Cerium Oxide Nanoparticles/Polyacrylonitrile Nanofibers as Impervious Barrier against Viral Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PAN NFs and CeO2 NP-Loaded PAN NFs
2.3. Characterization of CeO2 NPs
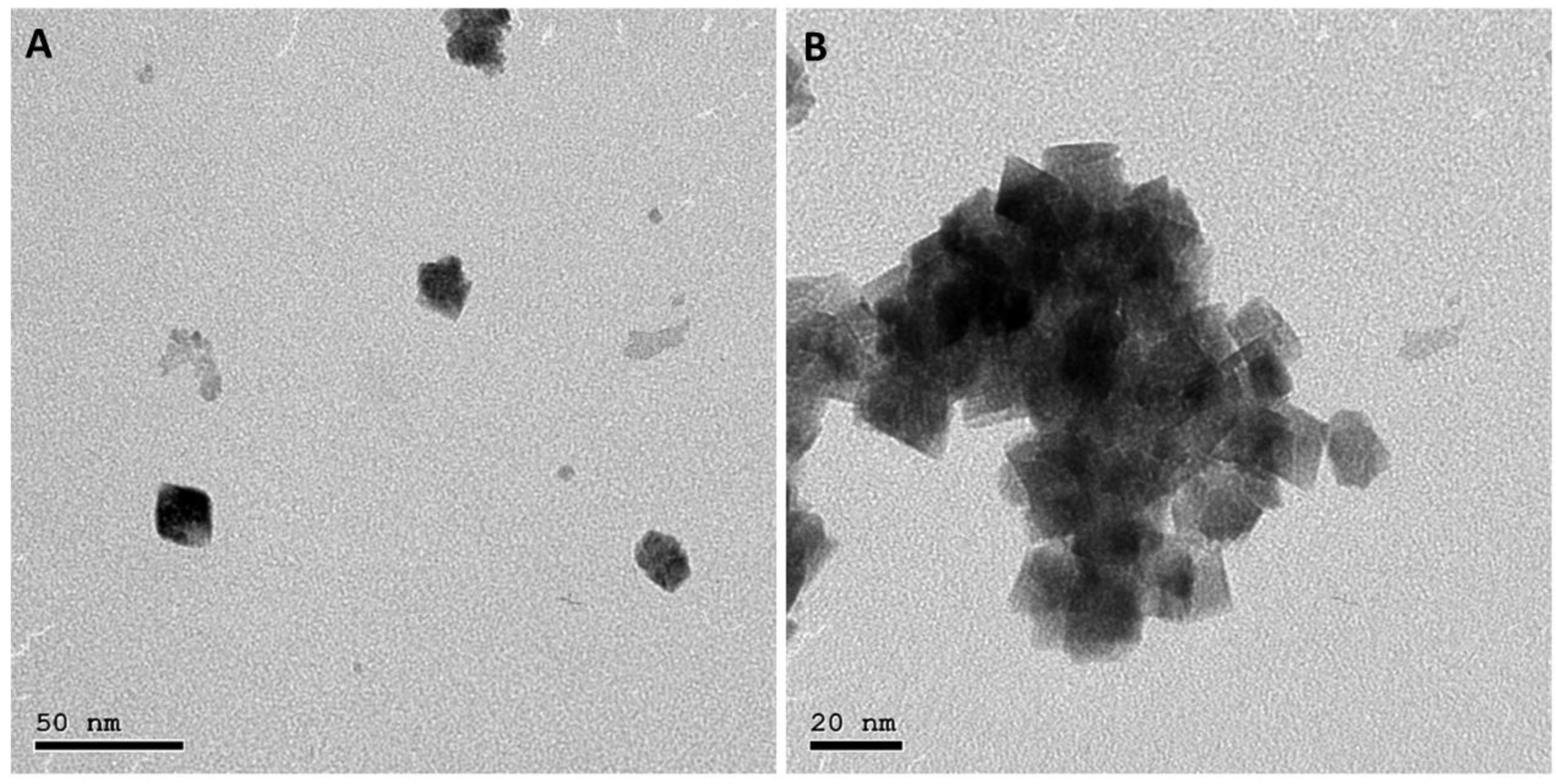
2.3.1. Transmission Electron Microscope (TEM)
2.3.2. Polydispersity Index (PDI) and Zeta Potential Determination
2.4. Characterization of PAN, and CeO2 NPs-Loaded PAN NFs
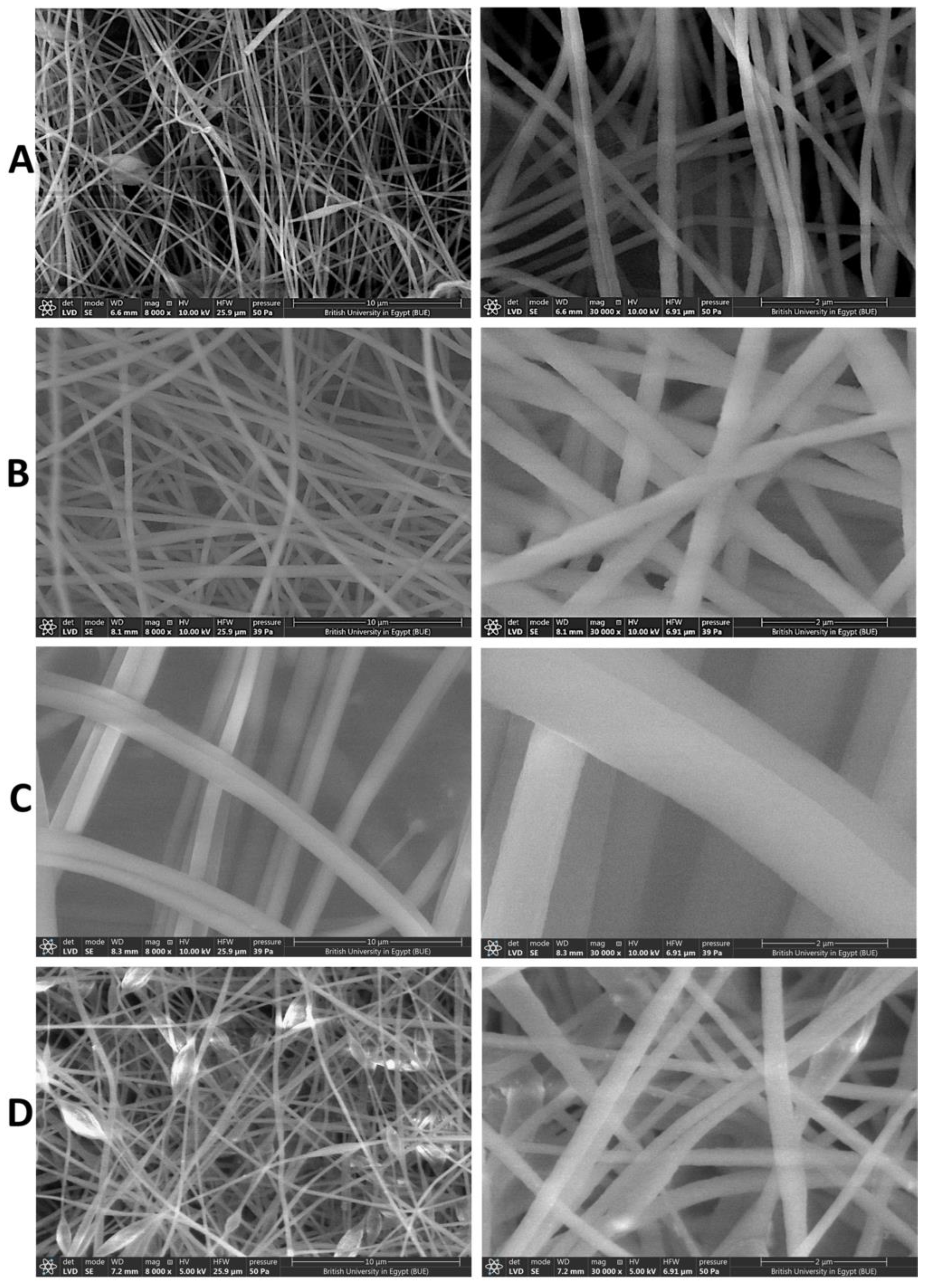
2.4.1. Scanning Electron Microscope (SEM)
2.4.2. Fourier-Transform Infrared Spectra (FT-IR)
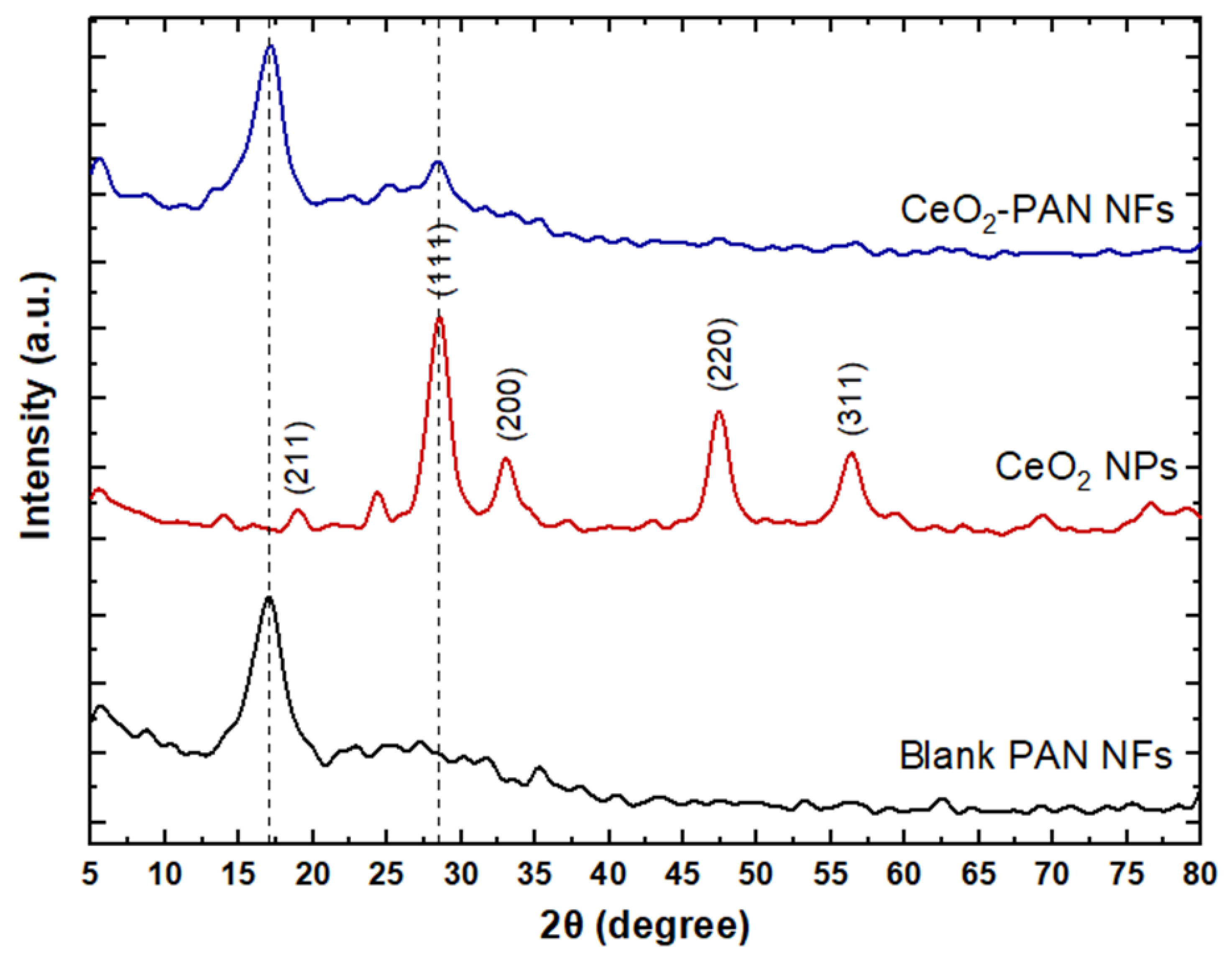
2.4.3. X-ray Diffractometer (XRD)
2.4.4. Mechanical Strength
2.5. Cell Culture and Cytotoxicity Assay
2.6. Antiviral Assay
2.6.1. Infectivity Assay
2.6.2. Titration of ADV-5 DNA in Cell Culture
2.6.3. Quantitation of ADV-5 DNA in Cell Culture
2.6.4. Antiviral Activity of CeO2 NP-Loaded PAN NFs
Adsorption Mechanism
Virucidal Mechanism
2.7. Statistical Analysis
3. Results and Discussion
3.1. Transmission Electron Microscope (TEM)
3.2. Polydispersity Index (PDI) and Zeta Potential of CeO2 NPs
3.3. Scanning Electron Microscope (SEM)
3.4. Fourier-Transform Infrared Spectra (FT-IR)
3.5. X-ray Diffraction (XRD)
3.6. Standard Uniaxial Tensile Test
3.7. Cytotoxicity Assay
3.8. Antiviral Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Packirisamy, G. Nano-based antiviral coatings to combat viral infections. Nano-Struct. Nano-Objects 2020, 24, 100620. [Google Scholar] [CrossRef]
- He, R.; He, S.; Li, X.; Shi, Y.; Liu, Q. How can heart disease patients prevent complications from viral infections? Eur. J. Prev. Cardiol. 2018, 25, 758. [Google Scholar] [CrossRef]
- CDC—Interim Infection Prevention and Control Recommendations for Healthcare Personnel during the Coronavirus Disease 2019 (COVID-19) Pandemic. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html (accessed on 4 July 2022).
- Metzgar, D.; Osuna, M.; Yingst, S.; Rakha, M.; Earhart, K.; Elyan, D.; Esmat, H.; Saad, M.D.; Kajon, A.; Wu, J.; et al. PCR analysis of Egyptian respiratory adenovirus isolates, including identification of species, serotypes, and coinfections. J. Clin. Microbiol. 2005, 43, 5743–5752. [Google Scholar] [CrossRef] [PubMed]
