Development of a Gold Nanoparticle-Linked Immunosorbent Assay of Staphylococcal Enterotoxin B Detection with Extremely High Sensitivity by Determination of Gold Atom Content Using Graphite Furnace Atomic Absorption Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of Gold Nanoparticle (AuNP) Seeds
2.3. Preparation of the Larger Particle Size Colloidal Gold
2.4. Preparation of AuNPs/HS-PEG-COOH/FMU-SEB-1 Detection mAb Bioconjugate
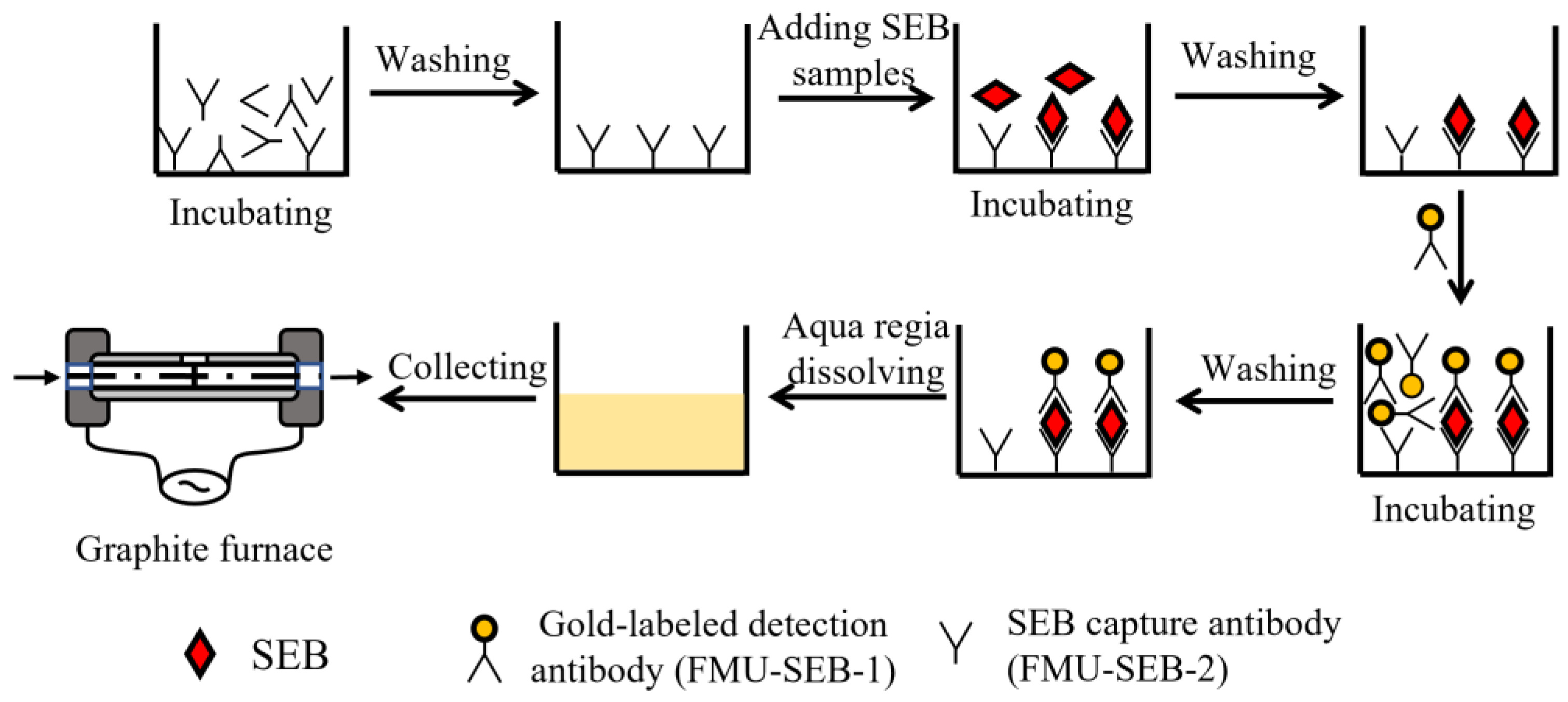
2.5. SEB Detection Using SEB Specific mAb Pair by ALISA
2.6. Sample Preparation
3. Result and Discussion
3.1. Characterization of Gold Nanoparticles of Different Sizes
3.2. Characterization of AuNP/HS-PEG-COOH and AuNP/HS-PEG-COOH/FMU-SEB-1
3.3. Establishment of ALISA Method and Sensitivity of SEB Detection
3.4. Precision and Accuracy of SEB Detection Using ALISA
3.5. Recoveries of SEB Detection Using ALISA in Various Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousavi, N.S.; Nasirizadeh, N.; Amani, J.; Halabian, R.; Imani Fooladi, A.A. An electrochemical aptasensor for staphylococcal enterotoxin B detection based on reduced graphene oxide and gold nano-urchins. Biosens. Bioelectron. 2019, 127, 221–228. [Google Scholar] [CrossRef]
- Xiong, X.; Shi, X.; Liu, Y.; Lu, L.; You, J. An aptamer-based electrochemical biosensor for simple and sensitive detection of staphylococcal enterotoxin B in milk. Anal. Methods 2018, 10, 365–370. [Google Scholar] [CrossRef]
- Chen, G.; Karauzum, H.; Long, H.; Carranza, D.; Holtsberg, F.W.; Howell, K.A.; Abaandou, L.; Zhang, B.; Jarvik, N.; Ye, W.; et al. Potent Neutralization of Staphylococcal Enterotoxin B In Vivo by Antibodies that Block Binding to the T-Cell Receptor. J. Mol. Biol. 2019, 431, 4354–4367. [Google Scholar] [CrossRef]
- Cai, X.L.; Luo, Y.X.; Zhu, C.Z.; Huang, D.M.; Song, Y. Rhodium nanocatalyst-based lateral flow immunoassay for sensitive detection of staphylococcal enterotoxin B. Sens. Actuators B Chem. 2022, 367, 132066. [Google Scholar] [CrossRef]
