Phosphatidylglycerol to Treat Chronic Skin Wounds in Diabetes
Abstract
:1. Introduction
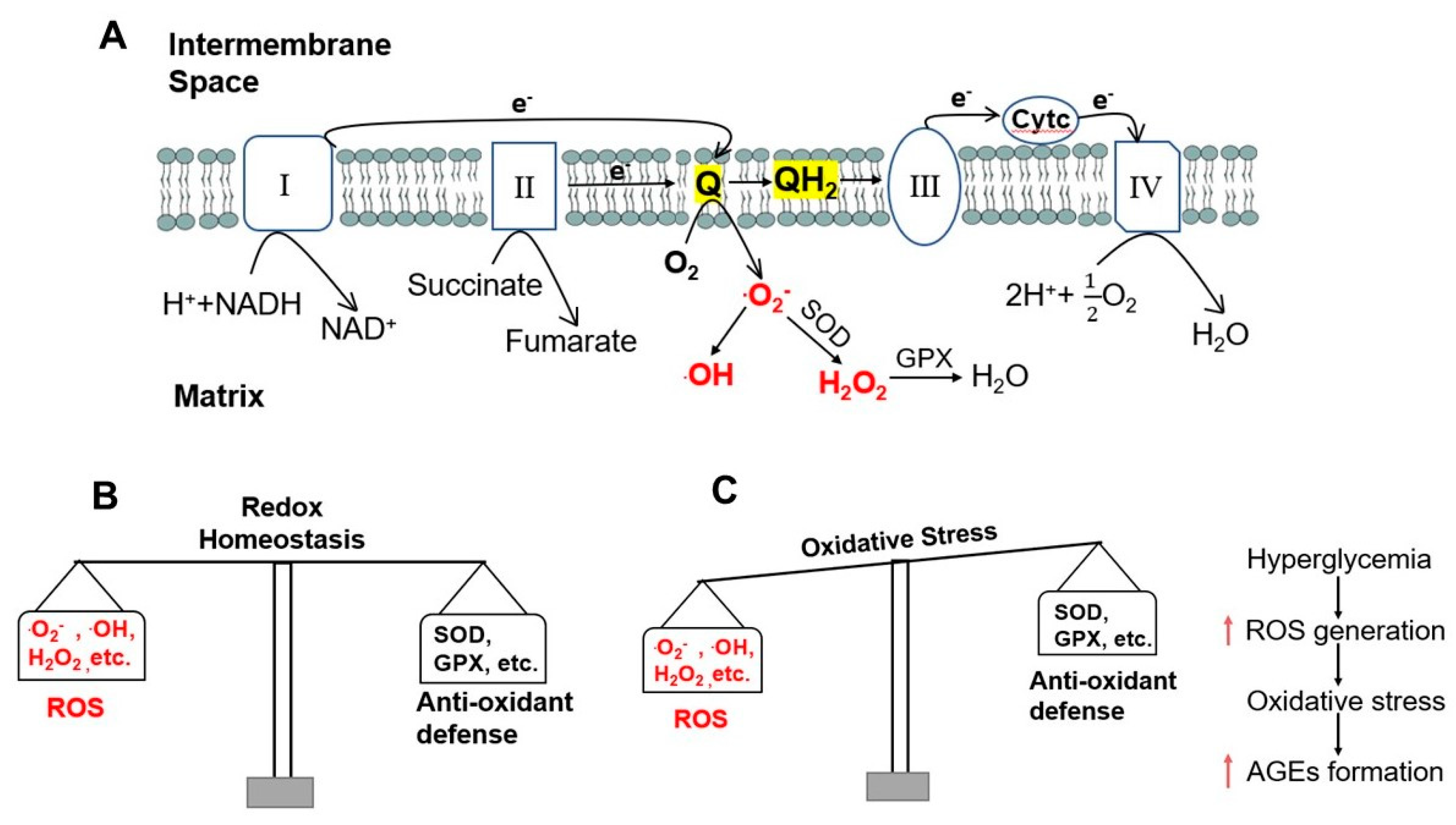
2. Mitochondria, ROS Generation, and Formation of AGEs in Diabetes
2.1. ROS Generation
2.2. The Role of Mitochondrial ROS and Hyperglycemia in the Formation of AGEs
2.3. Formation of AGEs
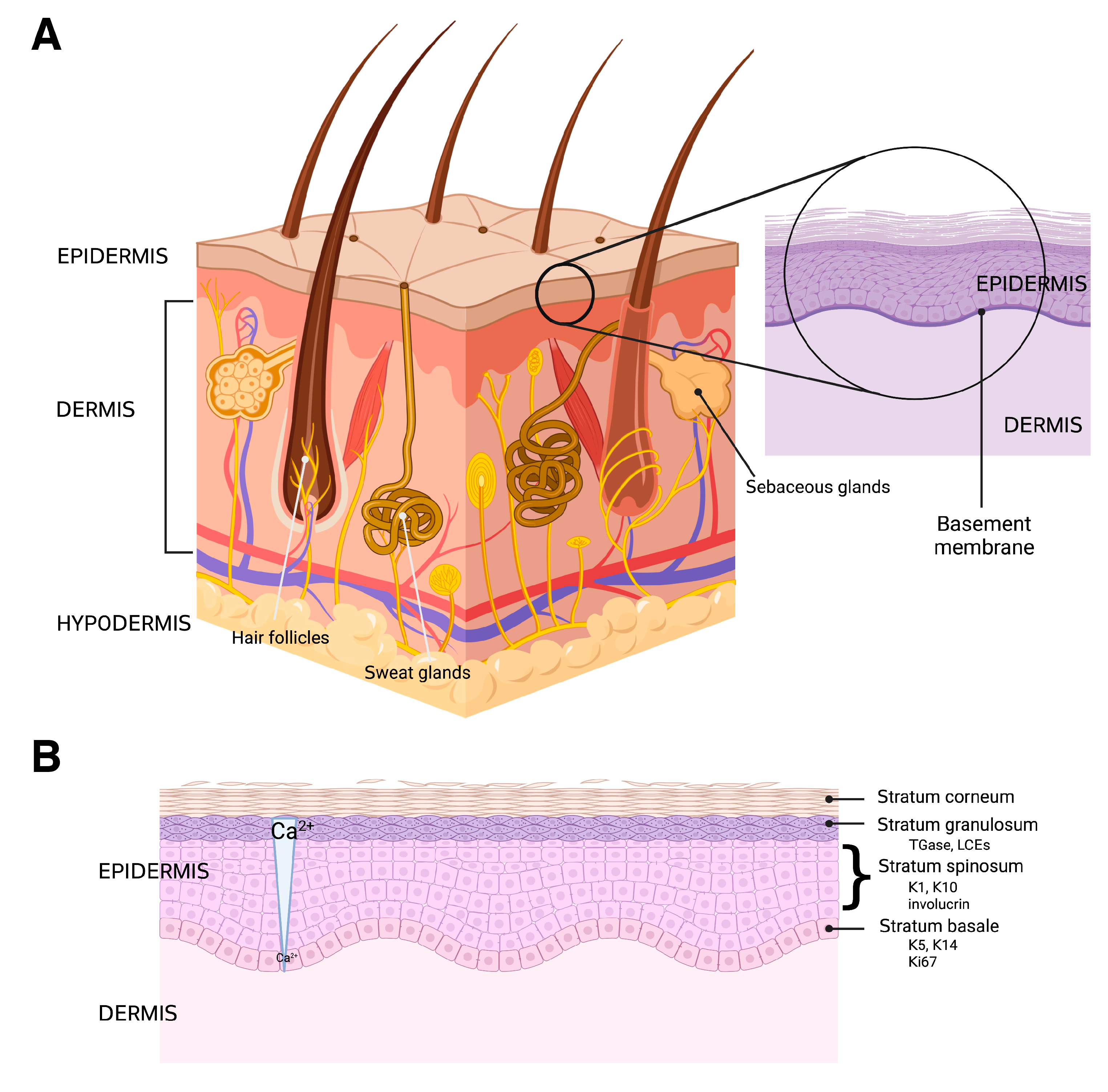
3. Skin Structure and Function
3.1. The Hypodermis and Dermis
3.2. The Epidermis
3.3. Skin Wound Healing
4. The Aquaporin 3-Phospholipase D2-Phosphatidylglycerol Signaling Pathway and Diabetes
4.1. Epidermal Aquaporin 3 (AQP3)
4.2. AQP3 and Diabetes
4.3. AQP3 and the Generation of the Lipid Signal, Phosphatidylglycerol (PG)
5. DOPG and Its Potential Beneficial Effects in Healing of Chronic Diabetic Wounds
5.1. DOPG and Inflammation
5.2. PG and Mitochondrial Function
5.3. Other Possible Targets of PG
5.4. Phospholipids, and in Particular PG, as Drug Delivery Systems
5.5. Dioloeoylphosphatidylglycerol to Treat Chronic Diabetic Wounds
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 30 April 2023).
- How to Care for Diabetic Ulcers and Sores. Available online: https://www.webmd.com/diabetes/diabetes-sores-ulcers-care (accessed on 30 April 2023).
- Dyer, J.M.; Miller, R.A. Chronic Skin Fragility of Aging: Current Concepts in the Pathogenesis, Recognition, and Management of Dermatoporosis. J. Clin. Aesthet. Dermatol. 2018, 11, 13–18. [Google Scholar]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet. Med. 2006, 23, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound. Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Insulin Effects on Target Tissues. Available online: https://encyclopedia.pub/entry/7702 (accessed on 30 April 2023).
