptamers to Membrane Lipid Rafts: Implications for Exosomal miRNAs Transfer from Cancer to Immune Cells. Int. J. Mol. Sci. 2020, 21, 8503. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Casu, A.; Kovacova, Z.; Petrilli, A.M.; Sideleva, O.; Tharp, W.G.; Pratley, R.E. Coordinated regulation of gene expression and microRNA changes in adipose tissue and circulating extracellular vesicles in response to pioglitazone treatment in humans with type 2 diabetes. Front. Endocrinol. 2022, 13, 955593. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, G.C. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 138–149. [Google Scholar] [PubMed]
- Liu, X.M.; Ma, L.; Schekman, R. Selective sorting of microRNAs into exosomes by phase-separated YBX1 condensates. eLife 2021, 10, e71982. [Google Scholar] [CrossRef]
- Moyano, S.; Musso, J.; Feliziani, C.; Zamponi, N.; Frontera, L.S.; Ropolo, A.S.; Lanfredi-Rangel, A.; Lalle, M.; Touz, M. Exosome Biogenesis in the Protozoa Parasite Giardia lamblia: A Model of Reduced Interorganellar Crosstalk. Cells 2019, 8, 1600. [Google Scholar] [CrossRef] [PubMed]
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J. Lipid Res. 2018, 59, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- lkommos-Zakhary, M.; Rajesh, N.; Beljanski, V. Exosome RNA Sequencing as a Tool in the Search for Cancer Biomarkers. Noncoding RNA 2022, 8, 75. [Google Scholar]
- Yokoi, A.; Villar-Prados, A.; Oliphint, P.A.; Zhang, J.; Song, X.; De Hoff, P.; Morey, R.; Liu, J.; Roszik, J.; Clise-Dwyer, K.; et al. Mechanisms of nuclear content loading to exosomes. Sci. Adv. 2019, 5, eaax8849. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.V.; da Rosa Soares, A.; Pereira, P. LAMP2A mediates the loading of proteins into endosomes and selects exosomal cargo. Autophagy 2022, 18, 2263–2265. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Osaki, F.; Hiragi, S.; Sakamaki, Y.; Fukuda, M. ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Rep. 2021, 22, e51475. [Google Scholar] [CrossRef]
- Gheytanchi, E.; Saeednejad Zanjani, L.; Ghods, R.; Abolhasani, M.; Shahin, M.; Vafaei, S.; Naseri, M.; Fattahi, F.; Madjd, Z. High expression of tumor susceptibility gene 101 (TSG101) is associated with more aggressive behavior in colorectal carcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 1631–1646. [Google Scholar] [CrossRef]
- Harada, T.; Yamamoto, H.; Kishida, S.; Kishida, M.; Awada, C.; Takao, T.; Kikuchi, A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017, 108, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Guney Eskiler, G.; Kazan, N.; Haciefendi, A.; Deveci Ozkan, A.; Ozdemir, K.; Ozen, M.; Kocer, H.B.; Yilmaz, F.; Kaleli, S.; Sahin, E.; et al. The prognostic and predictive values of differential expression of exosomal receptor tyrosine kinases and associated with the PI3K/AKT/mTOR signaling in breast cancer patients undergoing neoadjuvant chemotherapy. Clin. Transl. Oncol. 2023, 25, 460–472. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Trojanowicz, B.; Li, X.; Shi, D.; Zhan, C.; Wang, Z.; Chen, L. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric Cancer 2016, 19, 754–766. [Google Scholar] [CrossRef]
- Lv, L.H.; Wan, Y.L.; Lin, Y.; Zhang, W.; Yang, M.; Li, G.L.; Lin, H.M.; Shang, C.Z.; Chen, Y.J.; Min, J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012, 287, 15874–15885. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, Y.; Miao, F.; Chen, G.; Zhu, Y.; Wu, N.; Pang, L.; Chen, Z.; Chen, X. PDL1-positive exosomes suppress antitumor immunity by inducing tumor-specific CD8+ T cell exhaustion during metastasis. Cancer Sci. 2021, 112, 3437–3454. [Google Scholar] [CrossRef]
- Lee, C.H.; Bae, J.H.; Choe, E.J.; Park, J.M.; Park, S.S.; Cho, H.J.; Song, B.J.; Baek, M.C. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1. Theranostics 2022, 12, 1971–1987. [Google Scholar] [CrossRef]
- Huang, L.; Wang, F.; Wang, X.; Su, C.; Wu, S.; Yang, C.; Luo, M.; Zhang, J.; Fu, L. M2-like macrophage-derived exosomes facilitate metastasis in non-small-cell lung cancer by delivering integrin αVβ3. Med. Comm. 2022, 4, e191. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chang, C.; Guo, J.; Lincoln, V.; Liang, C.; Chen, M.; Woodley, D.T.; Li, W. Tumour-Secreted Hsp90α on External Surface of Exosomes Mediates Tumour—Stromal Cell Communication via Autocrine and Paracrine Mechanisms. Sci. Rep. 2019, 9, 15108. [Google Scholar] [CrossRef]