- Wu, K.H.; Huang, W.C.; Shyu, R.H.; Chang, S.C. Silver nanoparticle-base lateral flow immunoassay for rapid detection of Staphylococcal enterotoxin B in milk and honey. J. Inorg. Biochem. 2020, 210, 111163. [Google Scholar] [CrossRef]
- Xiong, X.; Luo, Y.; Lu, Y.; Xiong, X.; Li, Y.; Liu, Y.; Lu, L. Ultrasensitive detection of Staphylococcal enterotoxin B in milk based on target-triggered assembly of the flower like nucleic acid nanostructure. RSC Adv. 2019, 9, 42423–42429. [Google Scholar] [CrossRef]
- Shen, H.; Bai, J.; Zhao, X.; Lu, B.; Han, D.; Li, S.; Qin, K.; Ren, S.; Wang, Y.; Wang, M.; et al. Highly Ordered, Plasmonic Enhanced Inverse Opal Photonic Crystal for Ultrasensitive Detection of Staphylococcal Enterotoxin B. ACS Appl. Mater. Interfaces 2022, 14, 4637–4646. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, Z.; Liu, W.; Xu, Z.; He, H.; Ning, B.; Jiang, Y.; Gao, Z. Development of sandwich chemiluminescent immunoassay based on an anti-staphylococcal enterotoxin B Nanobody-Alkaline phosphatase fusion protein for detection of staphylococcal enterotoxin B. Anal. Chim. Acta 2020, 1108, 28–36. [Google Scholar] [CrossRef]
- Sun, S.; Yang, M.; Kostov, Y.; Rasooly, A. ELISA-LOC: Lab-on-a-chip for enzyme-linked immunodetection. Lab Chip 2010, 10, 2093–2100. [Google Scholar] [CrossRef]
- Khan, A.S.; Cao, C.J.; Thompson, R.G.; Valdes, J.J. A simple and rapid fluorescence-based immunoassay for the detection of staphylococcal enterotoxin B. Mol. Cell. Probes 2003, 17, 125–126. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, S.; Kim, S.G.; Lee, J.; Lee, D.G.; Jang, J.; Jeong, Y.S.; Song, D.H.; Min, J.K.; Park, J.G.; et al. A Sensitive Immunodetection Assay Using Antibodies Specific to Staphylococcal Enterotoxin B Produced by Baculovirus Expression. Biosensors 2022, 12, 787. [Google Scholar] [CrossRef]
