Immune Monitoring of Mycophenolate Mofetil Activity in Healthy Volunteers Using Ex Vivo T Cell Function Assays
Abstract
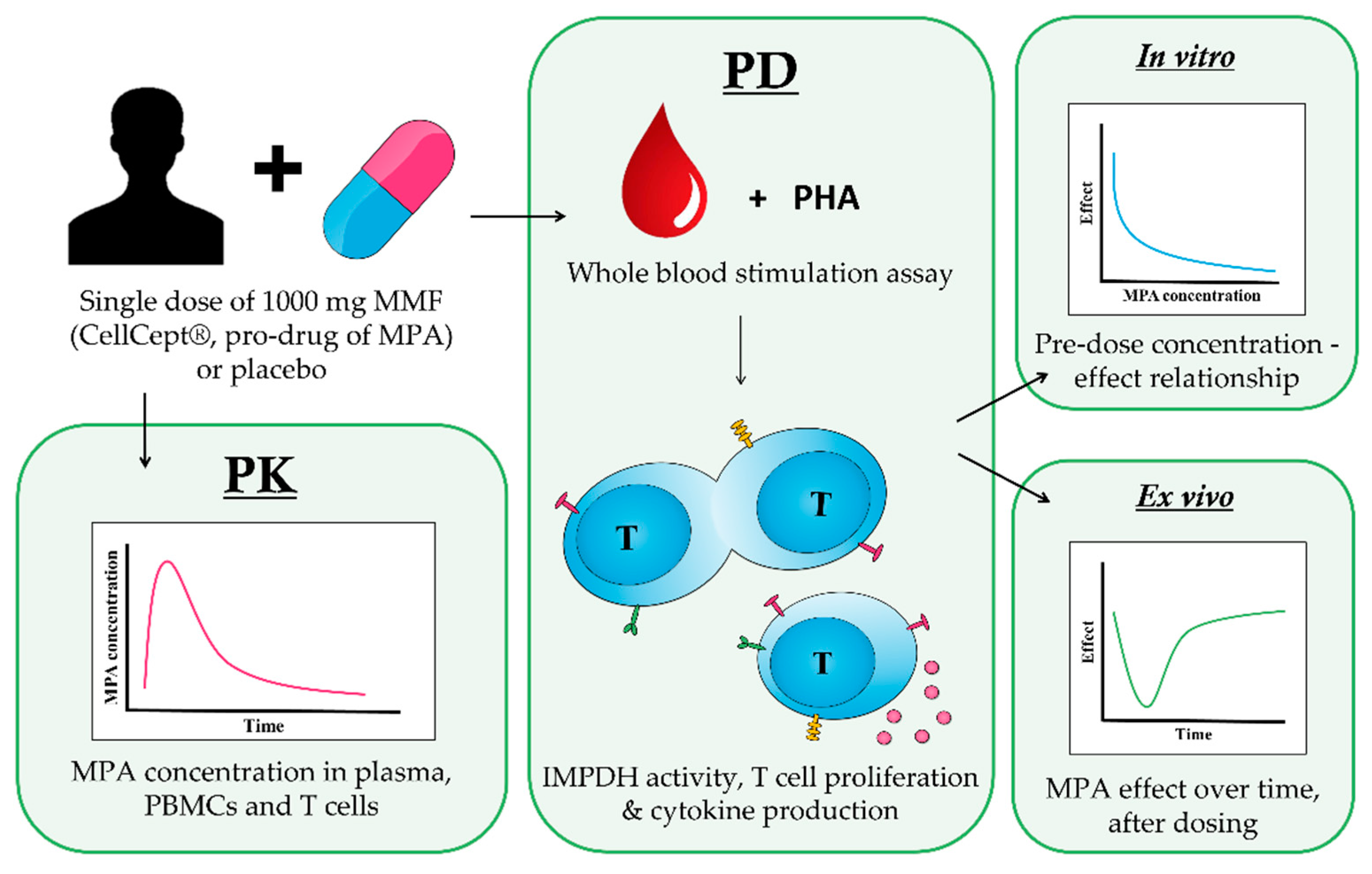
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Plasma and Intracellular PK
2.3. Cytokine Production and T Cell Proliferation
2.4. IMPDH Enzyme Activity
2.5. Data Analysis
3. Results
3.1. Subject Characteristics and Safety
3.2. Plasma and Intracellular Pharmacokinetics
3.3. IMPDH Enzymatic Activity
3.4. T Cell Proliferation
3.5. Cytokine Production
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Allison, A.C.; Eugui, E.M. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000, 47, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Prémaud, A.; Le Meur, Y.; Debord, J.; Szelag, J.C.; Rousseau, A.; Hoizey, G.; Toupance, O.; Marquet, P. Maximum a posteriori bayesian estimation of mycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods. Ther. Drug Monit. 2005, 27, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pawinski, T.; Hale, M.; Korecka, M.; Fitzsimmons, W.E.; Shaw, L.M. Limited sampling strategy for the estimation of mycophenolic acid area under the curve in adult renal transplant patients treated with concomitant tacrolimus. Clin. Chem. 2002, 48, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Bergan, S.; Brunet, M.; Hesselink, D.A.; Johnson-Davis, K.L.; Kunicki, P.K.; Lemaitre, F.; Marquet, P.; Molinaro, M.; Noceti, O.; Pattanaik, S.; et al. Personalized Therapy for Mycophenolate: Consensus Report by the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther. Drug Monit. 2021, 43, 150–200. [Google Scholar] [CrossRef] [PubMed]
- Le Meur, Y.; Büchler, M.; Thierry, A.; Caillard, S.; Villemain, F.; Lavaud, S.; Etienne, I.; Westeel, P.F.; Hurault de Ligny, B.; Rostaing, L.; et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surg. 2007, 7, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, T.; Hilbrands, L.B.; Vanrenterghem, Y.; Weimar, W.; de Fijter, J.W.; Squifflet, J.P.; Hené, R.J.; Verpooten, G.A.; Navarro, M.T.; Hale, M.D.; et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 1999, 68, 261–266. [Google Scholar] [CrossRef]
- Hale, M.D.; Nicholls, A.J.; Bullingham, R.E.; Hené, R.; Hoitsma, A.; Squifflet, J.P.; Weimar, W.; Vanrenterghem, Y.; Van de Woude, F.J.; Verpooten, G.A. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin. Pharmacol. Ther. 1998, 64, 672–683. [Google Scholar] [CrossRef]
- Gaston, R.S.; Kaplan, B.; Shah, T.; Cibrik, D.; Shaw, L.M.; Angelis, M.; Mulgaonkar, S.; Meier-Kriesche, H.U.; Patel, D.; Bloom, R.D. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: The Opticept trial. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surg. 2009, 9, 1607–1619. [Google Scholar] [CrossRef]
- Hwang, S.; Song, G.W.; Jung, D.H.; Park, G.C.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Kim, K.H.; Lee, S.G. Intra-individual variability of mycophenolic acid concentration according to renal function in liver transplant recipients receiving mycophenolate monotherapy. Ann. Hepato-Biliary-Pancreat. Surg. 2017, 21, 11–16. [Google Scholar] [CrossRef]