- Lee, H.M.; Choi, E.J.; Kim, J.H.; Kim, T.D.; Kim, Y.K.; Kang, C.; Gho, Y.S. A membranous form of ICAM-1 on exosomes efficiently blocks leukocyte adhesion to activated endothelial cells. Biochem. Biophys. Res. Commun. 2010, 397, 251–256. [Google Scholar] [CrossRef]
- Koh, E.; Lee, E.J.; Nam, G.H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef]
- Fujii, N.; Yashiro, M.; Hatano, T.; Fujikawa, H.; Motomura, H. CD9-positive Exosomes Derived from Cancer-associated Fibroblasts Might Inhibit the Proliferation of Malignant Melanoma Cells. Anticancer Res. 2023, 43, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zaro, J.; Shen, Y. Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate. Int. J. Nanomed. 2020, 15, 9355–9371. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Keramat, S.; Izadirad, M.; Chen, Z.S.; Soukhtanloo, M. The Potential Role of Exosomes in the Treatment of Brain Tumors, Recent Updates and Advances. Front. Oncol. 2022, 12, 869929. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qiu, Q.; Jing, X.; Song, Z.; Zhang, Y.; Wang, C.; Liu, K.; Ye, F.; Ji, X.; Luo, F.; et al. Cancer-associated fibroblast-derived exosome miR-181b-3p promotes the occurrence and development of colorectal cancer by regulating SNX2 expression. Biochem. Biophys. Res. Commun. 2023, 641, 177–185. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Zhang, Q.; Liu, Z.; Wang, T.; Wu, Z.; Wu, W. CAF-Released Exosomal miR-20a-5p Facilitates HCC Progression via the LIMA1-Mediated β-Catenin Pathway. Cells 2022, 11, 3857. [Google Scholar] [CrossRef]
- Luo, C.; Xin, H.; Zhou, Z.; Hu, Z.; Sun, R.; Yao, N.; Sun, Q.; Borjigin, U.; Wu, X.; Fan, J.; et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology 2022, 76, 982–999. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.J.; Peng, L.J.; Gao, H.F.; Liu, L.M.; Chen, H. M2 macrophage-derived exosomal miR-193b-3p promotes progression and glutamine uptake of pancreatic cancer by targeting TRIM62. Biol. Direct 2023, 18, 1. [Google Scholar] [CrossRef]
- Hu, R.; Xu, B.; Ma, J.; Li, L.; Zhang, L.; Wang, L.; Zhu, J.; Guo, T.; Zhang, H.; Wang, S. LINC00963 promotes the malignancy and metastasis of lung adenocarcinoma by stabilizing Zeb1 and exosomes-induced M2 macrophage polarization. Mol. Med. 2023, 29, 1. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Yue, C.; Ma, J.; Cao, L.; Lin, J.; Zhu, D.; An, R.; Lai, J.; Guo, Y. Human umbilical cord mesenchymal stem cell-derived exosomes carrying miR-1827 downregulate SUCNR1 to inhibit macrophage M2 polarization and prevent colorectal liver metastasis. Apoptosis 2023, 28, 549–565. [Google Scholar] [CrossRef]
- Bongolo, C.C.; Thokerunga, E.; Yan, Q.; Yacouba, M.B.M.; Wang, C. Exosomes Derived from microRNA-27a-3p Overexpressing Mesenchymal Stem Cells Inhibit the Progression of Liver Cancer through Suppression of Golgi Membrane Protein 1. Stem Cells Int. 2022, 2022, 9748714. [Google Scholar] [CrossRef]
- Ji, R.; Zhang, X.; Gu, H.; Ma, J.; Wen, X.; Zhou, J.; Qian, H.; Xu, W.; Qian, J.; Lin, J. miR-374a-5p: A New Target for Diagnosis and Drug Resistance Therapy in Gastric Cancer. Mol. Ther. Nucleic Acids 2019, 18, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, H.; Tian, Y.; Li, Y.; Li, J.; Zhong, X.; Yuan, X. LncRNA SNHG11 enhances bevacizumab resistance in colorectal cancer by mediating miR-1207-5p/ABCC1 axis. Anticancer Drugs 2022, 33, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Song, X.; Fan, B.; Li, M.; Zhang, A.; Pei, L. Exosomal circRNA Scm-like with four malignant brain tumor domains 2 (circ-SFMBT2) enhances the docetaxel resistance of prostate cancer via the microRNA-136-5p/tribbles homolog 1 pathway. Anticancer Drugs 2022, 33, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Xie, M.; Ding, K.; Xu, T.; Fang, Y.; Ma, P.; Shu, Y. Exosome-transmitted miR-769-5p confers cisplatin resistance and progression in gastric cancer by targeting CASP9 and promoting the ubiquitination degradation of p53. Clin. Transl. Med. 2022, 12, e780. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, L.; Zeng, C.; Wu, Y.; Tang, X.; Zhu, Y.; Tang, S. MiR-302c-5p affects the stemness and cisplatin resistance of nasopharyngeal carcinoma cells by regulating HSP90AA1. Anticancer Drugs 2023, 34, 135–143. [Google Scholar] [CrossRef]
- Semaan, L.; Zeng, Q.; Lu, Y.; Zhang, Y.; Zreik, M.M.; Chamseddine, M.B.; Chopp, M.; Zhang, Z.G.; Moonka, D. MicroRNA-214 enriched exosomes from human cerebral endothelial cells (hCEC) sensitize hepatocellular carcinoma to anti-cancer drugs. Oncotarget 2021, 12, 185–198. [Google Scholar] [CrossRef]