- Elmahdy, E.M.; Ahmed, N.I.; Shaheen, M.N.; Mohamed, E.C.B.; Loutfy, S.A. Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J. Water Health 2019, 17, 287–294. [Google Scholar] [CrossRef]
- Kinchington, P.R.; Romanowski, E.G.; Jerold Gordon, Y. Prospects for adenovirus antivirals. J. Antimicrob. Chemother. 2005, 55, 424–429. [Google Scholar] [CrossRef]
- Lynch, J.P., III; Kajon, A.E. Adenovirus: Epidemiology, global spread of novel serotypes, and advances in treatment and prevention. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2016. [Google Scholar]
- Cano-Vicent, A.; Tuñón-Molina, A.; Martí, M.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Antiviral face mask functionalized with solidified hand soap: Low-cost infection prevention clothing against enveloped viruses such as SARS-CoV-2. ACS Omega 2021, 6, 23495–23503. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Abdelhamid, S.G.; Metwally, A.A. Chloroquine and hydroxychloroquine for combating COVID-19: Investigating efficacy and hypothesizing new formulations using Bio/chemoinformatics tools. Inform. Med. Unlocked 2020, 21, 100446. [Google Scholar] [CrossRef]
- Akduman, C.; Akcakoca Kumbasar, E.P. Nanofibers in face masks and respirators to provide better protection. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Karmacharya, M.; Kumar, S.; Gulenko, O.; Cho, Y.K. Advances in Facemasks during the COVID-19 Pandemic Era. ACS Appl. Bio Mater. 2021, 4, 3891–3908. [Google Scholar] [CrossRef]
- Ganesan, A.; Jaiganesh, R. A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers. Carbohydr. Polym. Technol. Appl. 2022, 4, 100262. [Google Scholar]
- Liu, R.; Ji, D.; Zhou, G.; Liu, Z.; Xu, Q.; Ramakrishna, S. Electrospun nanofibers for personal protection in mines. Chem. Eng. J. 2021, 404, 126558. [Google Scholar] [CrossRef]
- Hathout, R.M.; Kassem, D.H. Positively Charged Electroceutical Spun Chitosan Nanofibers Can Protect Health Care Providers From COVID-19 Infection: An Opinion. Front. Bioeng. Biotechnol. 2020, 8, 885. [Google Scholar] [CrossRef]