- Tett, S.E.; Saint-Marcoux, F.; Staatz, C.E.; Brunet, M.; Vinks, A.A.; Miura, M.; Marquet, P.; Kuypers, D.R.; van Gelder, T.; Cattaneo, D. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant. Rev. 2011, 25, 47–57. [Google Scholar] [CrossRef]
- Sommerer, C.; Müller-Krebs, S.; Schaier, M.; Glander, P.; Budde, K.; Schwenger, V.; Mikus, G.; Zeier, M. Pharmacokinetic and pharmacodynamic analysis of enteric-coated mycophenolate sodium: Limited sampling strategies and clinical outcome in renal transplant patients. Br. J. Clin. Pharmacol. 2010, 69, 346–357. [Google Scholar] [CrossRef]
- Kuypers, D.R.; de Jonge, H.; Naesens, M.; de Loor, H.; Halewijck, E.; Dekens, M.; Vanrenterghem, Y. Current target ranges of mycophenolic acid exposure and drug-related adverse events: A 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin. Ther. 2008, 30, 673–683. [Google Scholar] [CrossRef]
- Jung, H.Y.; Lee, S.; Jeon, Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, Y.L.; Kim, H.K.; Huh, S.; Won, D.I.; et al. Mycophenolic Acid Trough Concentration and Dose Are Associated with Hematologic Abnormalities but Not Rejection in Kidney Transplant Recipients. J. Korean Med. Sci. 2020, 35, e185. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Glander, P.; Waiser, J.; Hambach, P.; Bachmann, F.; Budde, K.; Eckardt, K.U.; Friedersdorff, F.; Gaedeke, J.; Kron, S.; Lorkowski, C.; et al. Inosine 5′-Monophosphate Dehydrogenase Activity for the Longitudinal Monitoring of Mycophenolic Acid Treatment in Kidney Allograft Recipients. Transplantation 2021, 105, 916–927. [Google Scholar] [CrossRef]
- In ’t Veld, A.E.; Grievink, H.W.; Saghari, M.; Stuurman, F.E.; de Kam, M.L.; de Vries, A.P.J.; de Winter, B.C.M.; Burggraaf, J.; Cohen, A.F.; Moerland, M. Immunomonitoring of Tacrolimus in Healthy Volunteers: The First Step from PK- to PD-Based Therapeutic Drug Monitoring? Int. J. Mol. Sci. 2019, 20, 4710. [Google Scholar] [CrossRef]
- In ’t Veld, A.E.; Jansen, M.A.A.; Huisman, B.W.; Schoonakker, M.; de Kam, M.L.; Moes, D.; van Poelgeest, M.I.E.; Burggraaf, J.; Moerland, M. Monitoring of Ex Vivo Cyclosporin a Activity in Healthy Volunteers Using T Cell Function Assays in Relation to Whole Blood and Cellular Pharmacokinetics. Pharmaceutics 2022, 14, 1958. [Google Scholar] [CrossRef]
- Zwart, T.C.; Gokoel, S.R.M.; van der Boog, P.J.M.; de Fijter, J.W.; Kweekel, D.M.; Swen, J.J.; Guchelaar, H.J.; Moes, D. Therapeutic drug monitoring of tacrolimus and mycophenolic acid in outpatient renal transplant recipients using a volumetric dried blood spot sampling device. Br. J. Clin. Pharmacol. 2018, 84, 2889–2902. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Calculating correlation coefficients with repeated observations: Part 1--Correlation within subjects. BMJ 1995, 310, 446. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Corrigendum: Repeated Measures Correlation. Front. Psychol. 2019, 10, 1201. [Google Scholar] [CrossRef] [PubMed]