- Hwa, S.R.; Shek, T.S.; Jiang, C.D.; Wen, H.Y. Gold nanoparticle-based lateral flow assay for detection of staphylococcal enterotoxin B. Food Chem. 2010, 118, 462–466. [Google Scholar]
- Fathi, F.; Rashidi, M.R.; Omidi, Y. Ultra-sensitive detection by metal nanoparticles-mediated enhanced SPR biosensors. Talanta 2019, 192, 118–127. [Google Scholar] [CrossRef]
- Chang, X.; Cheng, Y.; Wang, X.; Wang, Y.; Liu, X.; Han, T.; Gao, Z.; Zhou, H. A novel ultrasensitive and fast aptamer biosensor of SEB based on AuNPs-assisted metal-enhanced fluorescence. Sci. Total Environ. 2023, 858, 159977. [Google Scholar] [CrossRef]
- Qin, T.; Hong, Y.; Han, D.; Li, S.; Ning, B.; Li, Z.; Wang, J.; Bai, J.; Gao, Z.; Peng, Y. Aptamer-based photonic crystals enable ultra-trace detection of staphylococcal enterotoxin B without labels. Food Chem. 2022, 391, 133271. [Google Scholar] [CrossRef]
- Chopra, A.; Swami, A.; Sharma, R.; Devi, N.; Mittal, S.; Sharma, R.K.; Wangoo, N. Femtomolar detection of staphylococcal enterotoxin ‘B’ using a fluorescent quantum dot based hybrid Apta-immunosensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 287, 122036. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, J.; Gong, B.; Matsumoto, K.; Hu, Z. Immunoassay by graphite furnace atomic absorption spectrometry using a metal chelate as a label. Anal. Chim. Acta 2001, 448, 165–172. [Google Scholar] [CrossRef]
- Liang, P.; Kang, C.; Yang, E.; Ge, X.; Du, D.; Lin, Y. A sensitive magnetic nanoparticle-based immunoassay of phosphorylated acetylcholinesterase using protein cage templated lead phosphate for signal amplification with graphite furnace atomic absorption spectrometry detection. Analyst 2016, 141, 2278–2283. [Google Scholar] [CrossRef]