- Hathout, R.M.; Kassem, D.H. Uniting Electroceutical and Cosmeceutical Interventions in Combating Coronavirus Using Ԑ-Poly-l-Lysine. Sci. Pharm. 2021, 89, 2. [Google Scholar] [CrossRef]
- Jarach, N.; Dodiuk, H.; Kenig, S. Polymers in the medical antiviral front-line. Polymers 2020, 12, 1727. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.Y.; Zarie, E.S.; Elbahri, M.; Young, T.H.; Boccaccini, A.R. Bovine Serum Albumin (BSA)/polyacrylonitrile (PAN) biohybrid nanofibers coated with a biomineralized calcium deficient hydroxyapatite (HA) shell for wound dressing. Mater. Sci. Eng. C 2020, 116, 111248. [Google Scholar] [CrossRef]
- Hussain, M.; Salam, A.; Arain, M.F.; Ullah, A.; Dao, A.T.; Vu-Manh, H.; Phan, D.N.; Ansari, A.S.; Khan, M.Q.; Javed, Z.; et al. Polyacrylonitrile Nanofibers Containing Viroblock as Promising Material for Protective Clothing. Appl. Sci. 2021, 11, 11469. [Google Scholar] [CrossRef]
- Ucar, N.; Kizildag, N.; Onen, A.; Karacan, I.; Eren, O. Polyacrylonitrile-polyaniline composite nanofiber webs: Effects of solvents, redoping process and dispersion technique. Fibers Polym. 2015, 16, 2223–2236. [Google Scholar] [CrossRef]
- Yang, A.; Cai, L.; Zhang, R.; Wang, J.; Hsu, P.C.; Wang, H.; Zhou, G.; Xu, J.; Cui, Y. Thermal management in nanofiber-based face mask. Nano Lett. 2017, 17, 3506–3510. [Google Scholar] [CrossRef]
- Emam, M.H.; Nageh, H.; Ali, F.; Taha, M.; ElShehaby, H.A.; Amin, R.; Kamoun, E.A.; Loutfy, S.A.; Kasry, A. Inhibition of SARS-CoV-2 spike protein entry using biologically modified polyacrylonitrile nanofibers: In vitro study towards specific antiviral masks. RSC Adv. 2022, 12, 16184–16193. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, S. Polyacrylonitrile Nanofiber Membranes Modified with Ni-Based Conductive Metal Organic Frameworks for Air Filtration and Respiration Monitoring. ACS Appl. Nano Mater. 2020, 3, 8192–8198. [Google Scholar] [CrossRef]
- Sohrabi, M.; Abbasi, M.; Sadighzadeh, A. Fabrication and evaluation of electrospun polyacrylonitrile/silver nanofiber membranes for air filtration and antibacterial activity. Polym. Bull. 2022, 80, 5481–5499. [Google Scholar] [CrossRef]