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Piboonpocanun, S.; Mitsuzawa, H.; Kuronuma, K.; Murphy, R.C.; Voelker, D.R. Pulmonary surfactant proteins and lipids as modulators of inflammation and innate immunity. Respirology 2006, 11, S2–S6. [Google Scholar] [CrossRef]
- Kandasamy, P.; Numata, M.; Berry, K.Z.; Fickes, R.; Leslie, C.C.; Murphy, R.C.; Voelker, D.R. Structural analogs of pulmonary surfactant phosphatidylglycerol inhibit toll-like receptor 2 and 4 signaling. J. Lipid Res. 2016, 57, 993–1005. [Google Scholar] [CrossRef]
- Kandasamy, P.; Zarini, S.; Chan, E.D.; Leslie, C.C.; Murphy, R.C.; Voelker, D.R. Pulmonary surfactant phosphatidylglycerol inhibits Mycoplasma pneumoniae-stimulated eicosanoid production from human and mouse macrophages. J. Biol. Chem. 2011, 286, 7841–7853. [Google Scholar] [CrossRef] [PubMed]
- Kuronuma, K.; Mitsuzawa, H.; Takeda, K.; Nishitani, C.; Chan, E.D.; Kuroki, Y.; Nakamura, M.; Voelker, D.R. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J. Biol. Chem. 2009, 284, 25488–25500. [Google Scholar] [CrossRef]
- Numata, M.; Chu, H.W.; Dakhama, A.; Voelker, D.R. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus-induced inflammation and infection. Proc. Natl. Acad. Sci. USA 2010, 107, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Choudhary, V.; Seremwe, M.; Edwards, J.G.; Wang, A.; Emmons, A.C.; Bollag, K.A.; Johnson, M.H.; Bollag, W.B. Soy Phosphatidylglycerol Reduces Inflammation in a Contact Irritant Ear Edema Mouse Model in vivo. J. Pharmacol. Exp. Ther. 2018, 366, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Uaratanawong, R.; Patel, R.R.; Patel, H.; Bao, W.; Hartney, B.; Cohen, E.; Chen, X.; Zhong, Q.; Isales, C.M.; et al. Phosphatidylglycerol Inhibits Toll-Like Receptor-Mediated Inflammation by Danger-Associated Molecular Patterns. J. Investig. Dermatol. 2019, 139, 868–877. [Google Scholar] [CrossRef]
- Klein, M.E.; Mauch, S.; Rieckmann, M.; Martinez, D.G.; Hause, G.; Noutsias, M.; Hofmann, U.; Lucas, H.; Meister, A.; Ramos, G.; et al. Phosphatidylserine (PS) and phosphatidylglycerol (PG) nanodispersions as potential anti-inflammatory therapeutics: Comparison of in vitro activity and impact of pegylation. Nanomedicine 2020, 23, 102096. [Google Scholar] [CrossRef]
- Klein, M.E.; Rieckmann, M.; Lucas, H.; Meister, A.; Loppnow, H.; Mader, K. Phosphatidylserine (PS) and phosphatidylglycerol (PG) enriched mixed micelles (MM): A new nano-drug delivery system with anti-inflammatory potential? Eur. J. Pharm. Sci. 2020, 152, 105451. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Griffith, S.; Chen, X.; Bollag, W.B. Pathogen-Associated Molecular Pattern-Induced TLR2 and TLR4 Activation Increases Keratinocyte Production of Inflammatory Mediators and is Inhibited by Phosphatidylglycerol. Mol. Pharmacol. 2020, 97, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Chao, Y.J.; Chang, W.H.; Chan, J.F.; Hsu, Y.H. Phosphatidylglycerol Incorporates into Cardiolipin to Improve Mitochondrial Activity and Inhibits Inflammation. Sci. Rep. 2018, 8, 4919. [Google Scholar] [CrossRef] [PubMed]
- Oxidative Phosphorylation. Available online: https://www.khanacademy.org/science/ap-biology/cellular-energetics/cellular-respiration-ap/a/oxidative-phosphorylation-etc (accessed on 30 April 2023).
- Generation of Reactive Oxygen Species by Mitochondria. Available online: https://encyclopedia.pub/entry/23268 (accessed on 30 April 2023).
- Nelson, D.l.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Bohm, M. Advanced glycation end products: Key players in skin aging? Derm.-Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef]
- Ahmad, S.; Farhan, M. Impact of Non-Enzymatic Glycation in Neurodegenerative Diseases: Role of Natural Products in Prevention. Adv. Neurobiol. 2016, 12, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Ljubimov, A.V. Diabetic complications in the cornea. Vision Res. 2017, 139, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.; Shiu, S.W.; Wong, Y.; Tam, X. Serum advanced glycation end products (AGEs) are associated with insulin resistance. Diabetes Metab. Res. Rev. 2011, 27, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Yamagishi, S.; Matsui, T.; Takeuchi, M.; Nitta, Y.; Kodama, N.; Mizoguchi, M.; Imaizumi, T. Serum levels of advanced glycation end products (AGEs) are independent correlates of insulin resistance in nondiabetic subjects. Cardiovasc. Ther. 2012, 30, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Junior, D.C.; Silva, K.S.; Michalani, M.L.; Yonamine, C.Y.; Esteves, J.V.; Fabre, N.T.; Thieme, K.; Catanozi, S.; Okamoto, M.M.; Seraphim, P.M.; et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci. Rep. 2018, 8, 8109. [Google Scholar] [CrossRef]
- Ikarashi, N.; Mizukami, N.; Kon, R.; Kaneko, M.; Uchino, R.; Fujisawa, I.; Fukuda, N.; Sakai, H.; Kamei, J. Study of the Mechanism Underlying the Onset of Diabetic Xeroderma Focusing on an Aquaporin-3 in a Streptozotocin-Induced Diabetic Mouse Model. Int. J. Mol. Sci. 2019, 20, 3782. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fat Cells. Available online: https://biologydictionary.net/fat-cells/ (accessed on 30 April 2023).