- Pober, Z.; Silverman, G.J. Modified radioimmunoassay determination for staphylococcal enterotoxin B in foods. Appl. Environ. Microb. 1977, 33, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Vokhmyanina, D.V.; Andreeva, K.D.; Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. “Artificial peroxidase” nanozyme–enzyme based lactate biosensor. Talanta 2020, 208, 120393. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Zhao, Q. A sensitive thrombin-linked sandwich immunoassay for protein targets using high affinity phosphorodithioate modified aptamer for thrombin labeling. Talanta 2020, 207, 20280. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Y.; Song, C.; Dong, B.; Liu, Z.; Zhang, K.; Li, H.; Sun, Y.; Wei, Y.; Yang, A.; et al. Highly sensitive microplate chemiluminescence enzyme immunoassay for the determination of staphylococcal enterotoxin B based on a pair of specific monoclonal antibodies and its application to various matrices. Anal. Chem. 2010, 82, 7758–7765. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. KONA Power Part. J. 2020, 37, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Godakhindi, V.S.; Kang, P.; Serre, M.; Revuru, N.A.; Zou, J.M.; Roner, M.R.; Levitz, R.; Kahn, J.S.; Randrianalisoa, J.; Qin, Z. Tuning the Gold Nanoparticle Colorimetric Assay by Nanoparticle Size, Concentration, and Size Combinations for Oligonucleotide Detection. ACS Sens. 2017, 2, 1627–1636. [Google Scholar] [CrossRef]
- Xu, G.; Sun, Y.; Zhang, Y.; Xia, L. Sulfite-triggered surface plasmon-catalyzed reduction of p-nitrothiophenol to p,p′-dimercaptoazobenzene. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2022, 264, 120282. [Google Scholar] [CrossRef]
- Mohd-Zahid, M.H.; Zulkifli, S.N.; Che Abdullah, C.A.; Lim, J.; Fakurazi, S.; Wong, K.K.; Zakaria, A.D.; Ismail, N.; Uskoković, V.; Mohamud, R.; et al. Gold nanoparticles conjugated with anti-CD133 monoclonal antibody and 5-fluorouracil chemotherapeutic agent as nanocarriers for cancer cell targeting. RSC Adv. 2021, 11, 16131–16141. [Google Scholar] [CrossRef]
- Zhu, X.S.; Tian, Y.; Dai, L.; Wang, Q.F.; Shi, M.Y.; Pang, C.; Wu, J.W.; Hu, J.Q.; Fan, H.B.; Song, C.J.; et al. The influence of hydrophilic decoration on X-ray excited luminescence nanoparticles to singlet oxygen production. Nano 2020, 15, 2050092. [Google Scholar] [CrossRef]