- Yao, S.; Yin, Y.; Jin, G.; Li, D.; Li, M.; Hu, Y.; Feng, Y.; Liu, Y.; Bian, Z.; Wang, X.; et al. Exosome-mediated delivery of miR-204-5p inhibits tumor growth and chemoresistance. Cancer Med. 2020, 9, 5989–5998. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Ning, Y.; Zheng, H.; Zhan, Y.; Wang, H.; Yang, Y.; Luo, J.; Wen, Q.; Zang, H.; et al. Exosome-mediated miR-7-5p delivery enhances the anticancer effect of Everolimus via blocking MNK/eIF4E axis in non-small cell lung cancer. Cell Death Dis. 2022, 13, 129. [Google Scholar] [CrossRef]
- Bamankar, S.; Londhe, V.Y. The Rise of Extracellular Vesicles as New Age Biomarkers in Cancer Diagnosis: Promises and Pitfalls. Technol. Cancer Res. Treat. 2023, 22, 15330338221149266. [Google Scholar] [CrossRef]
- Rui, T.; Zhang, X.; Guo, J.; Xiang, A.; Tang, N.; Liu, J.; Mao, Z. Serum-Exosome-Derived miRNAs Serve as Promising Biomarkers for HCC Diagnosis. Cancers 2022, 15, 205. [Google Scholar] [CrossRef]
- Asgari, R.; Rezaie, J. Differential Expression of Serum Exosomal miRNAs in Breast Cancer Patients and Healthy Controls. Adv. Pharm. Bull. 2022, 12, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Li, L.; Ke, X.; Chen, H.; Liu, Y.; Zheng, N.; Zheng, Y.; Ma, Y.; Zhou, Y.; Yang, J.; et al. Urinary exosomal long non-coding RNAs as noninvasive biomarkers for diagnosis of bladder cancer by RNA sequencing. Front. Oncol. 2022, 12, 976329. [Google Scholar] [CrossRef] [PubMed]
- Faur, C.I.; Roman, R.C.; Jurj, A.; Raduly, L.; Almășan, O.; Rotaru, H.; Chirilă, M.; Moldovan, M.A.; Hedeșiu, M.; Dinu, C. Salivary Exosomal MicroRNA-486-5p and MicroRNA-10b-5p in Oral and Oropharyngeal Squamous Cell Carcinoma. Medicina 2022, 58, 1478. [Google Scholar] [CrossRef]
- Jin, Z.; Song, Y.; Lian, C.; Gao, L. Decreased Serum Exosomal microRNA-134 Expression and Its Prognostic Value in Gastric Cancer. Ann. Clin. Lab. Sci. 2022, 52, 563–570. [Google Scholar] [PubMed]
- Xiao, Q.; Lin, C.; Peng, M.; Ren, J.; Jing, Y.; Lei, L.; Tao, Y.; Huang, J.; Yang, J.; Sun, M.; et al. Circulating plasma exosomal long non-coding RNAs LINC00265, LINC00467, UCA1, and SNHG1 as biomarkers for diagnosis and treatment monitoring of acute myeloid leukemia. Front. Oncol. 2022, 12, 1033143. [Google Scholar] [CrossRef]
- Liu, C.; Hu, C.; Chen, T.; Jiang, Y.; Zhang, X.; Liu, H.; Wang, Y.; Li, Z.; Hui, K.; Jiang, X. The role of plasma exosomal lnc-SNAPC5-3:4 in monitoring the efficacy of anlotinib in the treatment of advanced non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 2867–2879. [Google Scholar] [CrossRef]
- Tang, D.; Li, Y.; Tang, Y.; Zheng, H.; Luo, W.; Li, Y.; Li, Y.; Wang, Z.; Wu, S. Recognition of Glycometabolism-Associated lncRNAs as Prognosis Markers for Bladder Cancer by an Innovative Prediction Model. Front. Genet. 2022, 13, 918705. [Google Scholar] [CrossRef]
- Pan, Y.; Shao, S.; Sun, H.; Zhu, H.; Fang, H. Bile-derived exosome noncoding RNAs as potential diagnostic and prognostic biomarkers for cholangiocarcinoma. Front. Oncol. 2022, 12, 985089. [Google Scholar] [CrossRef]
- Song, Q.; Lv, X.; Ru, Y.; Dong, J.; Chang, R.; Wu, D.; Chen, L.; Wang, X.; Guo, X. Circulating exosomal gastric cancer-associated long noncoding RNA1 as a noninvasive biomarker for predicting chemotherapy response and prognosis of advanced gastric cancer: A multi-cohort, multi-phase study. eBioMedicine 2022, 78, 103971. [Google Scholar] [CrossRef]
- Sung, B.H.; Weaver, A.M. Visualization of Exosome Release and Uptake during Cell Migration Using the Live Imaging Reporter pHluorin_M153R-CD63. Methods Mol. Biol. 2023, 2608, 83–96. [Google Scholar]
- Xu, Y.; Liu, Y.; Yang, C.; Kang, L.; Wang, M.; Hu, J.; He, H.; Song, W.; Tang, H. Macrophages transfer antigens to dendritic cells by releasing exosomes containing dead-cell-associated antigens partially through a ceramide-dependent pathway to enhance CD4(+) T-cell responses. Immunology 2016, 149, 157–171. [Google Scholar] [CrossRef]
- Choezom, D.; Gross, J.C. Neutral sphingomyelinase 2 controls exosome secretion by counteracting V-ATPase-mediated endosome acidification. J. Cell Sci. 2022, 135, jcs259324. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Asef-Kabiri, L.; Sarvnaz, H.; Ghanavatinejad, A.; Rezayat, F.; Eskandari, N.; Akbari, M.E. Blockade of exosome release alters HER2 trafficking to the plasma membrane and gives a boost to Trastuzumab. Clin. Transl. Oncol. 2023, 25, 185–198. [Google Scholar] [CrossRef]