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurs. Assoc. 2011, 3, 203–213. Available online: https://journals.lww.com/jdnaonline/fulltext/2011/07000/anatomy_and_physiology_of_the_skin.3.aspx (accessed on 30 April 2023). [CrossRef]
- Hodge, B.D.; Sanvictores, T.; Brodell, R.T. Anatomy, Skin Sweat Glands; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Watabe, A.; Sugawara, T.; Kikuchi, K.; Yamasaki, K.; Sakai, S.; Aiba, S. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J. Dermatol. Sci. 2013, 72, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rieg, S.; Steffen, H.; Seeber, S.; Humeny, A.; Kalbacher, H.; Dietz, K.; Garbe, C.; Schittek, B. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J. Immunol. 2005, 174, 8003–8010. [Google Scholar] [CrossRef] [PubMed]
- Goletz, S.; Zillikens, D.; Schmidt, E. Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp. Dermatol. 2017, 26, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Mathew, S. Merkel Cells: A Collective Review of Current Concepts. Int. J. Appl. Basic Med. Res. 2019, 9, 9–13. [Google Scholar] [CrossRef]
- Merad, M.; Ginhoux, F.; Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008, 8, 935–947. [Google Scholar] [CrossRef]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans Cells—The Macrophage in Dendritic Cell Clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landen, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Bikle, D.D.; Xie, Z.; Tu, C.L. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 2012, 7, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Reina, O. Keratinocytes: Their Purpose, Their Subtypes and Their Lifecycle. Available online: https://www.tempobioscience.com/keratinocytes-their-purpose-their-subtypes-and-their-lifecycle/ (accessed on 30 April 2023).
- Albanesi, C.; Scarponi, C.; Giustizieri, M.L.; Girolomoni, G. Keratinocytes in inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 329–334. [Google Scholar] [CrossRef]
- Bowcock, A.M.; Krueger, J.G. Getting under the skin: The immunogenetics of psoriasis. Nat. Rev. Immunol. 2005, 5, 699–711. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Tomic-Canic, M. Role of keratinocytes in healing of chronic wounds. Surg. Technol. Int. 2008, 17, 105–112. [Google Scholar]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- The Reason Behind Moist Wound Environment. Available online:https://opendermatologyjournal.com/VOLUME/13/PAGE/34/FULLTEXT/ (accessed on 30 April 2023).
- Gu, L.H.; Coulombe, P.A. Keratin function in skin epithelia: A broadening palette with surprising shades. Curr. Opin. Cell Biol. 2007, 19, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Cario, M.; Pain, C.; Kaulanjan-Checkmodine, P.; Masia, D.; Delia, G.; Casoli, V.; Costet, P.; Goussot, J.F.; Guyonnet-Duperat, V.; Bibeyran, A.; et al. Epidermal keratin 5 expression and distribution is under dermal influence. Pigment Cell Melanoma Res. 2020, 33, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 Controls Breast Cancer Cell Migration by Regulating Hydrogen Peroxide Transport and Its Downstream Cell Signaling. Mol. Cell. Biol. 2016, 36, 1206–1218. [Google Scholar] [CrossRef]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the epidermis: More than skin deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef]
- Qin, H.; Zheng, X.; Zhong, X.; Shetty, A.K.; Elias, P.M.; Bollag, W.B. Aquaporin-3 in keratinocytes and skin: Its role and interaction with phospholipase D2. Arch. Biochem. Biophys. 2011, 508, 138–143. [Google Scholar] [CrossRef]
- Ma, T.; Hara, M.; Sougrat, R.; Verbavatz, J.M.; Verkman, A.S. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 2002, 277, 17147–17153. [Google Scholar] [CrossRef]
- Ma, T.; Song, Y.; Yang, B.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA 2000, 97, 4386–4391. [Google Scholar] [CrossRef]