- Wyantuti, S.; Hartati, Y.W.; Firdaus, M.L.; Panatarani, C.; Tjokronegoro, R. Fabrication of gold nanoparticles-modified glassy carbon electrode and its application for vol-tammetric detection of Cr (III). Int. J. Sci. Technol. Res. 2015, 4, 135–139. [Google Scholar]
- Jingyue, Z.; Bernd, F. Synthesis of Gold Nanoparticles via Chemical Reduction Methods; Nanocon: Oak Park, IL, USA, 2015; pp. 1–7. [Google Scholar]
- Retout, M.; Blond, P.; Jabin, I.; Bruylants, G. Ultrastable PEGylated Calixarene-Coated Gold Nanoparticles with a Tunable Bioconjugation Density for Biosensing Applications. Bioconjug. Chem. 2021, 32, 290–300. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Quantitative analysis of PEG-functionalized colloidal gold nanoparticles using charged aerosol detection. Anal. Bioanal. Chem. 2015, 407, 3705–3716. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.D.; Yang, Z.; Lu, W.; Zhang, R.; Huang, Q.; Tian, M.; Li, L.; Liang, D.; Li, C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 2009, 30, 1928–1936. [Google Scholar] [CrossRef]
- Pollitt, M.J.; Buckton, G.; Piper, R.; Brocchini, S. Measuring antibody coatings on gold nanoparticles by optical spectroscopy. RSC Adv. 2015, 5, 24521–24527. [Google Scholar] [CrossRef]
- Bragina, V.A.; Znoyko, S.L.; Orlov, A.V.; Pushkarev, A.V.; Nikitin, M.P.; Nikitin, P.I. Analytical Platform with Selectable Assay Parameters Based on Three Functions of Magnetic Nanoparticles: Demonstration of Highly Sensitive Rapid Quantitation of Staphylococcal Enterotoxin B in Food. Anal. Chem. 2019, 91, 9852. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Ma, X.; Xia, Y.; Wang, H.; Wang, Z. A highly sensitive fluorescence resonance energy transfer aptasensor for staphylococcal enterotoxin B detection based on exonuclease-catalyzed target recycling strategy. Anal. Chim. Acta 2013, 782, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, W.; Liu, L.; Xu, L.; Kuang, H.; Zhu, J.; Xu, C. Nanoshell-Enhanced Raman Spectroscopy on a Microplate for Staphylococcal Enterotoxin B Sensing. ACS Appl. Mater. Interfaces 2016, 8, 15591–15597. [Google Scholar] [CrossRef]