- Garrido, C.M.; Henkels, K.M.; Rehl, K.M.; Liang, H.; Zhou, Y.; Gutterman, J.U.; Cho, K.J. Avicin G is a potent sphingomyelinase inhibitor and blocks oncogenic K- and H-Ras signaling. Sci. Rep. 2020, 10, 9120. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, J.H. Graphene Oxide Enhances Biogenesis and Release of Exosomes in Human Ovarian Cancer Cells. Int. J. Nanomed. 2022, 17, 5697–5731. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, P.; Sharma, M.; Kim, S.; Singh, S.; Kridel, S.J.; Deep, G. Role of extracellular vesicles secretion in paclitaxel resistance of prostate cancer cells. Cancer Drug Resist. 2022, 5, 612–624. [Google Scholar] [CrossRef]
- Hekmatirad, S.; Moloudizargari, M.; Moghadamnia, A.A.; Kazemi, S.; Mohammadnia-Afrouzi, M.; Baeeri, M.; Moradkhani, F.; Asghari, M.H. Inhibition of Exosome Release Sensitizes U937 Cells to PEGylated Liposomal Doxorubicin. Front. Immunol. 2021, 12, 692654. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, Y.; Lan, Z.; Liu, Q.; Xu, J.; Zhu, M.; Yang, T.; Shi, R.; Gao, S.; Liang, G. Exosomal PD-L1 confers chemoresistance and promotes tumorigenic properties in esophageal cancer cells via upregulating STAT3/miR-21. Gene Ther. 2022, 30, 88–100. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, M.; Hu, Y.; Guo, H.; Zhang, Y.; Huang, Y.; Zhao, L.; Chai, Y.; Wang, Z. Blockade of exosome generation by GW4869 inhibits the education of M2 macrophages in prostate cancer. BMC Immunol. 2022, 23, 37. [Google Scholar] [CrossRef]
- Li, D.; Xia, L.; Huang, P.; Wang, Z.; Guo, Q.; Huang, C.; Leng, W.; Qin, S. Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis. Cell Death Discov. 2023, 9, 17. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, R.; Yang, Y.; Liang, C.; Yu, X.; Liu, Y.; Wang, T.; Yu, Y.; Deng, F. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J. Nanobiotechnol. 2021, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dong, C.; Jiang, K.; Sun, R.; Zhou, Y.; Yin, Z.; Lv, J.; Zhang, J.; Wang, Q.; Wang, L. Rab27B enhances drug resistance in hepatocellular carcinoma by promoting exosome-mediated drug efflux. Carcinogenesis 2020, 41, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, E.; Vorholt, D.; Blakemore, S.; Sackey, B.; Nolte, J.L.; Barbarino, V.; Schmitz, J.; Nickel, N.; Bachurski, D.; Lobastova, L.; et al. Extracellular vesicles and PD-L1 suppress macrophages, inducing therapy resistance in TP53-deficient B-cell malignancies. Blood 2022, 139, 3617–3629. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.W.; Kim, H.; Ahn, M.; Moustafa, A.A.; Zhou, H.; Barata, P.C.; Boulares, A.H.; Abdel-Mageed, A.B.; Krane, L.S. Combination of Tipifarnib and Sunitinib Overcomes Renal Cell Carcinoma Resistance to Tyrosine Kinase Inhibitors via Tumor-Derived Exosome and T Cell Modulation. Cancers 2022, 14, 903. [Google Scholar] [CrossRef]
- Luo, Y.H.; Yang, Y.P.; Chien, C.S.; Yarmishyn, A.A.; Adekunle Ishola, A.; Chien, Y.; Chen, Y.M.; Tsai, P.H.; Lin, T.W.; Wang, M.L.; et al. Circular RNA hsa_circ_0000190 Facilitates the Tumorigenesis and Immune Evasion by Upregulating the Expression of Soluble PD-L1 in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2021, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.J.; Lee, C.H.; Bae, J.H.; Park, J.M.; Park, S.S.; Baek, M.C. Atorvastatin Enhances the Efficacy of Immune Checkpoint Therapy and Suppresses the Cellular and Extracellular Vesicle PD-L1. Pharmaceutics 2022, 14, 1660. [Google Scholar] [CrossRef]
- Wang, G.; Xie, L.; Li, B.; Sang, W.; Yan, J.; Li, J.; Tian, H.; Li, W.; Zhang, Z.; Tian, Y.; et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat. Commun. 2021, 12, 5733. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Ly, T.B.; Wesseling, M.C.; Hittinger, M.; Torge, A.; Devitt, A.; Perrie, Y.; Bernhardt, I. Characterization of Microvesicles Released from Human Red Blood Cells. Cell. Physiol. Biochem. 2016, 38, 1085–1099. [Google Scholar] [CrossRef]
- Rosenthal, A.K.; Gohr, C.M.; Mitton-Fitzgerald, E.; Grewal, R.; Ninomiya, J.; Coyne, C.B.; Jackson, W.T. Autophagy modulates articular cartilage vesicle formation in primary articular chondrocytes. J. Biol. Chem. 2015, 290, 13028–13038. [Google Scholar] [CrossRef]
- Schillaci, O.; Fontana, S.; Monteleone, F.; Taverna, S.; Di Bella, M.A.; Di Vizio, D.; Alessandro, R. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: Their emerging role in tumor heterogeneity. Sci. Rep. 2017, 7, 4711. [Google Scholar] [CrossRef]