- Canalli Bortolassi, A.C.; Guerra, V.G.; Aguiar, M.L.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Composites Based on Nanoparticle and Pan Electrospun Nanofiber Membranes for Air Filtration and Bacterial Removal. Nanomaterials 2019, 9, 1740. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Kim, I.S. Copper oxide (CuO) loaded polyacrylonitrile (PAN) nanofiber membranes for antimicrobial breath mask applications. Curr. Res. Biotechnol. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.R.; Katiyar, V.K.; Gopinath, P. Graphene oxide/silver nanoparticle (GO/AgNP) impregnated polyacrylonitrile nanofibers for potential application in air filtration. Nano-Struct. Nano-Objects 2021, 26, 100708. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Y.; Li, Z.; Li, R.; Ren, X.; Huang, T.S. N-halamine antibacterial nanofibrous mats based on polyacrylonitrile and N-halamine for protective face masks. J. Eng. Fibers Fabr. 2019, 14, 1558925019843222. [Google Scholar] [CrossRef]
- Guo, S.; Yu, B.; Ahmed, A.; Cong, H.; Shen, Y. Synthesis of polyacrylonitrile/polytetrahydropyrimidine (PAN/PTHP) nanofibers with enhanced antibacterial and anti-viral activities for personal protective equipment. J. Hazard. Mater. 2022, 424, 127602. [Google Scholar] [CrossRef]
- Naragund, V.S.; Panda, P.K. Electrospun polyacrylonitrile nanofiber membranes for air filtration application. Int. J. Environ. Sci. Technol. 2021, 19, 10233–10244. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.; Ding, B. Ultra-light 3D nanofibre-nets binary structured nylon 6–polyacrylonitrile membranes for efficient filtration of fine particulate matter. J. Mater. Chem. A 2015, 3, 23946–23954. [Google Scholar] [CrossRef]
- Ince Yardimci, A.; Durmus, A.; Kayhan, M.; Tarhan, O. Antibacterial Activity of AgNO3 Incorporated Polyacrylonitrile/Polyvinylidene Fluoride (PAN/PVDF) Electrospun Nanofibrous Membranes and Their Air Permeability Properties. J. Macromol. Sci. Part B 2022, 61, 749–762. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef]