- Xu, Y.; Huo, B.; Li, C.; Peng, Y.; Tian, S.; Fan, L.; Bai, J.; Ning, B.; Gao, Z. Ultrasensitive detection of staphylococcal enterotoxin B in foodstuff through dual signal amplification by bio-barcode and real-time PCR. Food Chem. 2019, 283, 338–344. [Google Scholar] [CrossRef]
- Mondal, B.; Ramlal, S.; Lavu, P.S.; Kingston, J. Highly Sensitive Colorimetric Biosensor for Staphylococcal Enterotoxin B by a Label-Free Aptamer and Gold Nanoparticles Frontiers in Microbiology. Front. Microbiol. 2018, 9, 179. [Google Scholar] [CrossRef]


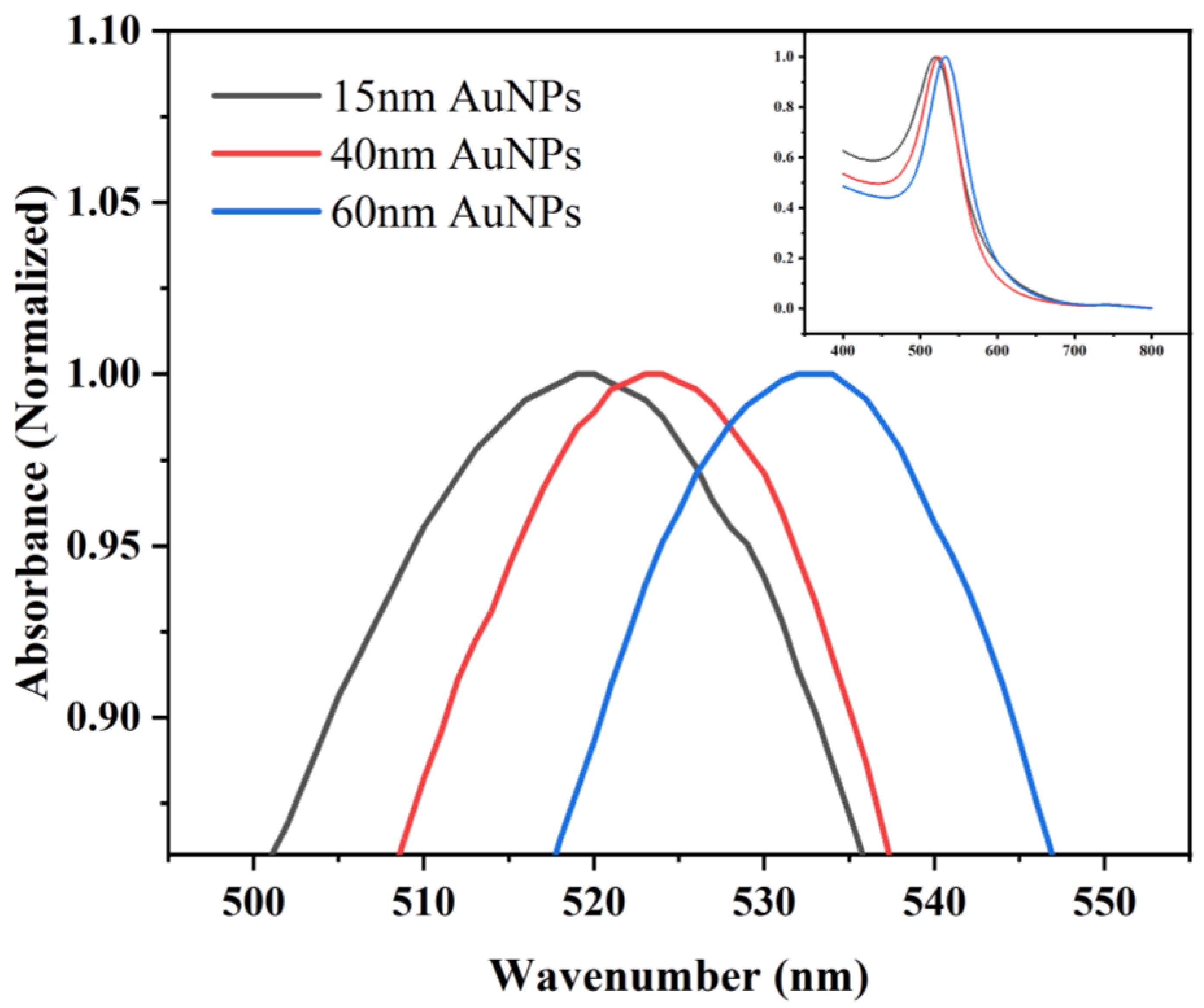
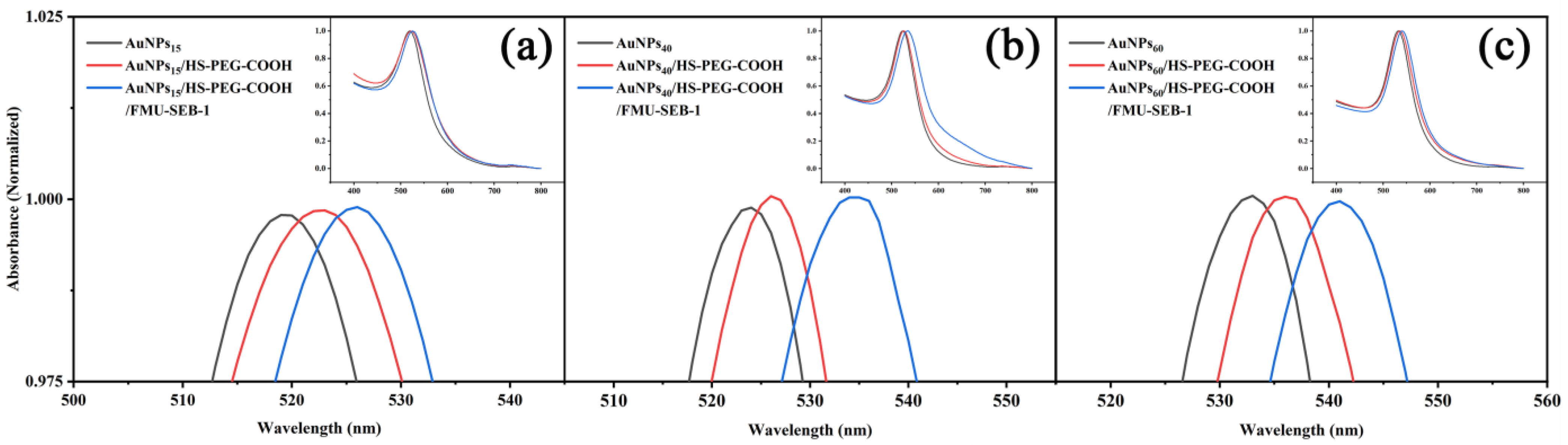


| Particle Size (nm) | Plasmon Absorption Peak (nm) | ||
|---|---|---|---|
| AuNPs | AuNPs/HS-PEG-COOH | AuNPs/HS-PEG-COOH/ FMU-SEB-1 | |
| 15 | 519.5 | 521.5 | 525.5 |
| 40 | 523.5 | 526 | 534.5 |
| 60 | 533 | 536 | 541 |
| Methods | Linear Range | Detection Limit | Detection Time (min) | Detection Sample | Recovery Rates (%) | Ref. |
|---|---|---|---|---|---|---|
| Lateral flow assay | N/A | 6 pg mL−1 | more than 60 | Milk, canned meat, baby food, canned mushrooms | N/A | [35] |
| Chemiluminescence immunoassay | 3.12–50 ng mL−1 | 1.44 ng mL−1 | more than 60 | Milk, water | 82.5–95.2 | [8] |
| FRET | 0.001–1 ng mL−1 | 0.3 pg mL−1 | more than 50 | Milk | 86.0–110.0 | [36] |
| SERS | 2–100 pg mL−1 | 1.3 pg mL−1 | more than 120 | Milk | 88.2–91.67 | [37] |
| Bio-barcode & real-time PCR | 0.001–100 ng mL−1 | 0.269 pg mL−1 | more than 150 | Milk, metamorphosed milk, water, orange juice | 89.17–110.29 | [38] |
| Colorimetric assay | 0.5–50 μg mL−1 | 0.5 ng mL−1 | 50 | Milk, cheese, ice-cream, chicken, pastries | N/A | [39] |