- Nakahigashi, K.; Kabashima, K.; Ikoma, A.; Verkman, A.S.; Miyachi, Y.; Hara-Chikuma, M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J. Investig. Dermatol. 2011, 131, 865–873. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Verkman, A.S. Roles of aquaporin-3 in the epidermis. J. Investig. Dermatol. 2008, 128, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell. Biol. 2008, 28, 326–332. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J. Mol. Med. 2008, 86, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Verkman, A.S. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 7360–7365. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S.; Honda, T.; Miyachi, Y.; Watanabe, T.; Verkman, A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-kappaB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015, 6, 7454. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Xie, D.; Zheng, X.; Zhong, X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: Production of a phosphatidylglycerol signaling lipid. J. Investig. Dermatol. 2007, 127, 2823–2831. [Google Scholar] [CrossRef]
- Choudhary, V.; Olala, L.O.; Qin, H.; Helwa, I.; Pan, Z.Q.; Tsai, Y.Y.; Frohman, M.A.; Kaddour-Djebbar, I.; Bollag, W.B. Aquaporin-3 re-expression induces differentiation in a phospholipase D2-dependent manner in aquaporin-3-knockout mouse keratinocytes. J. Investig. Dermatol. 2015, 135, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Huang, L.; Minematsu, T.; Yamamoto, Y.; Asada, M.; Nakagami, G.; Akase, T.; Nagase, T.; Oe, M.; Mori, T.; et al. Impaired aquaporin 3 expression in reepithelialization of cutaneous wound healing in the diabetic rat. Biol. Res. Nurs. 2013, 15, 347–355. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Kaneko, M.; Mizukami, N.; Kusunoki, Y.; Sugiyama, K. Relationship between Aging-Related Skin Dryness and Aquaporins. Int. J. Mol. Sci. 2017, 18, 1559. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, H.; Hu, X.; Chen, M.; Xie, H. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas. J. Dermatol. 2010, 51, 106–112. [Google Scholar] [CrossRef]
- Seleit, I.; Bakry, O.A.; El Rebey, H.S.; El-Akabawy, G.; Hamza, G. Is Aquaporin-3 a Determinant Factor of Intrinsic and Extrinsic Aging? An Immunohistochemical and Morphometric Study. Appl. Immunohistochem. Mol. Morphol. 2015, 25, 49–57. [Google Scholar] [CrossRef]
- Ikarashi, N.; Mizukami, N.; Pei, C.; Uchino, R.; Fujisawa, I.; Fukuda, N.; Kon, R.; Sakai, H.; Kamei, J. Role of Cutaneous Aquaporins in the Development of Xeroderma in Type 2 Diabetes. Biomedicines 2021, 9, 104. [Google Scholar] [CrossRef]
- Luo, Y.; Uaratanawong, R.; Choudhary, V.; Hardin, M.; Zhang, C.; Melnyk, S.; Chen, X.; Bollag, W.B. Advanced Glycation End Products and Activation of Toll-like Receptor-2 and -4 Induced Changes in Aquaporin-3 Expression in Mouse Keratinocytes. Int. J. Mol. Sci. 2023, 24, 1376. [Google Scholar] [CrossRef]
- Zheng, X.; Bollag, W.B. Aquaporin 3 colocates with phospholipase D2 in caveolin-rich membrane microdomains and is regulated by keratinocyte differentiation. J. Investig. Dermatol. 2003, 121, 1487–1495. [Google Scholar] [CrossRef]
- Zheng, X.; Ray, S.; Bollag, W.B. Modulation of phospholipase D-mediated phosphatidylglycerol formation by differentiating agents in primary mouse epidermal keratinocytes. Biochim. Biophys. Acta 2003, 1643, 25–36. [Google Scholar] [CrossRef]
- Arun, S.N.; Xie, D.; Howard, A.C.; Zhong, Q.; Zhong, X.; McNeil, P.L.; Bollag, W.B. Cell wounding activates phospholipase D in primary mouse keratinocytes. J. Lipid Res. 2013, 54, 581–591. [Google Scholar] [CrossRef]
- Xie, D.; Seremwe, M.; Edwards, J.G.; Podolsky, R.; Bollag, W.B. Distinct effects of different phosphatidylglycerol species on mouse keratinocyte proliferation. PLoS ONE 2014, 9, e107119. [Google Scholar] [CrossRef]
- Bollag, W.B.; Olala, L.O.; Xie, D.; Lu, X.; Qin, H.; Choudhary, V.; Patel, R.; Bogorad, D.; Estes, A.; Watsky, M. Dioloeoylphosphatidylglycerol accelerates corneal epithelial wound healing. Investig. Ophthamol. Vis. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Levin, M.H.; Verkman, A.S. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.E.; Bollag, R.J.; Fussell, N.; By, C.; Sheehan, D.J.; Bollag, W.B. Abnormal aquaporin-3 protein expression in hyperproliferative skin disorders. Arch. Dermatol. Res. 2011, 303, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J. Dermatol. Sci. 2018, 92, 97–105. [Google Scholar] [CrossRef]