- You, L.N.; Tai, Q.W.; Xu, L.; Hao, Y.; Guo, W.J.; Zhang, Q.; Tong, Q.; Zhang, H.; Huang, W.K. Exosomal LINC00161 promotes angiogenesis and metastasis via regulating miR-590-3p/ROCK axis in hepatocellular carcinoma. Cancer Gene Ther. 2021, 28, 719–736. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, Y.; Zhang, Y.; Zheng, D.; Yan, L.; Guo, M.; Mao, Y.; Yang, L. Exosomes from bone marrow-derived mesenchymal stem cells facilitate corneal wound healing via regulating the p44/42 MAPK pathway. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 261, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Kuninty, P.R.; Binnemars-Postma, K.; Jarray, A.; Pednekar, K.P.; Heinrich, M.A.; Pijffers, H.J.; Ten Hoopen, H.; Storm, G.; van Hoogevest, P.; den Otter, W.K.; et al. Cancer immune therapy using engineered ‛tail-flipping’ nanoliposomes targeting alternatively activated macrophages. Nat. Commun. 2022, 13, 4548. [Google Scholar] [CrossRef] [PubMed]
- Nezhadi, S.; Saadat, E.; Handali, S.; Dorkoosh, F. Nanomedicine and chemotherapeutics drug delivery: Challenges and opportunities. J. Drug Target 2021, 29, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Volovat, S.R.; Ursulescu, C.L.; Moisii, L.G.; Volovat, C.; Boboc, D.; Scripcariu, D.; Amurariti, F.; Stefanescu, C.; Stolniceanu, C.R.; Agop, M.; et al. The Landscape of Nanovectors for Modulation in Cancer Immunotherapy. Pharmaceutics 2022, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Varshney, A.; Bajaj, R.; Pokharkar, V. Exosomes as New Generation Vehicles for Drug Delivery: Biomedical Applications and Future Perspectives. Molecules 2022, 27, 7289. [Google Scholar] [CrossRef]
- Fu, W.; Li, T.; Chen, H.; Zhu, S.; Zhou, C. Research Progress in Exosome-Based Nanoscale Drug Carriers in Tumor Therapies. Front. Oncol. 2022, 12, 919279. [Google Scholar] [CrossRef]
- Kok, V.C.; Yu, C.C. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int. J. Nanomed. 2020, 15, 8019–8036. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef]
- Hou, C.; Wu, Q.; Xu, L.; Cui, R.; Ou, R.; Li, D.; Xu, Y. Exploiting the potential of extracellular vesicles as delivery vehicles for the treatment of melanoma. Front. Bioeng. Biotechnol. 2022, 10, 1054324. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.I.; Khan, M.U.; Khan, N.M.; Bungau, S.; Hassan, S.S.U. Applications of Extracellular Vesicles in Nervous System Disorders: An Overview of Recent Advances. Bioengineering 2022, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, N.; Sugaya, K. Exosome mediated Tom40 delivery protects against hydrogen peroxide-induced oxidative stress by regulating mitochondrial function. PLoS ONE 2022, 17, e0272511. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, J. Mesenchymal stem cell-derived exosomes containing miR-145-5p reduce inflammation in spinal cord injury by regulating the TLR4/NF-κB signaling pathway. Cell Cycle 2021, 20, 993–1009. [Google Scholar] [CrossRef]
- Gomes, E.R.; Souza, F.R.; Cassali, G.D.; Sabino, A.P.; Barros, A.L.B.; Oliveira, M.C. Investigation of the Antitumor Activity and Toxicity of Tumor-Derived Exosomes Fused with Long-Circulating and pH-Sensitive Liposomes Containing Doxorubicin. Pharmaceutics 2022, 14, 2256. [Google Scholar] [CrossRef]
- Zhou, G.; Gu, Y.; Zhu, Z.; Zhang, H.; Liu, W.; Xu, B.; Zhou, F.; Zhang, M.; Hua, K.; Wu, L.; et al. Exosome Mediated Cytosolic Cisplatin Delivery through Clathrin-Independent Endocytosis and Enhanced Anti-cancer Effect via Avoiding Endosome Trapping in Cisplatin-Resistant Ovarian Cancer. Front. Med. 2022, 9, 810761. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, K.; Zhang, T.; Gao, G.C.; Xu, H. Natural killer cell-derived exosome-entrapped paclitaxel can enhance its anti-tumor effect. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5703–5713. [Google Scholar] [PubMed]
- Zhang, X.; Liu, L.; Tang, M.; Li, H.; Guo, X.; Yang, X. The effects of umbilical cord-derived macrophage exosomes loaded with cisplatin on the growth and drug resistance of ovarian cancer cells. Drug Dev. Ind. Pharm. 2020, 46, 1150–1162. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Jin, L.; Guo, P.; Zhang, Z.; Zhanghuang, C.; Tan, X.; Mi, T.; Liu, J.; Wu, X.; et al. Exosome mimetics derived from bone marrow mesenchymal stem cells deliver doxorubicin to osteosarcoma in vitro and in vivo. Drug Deliv. 2022, 29, 3291–3303. [Google Scholar] [CrossRef]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020, 261, 118369. [Google Scholar] [CrossRef]