| ALISA | 0.125–32 pg mL−1 | 0.125 pg mL−1 | about 150 | River water, milk, orange juice, apple juice, human serum, ketchup, blueberry, soybean, roast beef, cured ham, peanut butter | 92.7–95.0 | ALISA method |
| Concn Concentration of SEB (pg/mL) | Mean Measured Concentration (pg/mL) | CV (%) |
|---|---|---|
| intra-assay (n = 8) | ||
| 2 | 1.84 ± 0.08 | 4.4 |
| 8 | 7.70 ± 0.41 | 5.3 |
| 20 | 18.82 ± 1.36 | 7.2 |
| inter-assay (n = 8) | ||
| 2 | 1.84 ± 0.23 | 12.3 |
| 8 | 8. 11 ± 0.88 | 10.8 |
| 20 | 20.07 ± 2.20 | 11.0 |
| Spike Levels (pg/mL) | Mean Measured Concentration (pg/mL) | Mean Recovery (%) |
|---|---|---|
| 2 | 1.90 ± 0.10 | 95.0 ± 4.8 |
| 8 | 7.50 ± 0.48 | 93.8 ± 6.0 |
| 20 | 18.55 ± 1.48 | 92.7 ± 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Liu, Y.; Hu, J.; Zhu, Y.; Ma, Z.; Xi, J.; Cui, M.; Ren, L.; Fan, L. Development of a Gold Nanoparticle-Linked Immunosorbent Assay of Staphylococcal Enterotoxin B Detection with Extremely High Sensitivity by Determination of Gold Atom Content Using Graphite Furnace Atomic Absorption Spectrometry. Pharmaceutics 2023, 15, 1493. https://doi.org/10.3390/pharmaceutics15051493
Song C, Liu Y, Hu J, Zhu Y, Ma Z, Xi J, Cui M, Ren L, Fan L. Development of a Gold Nanoparticle-Linked Immunosorbent Assay of Staphylococcal Enterotoxin B Detection with Extremely High Sensitivity by Determination of Gold Atom Content Using Graphite Furnace Atomic Absorption Spectrometry. Pharmaceutics. 2023; 15(5):1493. https://doi.org/10.3390/pharmaceutics15051493
Chicago/Turabian StyleSong, Chaojun, Yutao Liu, Jinwei Hu, Yupu Zhu, Zhengjun Ma, Jiayue Xi, Minxuan Cui, Leiqi Ren, and Li Fan. 2023. "Development of a Gold Nanoparticle-Linked Immunosorbent Assay of Staphylococcal Enterotoxin B Detection with Extremely High Sensitivity by Determination of Gold Atom Content Using Graphite Furnace Atomic Absorption Spectrometry" Pharmaceutics 15, no. 5: 1493. https://doi.org/10.3390/pharmaceutics15051493