- Paolino, G.; Buratta, S.; Mercuri, S.R.; Pellegrino, R.M.; Urbanelli, L.; Emiliani, C.; Bertuccini, L.; Iosi, F.; Huber, V.; Brianti, P.; et al. Lipidic Profile Changes in Exosomes and Microvesicles Derived From Plasma of Monoclonal Antibody-Treated Psoriatic Patients. Front. Cell Dev. Biol. 2022, 10, 923769. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Wolk, K. Research in practice: IL-22 and IL-20: Significance for epithelial homeostasis and psoriasis pathogenesis. J. Dtsch. Dermatol. Ges. 2011, 9, 518–523. [Google Scholar] [CrossRef]
- Chun, K.H.; Seong, S.Y. CD14 but not MD2 transmit signals from DAMP. Int. Immunopharmacol. 2010, 10, 98–106. [Google Scholar] [CrossRef]
- Muroi, M.; Ohnishi, T.; Tanamoto, K. Regions of the mouse CD14 molecule required for toll-like receptor 2- and 4-mediated activation of NF-kappa B. J. Biol. Chem. 2002, 277, 42372–42379. [Google Scholar] [CrossRef]
- Van Bergenhenegouwen, J.; Plantinga, T.S.; Joosten, L.A.; Netea, M.G.; Folkerts, G.; Kraneveld, A.D.; Garssen, J.; Vos, A.P. TLR2 & Co: A critical analysis of the complex interactions between TLR2 and coreceptors. J. Leukoc. Biol. 2013, 94, 885–902. [Google Scholar] [CrossRef]
- Fowler, T.; Choudhary, V.; Melnyk, S.; Farsi, M.; Chang, L.Y.; Fortingo, N.; Chen, X.; Watsky, M.A.; Bollag, W.B. Dioleoylphosphatidylglycerol heat shock protein B4-induced inflammatory pathways in vitro. Int. J. Mol. Sci. 2023, 24, 5839. [Google Scholar] [CrossRef]
- Numata, M.; Voelker, D.R. Anti-inflammatory and anti-viral actions of anionic pulmonary surfactant phospholipids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159139. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Kandasamy, P.; Nagashima, Y.; Posey, J.; Hartshorn, K.; Woodland, D.; Voelker, D.R. Phosphatidylglycerol suppresses influenza A virus infection. Am. J. Respir Cell Mol. Biol. 2012, 46, 479–487. [Google Scholar] [CrossRef]
- Numata, M.; Nagashima, Y.; Moore, M.L.; Berry, K.Z.; Chan, M.; Kandasamy, P.; Peebles, R.S., Jr.; Murphy, R.C.; Voelker, D.R. Phosphatidylglycerol provides short-term prophylaxis against respiratory syncytial virus infection. J. Lipid Res. 2013, 54, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Remor, A.P.; de Matos, F.J.; Ghisoni, K.; da Silva, T.L.; Eidt, G.; Burigo, M.; de Bem, A.F.; Silveira, P.C.; de Leon, A.; Sanchez, M.C.; et al. Differential effects of insulin on peripheral diabetes-related changes in mitochondrial bioenergetics: Involvement of advanced glycosylated end products. Biochim. Biophys. Acta 2011, 1812, 1460–1471. [Google Scholar] [CrossRef]
- Yang, C.T.; Meng, F.H.; Chen, L.; Li, X.; Cen, L.J.; Wen, Y.H.; Li, C.C.; Zhang, H. Inhibition of Methylglyoxal-Induced AGEs/RAGE Expression Contributes to Dermal Protection by N-Acetyl-L-Cysteine. Cell Physiol. Biochem. 2017, 41, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, B.T.; Schlotzer-Schrehardt, U.; Skeie, J.M.; Burckart, K.A.; Schmidt, G.A.; Reed, C.R.; Zimmerman, M.B.; Kruse, F.E.; Greiner, M.A. Mitochondrial and Morphologic Alterations in Native Human Corneal Endothelial Cells Associated With Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2130–2138. [Google Scholar] [CrossRef]
- Qu, X.; Yu, H.; Jia, B.; Yu, X.; Cui, Q.; Liu, Z.; Sun, C.; Chu, Y. Association of downregulated HDAC 2 with the impaired mitochondrial function and cytokine secretion in the monocytes/macrophages from gestational diabetes mellitus patients. Cell Biol. Int. 2016, 40, 642–651. [Google Scholar] [CrossRef]
- Lou, P.H.; Lucchinetti, E.; Scott, K.Y.; Huang, Y.; Gandhi, M.; Hersberger, M.; Clanachan, A.S.; Lemieux, H.; Zaugg, M. Alterations in fatty acid metabolism and sirtuin signaling characterize early type-2 diabetic hearts of fructose-fed rats. Physiol. Rep. 2017, 5, e13388. [Google Scholar] [CrossRef]
- Dugail, I.; Kayser, B.D.; Lhomme, M. Specific roles of phosphatidylglycerols in hosts and microbes. Biochimie 2017, 141, 47–53. [Google Scholar] [CrossRef]