- Grilli, M. Metal Oxides. Metals 2020, 10, 820. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Neal, C.J.; Fox, C.R.; Sakthivel, T.S.; Kumar, U.; Fu, Y.; Drake, C.; Parks, G.D.; Seal, S. Metal-Mediated Nanoscale Cerium Oxide Inactivates Human Coronavirus and Rhinovirus by Surface Disruption. ACS Nano 2021, 15, 14544–14556. [Google Scholar] [CrossRef]
- Vazirov, R.A.; Sokovnin, S.Y.; Ilves, V.G.; Bazhukova, I.N.; Pizurova, N.; Kuznetsov, M.V. Physicochemical characterization and antioxidant properties of cerium oxide nanoparticles. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Yadavalli, T.; Shukla, D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 219–230. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Madhukar, M.; Das, P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium oxide nanoparticles: Properties, biosynthesis and biomedical application. RSC Adv. 2020, 10, 27194–27214. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L. Cerium oxide nanoparticles and their efficient antibacterial application in vitro against gram-positive and gram-negative pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef]
- Ulker, D.; Abacioglu, N.; Sehirli, A.O. Cerium Oxide (CeO2) Nanoparticles Could Have Protective Effect Against COVID-19. Lett. Appl. NanoBioSci. 2022, 12, 12. [Google Scholar]
- Tumkur, P.P.; Gunasekaran, N.K.; Lamani, B.R.; Nazario Bayon, N.; Prabhakaran, K.; Hall, J.C.; Ramesh, G.T. Cerium Oxide Nanoparticles: Synthesis and Characterization for Biosafe Applications. Nano Manuf. 2021, 1, 176–189. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-Charge-Dependent Cell Localization and Cytotoxicity of Cerium Oxide Nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef]
- Hathout, R.M.; Gad, H.A.; Abdel-Hafez, S.M.; Nasser, N.; Khalil, N.; Ateyya, T.; Amr, A.; Yasser, N.; Nasr, S.; Metwally, A.A. Gelatinized core liposomes: A new Trojan horse for the development of a novel timolol maleate glaucoma medication. Int. J. Pharm. 2019, 556, 192–199. [Google Scholar] [CrossRef]