- Uslu, D.; Abas, B.I.; Demirbolat, G.M.; Cevik, O. Effect of platelet exosomes loaded with doxorubicin as a targeted therapy on triple-negative breast cancer cells. Mol. Divers. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Chen, W.; Lin, W.; Yu, N.; Zhang, L.; Wu, Z.; Chen, Y.; Li, Z.; Gong, F.; Li, N.; Chen, X.; et al. Activation of Dynamin-Related Protein 1 and Induction of Mitochondrial Apoptosis by Exosome-Rifampicin Nanoparticles Exerts Anti-Osteosarcoma Effect. Int. J. Nanomed. 2022, 17, 5431–5446. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, Y.; Li, L.; Luo, H.; Cao, T.; Tu, B. Exosomal miR-22-3p from Mesenchymal Stem Cells Inhibits the Epithelial-Mesenchymal Transition (EMT) of Melanoma Cells by Regulating LGALS1. Front. Biosci. 2022, 27, 275. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, L.; Piontek, K.; Sakaguchi, M.; Selaru, F.M. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology 2018, 67, 940–954. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Zhang, X.; Yan, N.; Li, X. Exosomal miR-126 blocks the development of non-small cell lung cancer through the inhibition of ITGA6. Cancer Cell Int. 2020, 20, 574. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Xu, W.; Zhang, X.; Jiang, J. Engineered exosome-mediated delivery of circDIDO1 inhibits gastric cancer progression via regulation of MiR-1307-3p/SOCS2 Axis. J. Transl. Med. 2022, 20, 326. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yang, N.; Yin, C.; Zeng, Y.; Zhu, X. Engineered Exosomes Loaded with miR-563 Inhibit Lung Cancer Growth. J. Oncol. 2022, 2022, 6141857. [Google Scholar] [CrossRef]
- Deng, W.; Meng, Y.; Wang, B.; Wang, C.X.; Hou, C.X.; Zhu, Q.H.; Tang, Y.T.; Ye, J.H. In vitro experimental study on the formation of microRNA-34a loaded exosomes and their inhibitory effect in oral squamous cell carcinoma. Cell Cycle 2022, 21, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yang, Y.; Yang, L.; Yan, X.; Shen, Z.; Liu, J.; Xue, J.; Zhao, W.; Liu, X. miR-371b-5p-Engineered Exosomes Enhances Tumor Inhibitory Effect. Front. Cell Dev. Biol. 2021, 9, 750171. [Google Scholar] [CrossRef]
- Hareendran, S.; Albraidy, B.; Yang, X.; Liu, A.; Breggia, A.; Chen, C.C.; Loh, Y.P. Exosomal Carboxypeptidase E (CPE) and CPE-shRNA-Loaded Exosomes Regulate Metastatic Phenotype of Tumor Cells. Int. J. Mol. Sci. 2022, 23, 3113. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Ma, Q.; Wei, M.; Liu, X.; Qi, Y.; Li, C.; Dong, L.; Zhang, H. si-PDGFRβ-Loaded Exosomes Suppress the Progression of Glioma by Inhibiting the Oxidative Associated PI3K/Akt/EZH2 Signaling Pathway. Oxidative Med. Cell Longev. 2022, 2022, 5081439. [Google Scholar] [CrossRef]
- Lin, X.; Lin, L.; Wu, J.; Jiang, W.; Wu, J.; Yang, J.; Chen, C. A targeted siRNA-loaded PDL1-exosome and functional evaluation against lung cancer. Thorac. Cancer 2022, 13, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dai, G.; Wu, Y.; Zhang, M.; Yang, M.; Wang, X.; Song, M.; Li, X.; Xia, R.; Wu, Z. iRGD-modified exosomes-delivered BCL6 siRNA inhibit the progression of diffuse large B-cell lymphoma. Front. Oncol. 2022, 12, 822805. [Google Scholar] [CrossRef]
- Ni, H.; Zhang, H.; Li, L.; Huang, H.; Guo, H.; Zhang, L.; Li, C.; Xu, J.X.; Nie, C.P.; Li, K.; et al. T cell-intrinsic STING signaling promotes regulatory T cell induction and immunosuppression by upregulating FOXP3 transcription in cervical cancer. J. Immunother. Cancer 2022, 10, e005151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, Y.; Li, B.; Sugimoto, H.; Yang, S.; Yang, C.; LeBleu, V.S.; McAndrews, K.M.; Kalluri, R. Therapeutic targeting of STAT3 with small interference RNAs and antisense oligonucleotides embedded exosomes in liver fibrosis. FASEB J. 2021, 35, e21557. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2015, 5, e1071008. [Google Scholar] [CrossRef]
- Khezri, K.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Sharifi, S.; Shahi, S.; Ahmadian, E.; Eftekhari, A.; Dalir Abdolahinia, E.; Lotfipour, F. Osteogenic Differentiation of Mesenchymal Stem Cells via Curcumin-Containing Nanoscaffolds. Stem Cells Int. 2021, 2021, 1520052. [Google Scholar] [CrossRef]
- Wu, K.; Xing, F.; Wu, S.Y.; Watabe, K. Extracellular vesicles as emerging targets in cancer: Recent development from bench to bedside. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 538–563. [Google Scholar] [CrossRef]