- Schlame, M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 2008, 49, 1607–1620. [Google Scholar] [CrossRef] [PubMed]
- Barth Syndrome. Available online: https://medlineplus.gov/genetics/condition/barth-syndrome/#description (accessed on 30 April 2023).
- Falabella, M.; Vernon, H.J.; Hanna, M.G.; Claypool, S.M.; Pitceathly, R.D.S. Cardiolipin, Mitochondria, and Neurological Disease. Trends Endocrinol. Metab. 2021, 32, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef]
- Pizzuto, M.; Lonez, C.; Baroja-Mazo, A.; Martinez-Banaclocha, H.; Tourlomousis, P.; Gangloff, M.; Pelegrin, P.; Ruysschaert, J.M.; Gay, N.J.; Bryant, C.E. Saturation of acyl chains converts cardiolipin from an antagonist to an activator of Toll-like receptor-4. Cell. Mol. Life Sci. 2019, 76, 3667–3678. [Google Scholar] [CrossRef]
- Furse, S. Is phosphatidylglycerol essential for terrestrial life? J. Chem. Biol. 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Sato, N.; Hagio, M.; Wada, H.; Tsuzuki, M. Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc. Natl. Acad. Sci. USA 2000, 97, 10655–10660. [Google Scholar] [CrossRef]
- Bogos, B.; Ughy, B.; Domonkos, I.; Laczko-Dobos, H.; Komenda, J.; Abasova, L.; Cser, K.; Vass, I.; Sallai, A.; Wada, H.; et al. Phosphatidylglycerol depletion affects photosystem II activity in Synechococcus sp. PCC 7942 cells. Photosynth. Res. 2010, 103, 19–30. [Google Scholar] [CrossRef]
- Kruse, O.; Hankamer, B.; Konczak, C.; Gerle, C.; Morris, E.; Radunz, A.; Schmid, G.H.; Barber, J. Phosphatidylglycerol is involved in the dimerization of photosystem II. J. Biol. Chem. 2000, 275, 6509–6514. [Google Scholar] [CrossRef]
- Shaban, H.; Borras, C.; Vina, J.; Richter, C. Phosphatidylglycerol potently protects human retinal pigment epithelial cells against apoptosis induced by A2E, a compound suspected to cause age-related macula degeneration. Exp. Eye Res. 2002, 75, 99–108. [Google Scholar] [CrossRef]
- Piccotti, L.; Marchetti, C.; Migliorati, G.; Roberti, R.; Corazzi, L. Exogenous phospholipids specifically affect transmembrane potential of brain mitochondria and cytochrome C release. J. Biol. Chem. 2002, 277, 12075–12081. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yao, M.; Wang, R.; Shi, Y.; Hou, L.; Hou, Z.; Lian, K.; Zhang, N.; Wang, Y.; Li, W.; et al. Profile of cardiac lipid metabolism in STZ-induced diabetic mice. Lipids Health Dis. 2018, 17, 231. [Google Scholar] [CrossRef]
- Hatch, G.M.; Cao, S.G.; Angel, A. Decrease in cardiac phosphatidylglycerol in streptozotocin-induced diabetic rats does not affect cardiolipin biosynthesis: Evidence for distinct pools of phosphatidylglycerol in the heart. Biochem. J. 1995, 306 Pt 3, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.R.; Fields, A.P. Phosphatidylglycerol is a physiologic activator of nuclear protein kinase C. J. Biol. Chem. 1998, 273, 11514–11520. [Google Scholar] [CrossRef] [PubMed]
- Gökmen-Polar, Y.; Fields, A.P. Mapping of a molecular determinant for protein kinase C bII isozyme function. J. Biol. Chem. 1998, 273, 20261–20266. [Google Scholar] [CrossRef]
- Klemm, D.J.; Elias, L. Phosphatidylglycerol-modulated protein kinase activity from human spleen. II. Interaction with phospholipid vesicles. Arch. Biochem. Biophys. 1988, 265, 506–513. [Google Scholar] [CrossRef]
- Klemm, D.J.; Elias, L. Purification and assay of a phosphatidylglycerol-stimulated protein kinase from murine leukemic cells and its perturbation in response to IL-3 and PMA treatment. Exp. Hematol. 1988, 16, 855–860. [Google Scholar] [PubMed]
- Klemm, D.J.; Kazim, A.L.; Elias, L. Phosphatidylglycerol-modulated protein kinase activity from human spleen. I. Enzyme purification and properties. Arch. Biochem. Biophys. 1988, 265, 496–505. [Google Scholar] [CrossRef]
- Yachida, N.; Hoshino, F.; Murakami, C.; Ebina, M.; Miura, Y.; Sakane, F. Saturated fatty acid- and/or monounsaturated fatty acid-containing phosphatidic acids selectively interact with heat shock protein 27. J. Biol. Chem. 2023, 299, 103019. [Google Scholar] [CrossRef]