- Farid, M.M.; Hathout, R.M.; Fawzy, M.; Abou-Aisha, K. Silencing of the scavenger receptor (Class B—Type 1) gene using siRNA-loaded chitosan nanaoparticles in a HepG2 cell model. Colloids Surf. B Biointerfaces 2014, 123, 930–937. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Abdel-Salam, A.I.; Moatasim, Y.; Gomaa, M.R.; Fattah, N.F.A.; Emam, M.H.; Ali, F.; ElShehaby, H.A.; Ragab, E.A.; El-Din, H.M.A.; et al. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. RSC Adv. 2020, 15, 2699. [Google Scholar] [CrossRef]
- Loutfy, S.A.; El-Din, H.M.A.; Elberry, M.H.; Allam, N.G.; Hasanin, M.T.M.; Abdellah, A.M. Synthesis, characterization and cytotoxic evaluation of chitosan nanoparticles: In vitro liver cancer model. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035008. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nageh, H.; Emam, M.H.; Ali, F.; Abdel Fattah, N.F.; Taha, M.; Amin, R.; Kamoun, E.A.; Loutfy, S.A.; Kasry, A. Zinc Oxide Nanoparticle-Loaded Electrospun Polyvinylidene Fluoride Nanofibers as a Potential Face Protector against Respiratory Viral Infections. ACS Omega 2022, 7, 14887–14896. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.A.; Morales, G.E.; Forero, J.E.; Roldan, J. Cytotoxic and antiviral activities of Colombian medicinal plant extracts of the Euphorbia genus. Memórias Inst. Oswaldo Cruz 2002, 97, 541–546. [Google Scholar] [CrossRef]
- Jauhari, J.; AJ, S.; Nawawi, Z.; Sriyanti, I. Synthesis and characteristics of polyacrylonitrile (PAN) nanofiber membrane using electrospinning method. J. Chem. Technol. Metall. 2021, 56, 698–703. [Google Scholar]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Korycka, P.; Mirek, A.; Kramek-Romanowska, K.; Grzeczkowicz, M.; Lewińska, D. Effect of electrospinning process variables on the size of polymer fibers and bead-on-string structures established with a 23 factorial design. Beilstein J. Nanotechnol. 2018, 9, 2466–2478. [Google Scholar] [CrossRef]
- Jabur, A.R.; Najim, M.A.; Abd Al-Rahman, S.A. Study the effect of flow rate on some physical properties of different polymeric solutions. J. Phys. Conf. Ser. 2018, 1003, 012069. [Google Scholar] [CrossRef]