- Akbari, A.; Nazari-Khanamiri, F.; Ahmadi, M.; Shoaran, M.; Rezaie, J. Engineered Exosomes for Tumor-Ta. rgeted Drug Delivery: A Focus on Genetic and Chemical Functionalization. Pharmaceutics 2022, 15, 66. [Google Scholar] [CrossRef]
- Kar, R.; Dhar, R.; Mukherjee, S.; Nag, S.; Gorai, S.; Mukerjee, N.; Mukherjee, D.; Vatsa, R.; Chandrakanth Jadhav, M.; Ghosh, A.; et al. Exosome-Based Smart Drug Delivery Tool for Cancer Theranostics. ACS Biomater. Sci. Eng. 2023, 9, 577–594. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, Y.; Jiang, W.; Shen, J.; Yao, X.; He, X.; Li, L.; Fu, B.; Liu, X. Biological Features of Extracellular Vesicles and Challenges. Front. Cell Dev. Biol. 2022, 10, 816698. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Chodari, L.; Dilsiz Aytemir, M.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxidative Med. Cell Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef] [PubMed]


| Proteins | Origin of Exosomes | Effect | Ref. |
|---|---|---|---|
| B7H4 | Glioblastoma cells | Enhances tumor growth by increasing the expression of FOXP3, which induces immune evasion. | [17] |
| RAB27A | Gastric cancer cells | Secretion of exosomes ↑ Peritoneal metastasis ↑ | [46] |
| TSG101 | Colorectal cancer cells | Enhances proliferation and migration of lung cancer cells via Wnt5b | [61] |
| CD97 | Gastric cancer cells | Enhances proliferation and metastatic potential of gastric cancer cells | [63] |
| Heat shock proteins (Hsp60, Hsp70, Hsp90) | Hepatocellular carcinoma cells | Suppresses NK cell function | [64] |
| Integrins αV and β3 | M2 macrophages | Enhances the metastatic potential of NSCLCs | [67] |
| HSP90 | Breast cancer cells | Recruits stromal cells such as fibroblasts | [68] |
| ICAM-1 (membrane form) | Prostate cancer cells/Breast cancer cells | Suppresses adhesion of leukocytes to endothelial cells | [69] |
| CD47 | HEK293T cells | Inhibits the phagocytic effect of macrophages | [70] |
| CD9 | Cancer associated fibroblasts | Inhibits the proliferation of melanoma cells | [71] |
| Exosomal Cargo | Sources of Exosomes | Target | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|
| miR-181b-3p | Cancer associated fibroblasts | Colorectal cancer cells | Enhances progression of colorectal cancer | SNX2 ↓ | [74] |
| miR-20a-5p | Cancer associated fibroblasts | Hepatic cancer cells | Enhances progression of hepatic cancer cells | LIMA1 ↓ Wnt/β-Catenin ↓ | [75] |
| miR-183-5p | Intrahepatic cholangiocarcinoma | intrahepatic cholangiocarcinoma cells | Enhances progression of cholangiocarcinoma cells | Increases the number of PD-L1 expressing macrophages | [76] |
| miR-193b-3p | M2 macrophages | Pancreatic cancer cells | Enhances proliferation of cancer cells | TRIM62 ↓ | [77] |
| LINC00963 | Lung adenocarcinoma cells | Lung adenocarcinoma cells | Induces M2 polarization of macrophages, enhances metastasis of lung adenocarcinoma cells. | SIAH1 ↓ ZEB1 ↑ | [78] |
| miR-10527-5p | Plasma and serum of esophageal squamous cell carcinoma (ESCC) patients | esophageal squamous cell carcinoma | Inhibits lymphatic metastasis of ESCC | Rab10 ↓ | [9] |
| miR-1827 | Human umbilical cord mesenchymal stem cells | Colorectal cancer cells | Suppresses metastatic potential | SUCNR1 ↓ | [79] |
| miR-27a-3p | Mesenchymal stem cells | Hepatic cancer cells | Inhibits progression of hepatic cancer | GOLM1 ↓ | [80] |
| circ_0063526 | CDDP-resistant Gastric cancer cells | CDDP-sensitive gastric cancer cells | Enhances CDDP resistance | miR-449 ↓ SHMT2 ↑ | [16] |
| miR-374a-5p | Serum of gastric cancer patients | Gastric cancer cells | Enhances resistance to oxaliplatin | NeuroD1 ↓ | [81] |
| Lnc RNA SNHG11 | Bevacizumab-resistant Colorectal cancer cells | Colorectal cancer cells | Enhances Bevacizumab resistance | MiR-1207-5p ↓ ABCC1 ↑ | [82] |
| circSFMBT2 | Docetaxel-resistant prostate cancer cells | Prostate cancer cells | Enhances resistance to doxorubicin | miR-136-5p ↓ TRIB1 ↑ | [83] |
| miR-769-5p | CDDP-resistant Gastric cancer cells | CDDP-sensitive gastric cancer cells | Enhances CDDP resistance | CASP9 ↓ P53 degradation by ubiquitination | [84] |
| miR-214 | Human cerebral endothelial cells | HCC | Enhances the sensitivity to oxaliplatin and sorafenib | P-gp ↓ Splicing factor 3B subunit 3 (SF3B3) ↓ | [86] |
| miR-204-5p | HEK293T cells | Colorectal cancer cells | Enhances the sensitivity to 5-FU | RAB22A ↓ Bcl-2 ↓ | [87] |
| miR-7-5p | NSCLC | NSCLC | Enhances the sensitivity to Everolimus | MNK/eIF4E ↓ mTOR ↓ | [88] |