- Dores-Silva, P.R.; Cauvi, D.M.; Coto, A.L.S.; Kiraly, V.T.R.; Borges, J.C.; De Maio, A. Interaction of HSPA5 (Grp78, BIP) with negatively charged phospholipid membranes via oligomerization involving the N-terminal end domain. Cell Stress Chaperones 2020, 25, 979–991. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Ducat, E.; Evrard, B.; Peulen, O.; Piel, G. Cellular uptake of liposomes monitored by confocal microscopy and flow cytometry. J. Drug Del. Sci. Technol. 2011, 21, 469–477. [Google Scholar] [CrossRef]
- Kandregula, B.; Narisepalli, S.; Chitkara, D.; Mittal, A. Exploration of Lipid-Based Nanocarriers as Drug Delivery Systems in Diabetic Foot Ulcer. Mol. Pharm. 2022, 19, 1977–1998. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Greer, N.; Foman, N.; Dorrian, J.; Fitzgerald, P.; MacDonald, R.; Rutks, I.; Wilt, T. Advanced wound care therapies for non-healing diabetic, venous, and arterial ulcers: A systematic review. Ann. Intern. Med. 2013, 159, 532–542. [Google Scholar] [CrossRef]
- Garber, S.L.; Rintala, D.H. Pressure ulcers in veterans with spinal cord injury: A retrospective study. J. Rehabil. Res. Dev. 2003, 40, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, J.; Zhang, X.; Liu, Z.; Yang, Y.; Gong, Q.; Ren, B. The Role of HMGB1 in the Pathogenesis of Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 2543268. [Google Scholar] [CrossRef]
- Erridge, C. Endogenous ligands of TLR2 and TLR4: Agonists or assistants? J. Leukoc. Biol. 2010, 87, 989–999. [Google Scholar] [CrossRef]
- Products with Phosphatidylglycerol. Available online: https://incidecoder.com/ingredients/phosphatidylglycerol (accessed on 30 April 2023).
- Fortingo, N.; Melnyk, S.; Sutton, S.H.; Watsky, M.A.; Bollag, W.B. Innate Immune System Activation, Inflammation and Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 14933. [Google Scholar] [CrossRef]
- Bollag, W.B.; Gonzales, J.N. Phosphatidylglycerol and surfactant: A potential treatment for COVID-19? Med. Hypotheses 2020, 144, 110277. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]


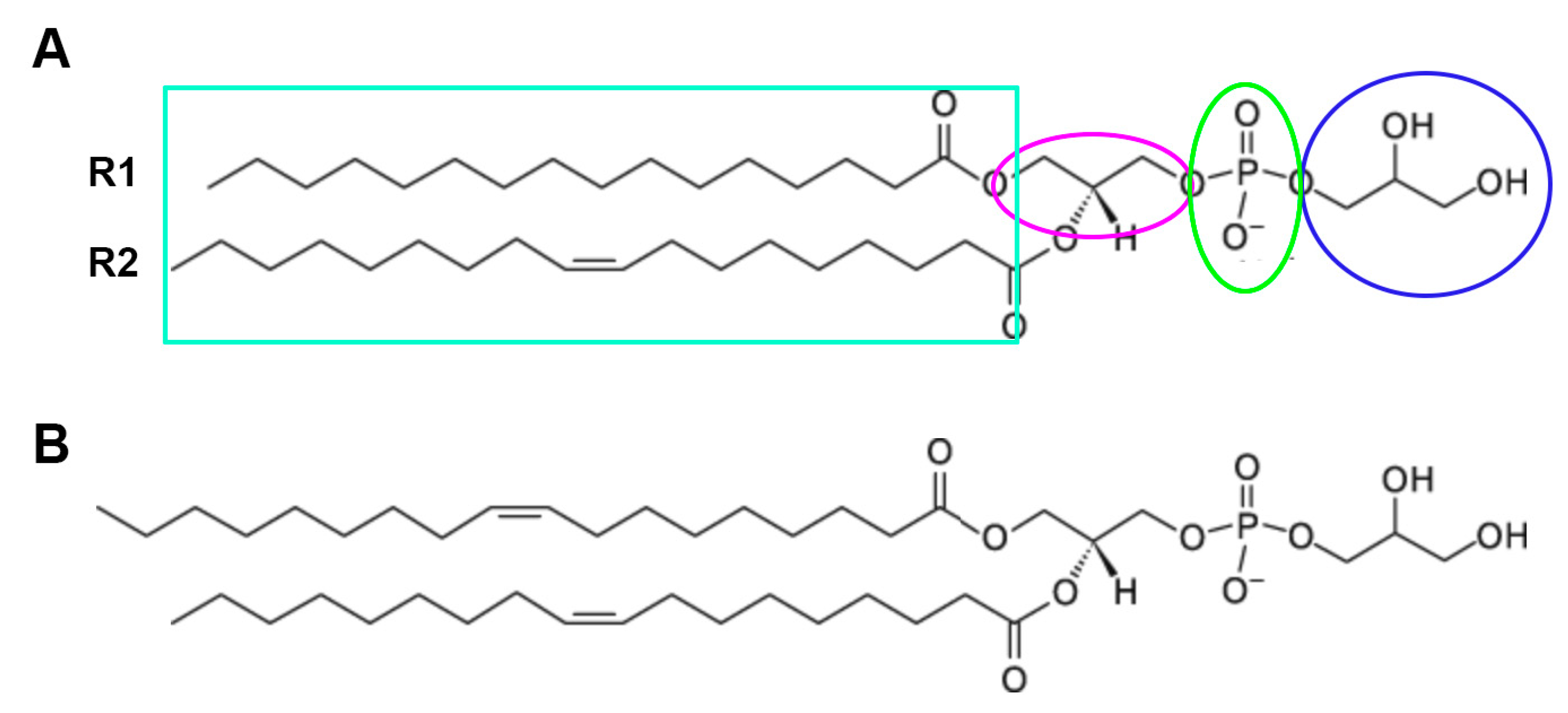

| Fatty Acid | Percentage |
|---|---|
| Palmitic acid (16:0) 2 | 32.9 |
| Palmitoleic acid (16:1) | 0.9 |
| Stearic acid (18:0) | 12.2 |
| Oleic acid (18:1) | 30.2 |
| Linoleic acid (18:2) | 18.7 |
| Arachidonic acid (20:4) | 3.5 |
| Docosatetraenoic acid (22:4) | 0.9 |
| Docosapentaenoic acid (22:5) | 0.7 |
| PG Species | Number of Carbons: Number of Double Bonds in R1; R2 Fatty Acids | Effect on Keratinocyte Proliferation (at Concentrations from 6.25–100 µg/mL) |
|---|---|---|
| egg-derived phosphatidylglycerol | see Table 1 | ↑ Slowly proliferating cells ↓ Rapidly proliferating cells |
| dipalmitoylphosphatidylglycerol | 16:0; 16:0 | ns |
| dioleoylphosphatidylglycerol | 18:1; 18:1 | ↑ |
| 1-palmitoyl-2-oleoyl-phosphatidylglycerol | 16:0; 18:1 | ↑ |
| distearoylphosphatidylglycerol | 18:0; 18:0 | ↑ |
| 1-palmitoyl-2-linoleoyl-phosphatidylglcerol | 16:0; 18:2 | ↓ |
| dilinoleoylphosphatidylglycerol | 18:2; 18:2 | ↓ |
| 1-palmitoyl-2-arachidonoyl-phosphatidylglycerol | 16:0; 20:4 | ↓ |
| dihexanoylphosphatidylglycerol | 6:0; 6:0 | ns |
| soy-derived phosphatidylglycerol | 17% 16:0, 6% 18:0, 13% 18:1, 59% 18:2 and 5% 18:3 | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Vivaldi Marrero, E.; Choudhary, V.; Bollag, W.B. Phosphatidylglycerol to Treat Chronic Skin Wounds in Diabetes. Pharmaceutics 2023, 15, 1497. https://doi.org/10.3390/pharmaceutics15051497
Luo Y, Vivaldi Marrero E, Choudhary V, Bollag WB. Phosphatidylglycerol to Treat Chronic Skin Wounds in Diabetes. Pharmaceutics. 2023; 15(5):1497. https://doi.org/10.3390/pharmaceutics15051497
Chicago/Turabian StyleLuo, Yonghong, Edymarie Vivaldi Marrero, Vivek Choudhary, and Wendy B. Bollag. 2023. "Phosphatidylglycerol to Treat Chronic Skin Wounds in Diabetes" Pharmaceutics 15, no. 5: 1497. https://doi.org/10.3390/pharmaceutics15051497
APA StyleLuo, Y., Vivaldi Marrero, E., Choudhary, V., & Bollag, W. B. (2023). Phosphatidylglycerol to Treat Chronic Skin Wounds in Diabetes. Pharmaceutics, 15(5), 1497. https://doi.org/10.3390/pharmaceutics15051497