- Bode-Aluko, C.A.; Pereao, O.; Kyaw, H.H.; Al-Naamani, L.; Al-Abri, M.Z.; Myint, M.T.Z.; Rossouw, A.; Fatoba, O.; Petrik, L.; Dobretsov, S. Photocatalytic and antifouling properties of electrospun TiO2 polyacrylonitrile composite nanofibers under visible light. Mater. Sci. Eng. B 2021, 264, 114913. [Google Scholar] [CrossRef]
- Zandavar, H.; Pourmortazavi, S.M.; Mirsadeghi, S. Highly-efficient capture of chromium (VI) ions on electrospun polyacrylonitrile/diaminoglyoxime nanofiber: Thermal stability, decomposition kinetics and tensile strength. J. Mater. Res. Technol. 2021, 13, 25–37. [Google Scholar] [CrossRef]
- Jenab, A.; Roghanian, R.; Ghorbani, N.; Ghaedi, K.; Emtiazi, G. The efficacy of electrospun PAN/Kefiran nanofiber and kefir in mammalian cell culture: Promotion of PC12 cell growth, anti-MCF7 breast cancer cells activities, and cytokine production of PBMC. Int. J. Nanomed. 2020, 15, 717. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T.P. Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method. Rev. Mex. Fís. 2016, 62, 496–499. [Google Scholar]
- Vazquez-Velez, E.; Lopez-Zarate, L.; Martinez-Valencia, H. Electrospinning of polyacrylonitrile nanofibers embedded with zerovalent iron and cerium oxide nanoparticles, as Cr (VI) adsorbents for water treatment. J. Appl. Polym. Sci. 2020, 137, 48663. [Google Scholar] [CrossRef]
- Safwat, S.; Ishak, R.A.; Hathout, R.M.; Mortada, N.D. Statins Anticancer Targeted Delivery Systems: Re-Purposing an Old Molecule. J. Pharm. Pharm. 2017, 69, 613–624. [Google Scholar] [CrossRef]
- Qiao, M.; Kong, H.; Ding, X.; Hu, Z.; Zhang, L.; Cao, Y.; Yu, M. Study on the changes of structures and properties of PAN fibers during the cyclic reaction in supercritical carbon dioxide. Polymers 2019, 11, 402. [Google Scholar] [CrossRef]
- Ghaffari, T.; Hamedi-Rad, F. Effect of Silver Nano-particles on Tensile Strength of Acrylic Resins. J. Dent. Res. Dent. Clin. Dent. Prospect. 2015, 9, 40. [Google Scholar] [CrossRef]
- Shirkavand, S.; Moslehifard, E. Effect of TiO2 Nanoparticles on Tensile Strength of Dental Acrylic Resins. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 197. [Google Scholar]