| miRNAs/Lnc RNAs | Cancer Types/Sources | Diagnosis/Prognosis | Expression | Ref. |
|---|---|---|---|---|
| miR-320d, miR-4479, and miR-6763-5p | Epithelial ovarian cancer/Plasma | Diagnosis | Downregulated in cancer patients | [4] |
| miR-122-5p, let-7d-5p, and miR-425-5p | Hepatocellular carcinoma/Serum | Diagnosis | Increased in cancer patients | [90] |
| miR-21, miR-155, miR-182, miR-373, and miR-126 | Breast cancer/Serum | Diagnosis | Increased in cancer patients | [91] |
| MKLN1-AS, TALAM1, TTN-AS1 and UCA1 | Bladder cancer/Urine | Diagnosis | Increased in cancer patients | [92] |
| miR-10b-5p and miR-486-5p | Oral and oropharyngeal cancer/Saliva | Diagnosis | miR-486-5p is elevated and miR-10b-5p is decreased | [93] |
| miR-134 | Gastric cancer/Serum | Diagnosis | Downregulated in cancer patients | [94] |
| LINC00265, LINC00467, and UCA1 | Acute myeloid leukemia/Plasma | Diagnosis | Downregulated in cancer patients | [95] |
| lnc-SNAPC5-3:4 | NSCLC/Plasma | Prognosis | High level can predict favorable response to anlotinib | [96] |
| lncRNA AL355353.1, AC011468.1, and AL354919.2 | Bladder cancer/Urine | Prognosis | High level can predict unfavorable prognosis | [97] |
| miR-200c-3p | Cholangiocarcinoma/Serum | Prognosis | High level can predict unfavorable prognosis | [98] |
| lncRNA-GC1 | Gastric cancer | Prognosis | Low level can predict favorable response to 5-FU treatment | [99] |
| Title | Status | Condition or Disease | Prospective Outcome Measures | Dates | ID/Purpose |
|---|---|---|---|---|---|
| Serum Exosomal Long Noncoding RNAs as Potential Biomarkers for Lung Cancer Diagnosis | Completed | Lung cancer |
|
| NCT03830619/Diagnosis |
| Interrogation of Exosome-mediated Intercellular Signaling in Patients with Pancreatic Cancer | Recruiting | Pancreatic cancer |
|
| NCT02393703/Diagnosis |
| A Pilot Study of Circulating Exosome RNA as Diagnostic and Prognostic Markers in Lung Metastases of Primary High-Grade Osteosarcoma | Active, not recruiting | Osteosarcoma, lung metastases |
|
| NCT03108677/Diagnosis and prognosis |
| Clinical Validation of a Urinary Exosome Gene Signature in Men Presenting for Suspicion of Prostate Cancer | Completed | Prostate cancer |
|
| NCT02702856/Diagnosis |
| Use of Circulating Exosomal lncRNA-GC1 as Blood Biomarker for Early Detection and Monitoring Gastric cancer | Recruiting | Gastric cancer |
|
| NCT05397548/Diagnosis |
| A Prospective, Multicenter Cohort Study of Urinary Exosome lncRNAs for Preoperative Diagnosis of Lymphatic Metastasis in Patients with Bladder Cancer | Not yet recruiting | Bladder cancer |
|
| NCT05270174/Diagnosis |
| A Prospective Study of Predicting Prognosis and Recurrence of Thyroid Cancer Via New Biomarkers, Urinary Exosomal Thyroglobulin and Galectin-3 | Active, not recruiting | Thyroid cancer |
|
| NCT03488134/Prognosis |
| Title | Arms/Interventions | Study Design | Types of Cancers | Phase | Study Dates | NCT Number |
|---|---|---|---|---|---|---|
| Phase I Study of Mesenchymal Stromal Cells-Derived Exosomes with KrasG12D siRNA for Metastatic Pancreas Cancer Patients Harboring KrasG12D Mutation |
| Outcome measures:
| Metastatic Pancreatic Adenocarcinoma | Phase 1 |
| NCT03608631 |
| Phase I Clinical Trial Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Malignant Colon Tissue | Arm1: curcumin alone Arm 2: curcumin with plant exosome Arm 3: no treatment | Outcome measures:
| Colon cancer | Phase 1 |
| NCT01294072 |
| An Open, Dose-escalation Clinical Study of Chimeric Exosomal Tumor Vaccines for Recurrent or Metastatic Bladder Cancer |
|
| Bladder cancer | Early phase 1 |
| NCT05559177 |
| Phase II Trial of a Vaccination with Tumor Antigen-loaded Dendritic Cell-derived Exosomes on Patients with Unresectable Non-Small Cell Lung Cancer Responding to Induction Chemotherapy |
|
| Non-small-cell lung cancer | Phase 2 |
| NCT01159288 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Shim, K.; Jeoung, D. Exosomes: Diagnostic and Therapeutic Implications in Cancer. Pharmaceutics 2023, 15, 1465. https://doi.org/10.3390/pharmaceutics15051465
Jo H, Shim K, Jeoung D. Exosomes: Diagnostic and Therapeutic Implications in Cancer. Pharmaceutics. 2023; 15(5):1465. https://doi.org/10.3390/pharmaceutics15051465
Chicago/Turabian StyleJo, Hyein, Kyeonghee Shim, and Dooil Jeoung. 2023. "Exosomes: Diagnostic and Therapeutic Implications in Cancer" Pharmaceutics 15, no. 5: 1465. https://doi.org/10.3390/pharmaceutics15051465