- Hathout, R.M.; Metwally, A.A. Gelatin Nanoparticles. Methods Mol. Biol 2019, 2000, 71–78. [Google Scholar]
- Desai, C. Meyler’s side effects of drugs: The international encyclopedia of adverse drug reactions and interactions. Indian J. Pharmacol. 2016, 48, 224. [Google Scholar]
- Neurath, A.R.; Strick, N.; Li, Y.Y. Anti-HIV-1 activity of anionic polymers: A comparative study of candidate microbicides. BMC Infect. Dis. 2002, 2, 27. [Google Scholar] [CrossRef]




| Formula Code | PAN Concentration (%, w/v) | CeO2 NP Concentration (%, w/v) | Voltage (kV) | Feed Rate (mL/h) | Distance between Needle and Collector (cm) |
|---|---|---|---|---|---|
| F1 | 6 | _ | 28 | 0.6 | 15 |
| F2 | 8 | _ | 26 | 1.4 | 15 |
| F3 | 10 | _ | 29 | 1 | 15 |
| F4 | 8 | 0.25 | 26 | 0.5 | 15 |
| PAN NFs | CeO2-PAN NFs | |
|---|---|---|
| Breaking strain (%) | 25.6 ± 1.41 | 7.33 ± 1.6 |
| Max. displacement (mm) | 12.7 ± 3.29 | 12.1 ± 2.69 |
| Tensile strain (%) | 395.5 ± 1.5 | 325.63 ± 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emam, M.H.; Elezaby, R.S.; Swidan, S.A.; Loutfy, S.A.; Hathout, R.M. Cerium Oxide Nanoparticles/Polyacrylonitrile Nanofibers as Impervious Barrier against Viral Infections. Pharmaceutics 2023, 15, 1494. https://doi.org/10.3390/pharmaceutics15051494
Emam MH, Elezaby RS, Swidan SA, Loutfy SA, Hathout RM. Cerium Oxide Nanoparticles/Polyacrylonitrile Nanofibers as Impervious Barrier against Viral Infections. Pharmaceutics. 2023; 15(5):1494. https://doi.org/10.3390/pharmaceutics15051494
Chicago/Turabian StyleEmam, Merna H., Reham S. Elezaby, Shady A. Swidan, Samah A. Loutfy, and Rania M. Hathout. 2023. "Cerium Oxide Nanoparticles/Polyacrylonitrile Nanofibers as Impervious Barrier against Viral Infections" Pharmaceutics 15, no. 5: 1494. https://doi.org/10.3390/pharmaceutics15051494
APA StyleEmam, M. H., Elezaby, R. S., Swidan, S. A., Loutfy, S. A., & Hathout, R. M. (2023). Cerium Oxide Nanoparticles/Polyacrylonitrile Nanofibers as Impervious Barrier against Viral Infections. Pharmaceutics, 15(5), 1494. https://doi.org/10.3390/pharmaceutics15051494