- Bullingham, R.; Monroe, S.; Nicholls, A.; Hale, M. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. J. Clin. Pharmacol. 1996, 36, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kees, M.G.; Steinke, T.; Moritz, S.; Rupprecht, K.; Paulus, E.M.; Kees, F.; Bucher, M.; Faerber, L. Omeprazole impairs the absorption of mycophenolate mofetil but not of enteric-coated mycophenolate sodium in healthy volunteers. J. Clin. Pharmacol. 2012, 52, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, Y.; Zhu, Z.; Feng, G.; Sun, Z.; Zhang, X. Pharmacokinetic Comparison of Two Mycophenolate Mofetil Formulations in Kidney Transplant Recipients. Ther. Drug Monit. 2018, 40, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Grinyó, J.M.; Ekberg, H.; Mamelok, R.D.; Oppenheimer, F.; Sánchez-Plumed, J.; Gentil, M.A.; Hernandez, D.; Kuypers, D.R.; Brunet, M. The pharmacokinetics of mycophenolate mofetil in renal transplant recipients receiving standard-dose or low-dose cyclosporine, low-dose tacrolimus or low-dose sirolimus: The Symphony pharmacokinetic substudy. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2009, 24, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.; Mourad, M.; Capron, A.; Tshinanu, F.M.; Vincent, M.F.; Wallemacq, P. Plasma and intracellular pharmacokinetic-pharmacodynamic analysis of mycophenolic acid in de novo kidney transplant patients. Clin. Biochem. 2015, 48, 401–405. [Google Scholar] [CrossRef]
- Budde, K.; Glander, P.; Bauer, S.; Braun, K.; Waiser, J.; Fritsche, L.; Mai, I.; Roots, I.; Neumayer, H.H. Pharmacodynamic monitoring of mycophenolate mofetil. Clin. Chem. Lab. Med. 2000, 38, 1213–1216. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Molinaro, M.; Libetta, C.; Tinelli, C.; Cosmai, L.; Valentini, G.; Dal Canton, A.; Regazzi, M. Inosine monophosphate dehydrogenase variability in renal transplant patients on long-term mycophenolate mofetil therapy. Br. J. Clin. Pharmacol. 2010, 69, 38–50. [Google Scholar] [CrossRef]
- Mourad, M.; Malaise, J.; Chaib Eddour, D.; De Meyer, M.; König, J.; Schepers, R.; Squifflet, J.P.; Wallemacq, P. Pharmacokinetic basis for the efficient and safe use of low-dose mycophenolate mofetil in combination with tacrolimus in kidney transplantation. Clin. Chem. 2001, 47, 1241–1248. [Google Scholar] [CrossRef]
- Okour, M.; Jacobson, P.A.; Ahmed, M.A.; Israni, A.K.; Brundage, R.C. Mycophenolic Acid and Its Metabolites in Kidney Transplant Recipients: A Semimechanistic Enterohepatic Circulation Model to Improve Estimating Exposure. J. Clin. Pharmacol. 2018, 58, 628–639. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Dimitrov, S.; Benedict, C.; Heutling, D.; Westermann, J.; Born, J.; Lange, T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood 2009, 113, 5134–5143. [Google Scholar] [CrossRef]
- Wang, P.; Xie, H.; Zhang, Q.; Tian, X.; Feng, Y.; Qin, Z.; Yang, J.; Shang, W.; Feng, G.; Zhang, X. Population Pharmacokinetics of Mycophenolic Acid in Renal Transplant Patients: A Comparison of the Early and Stable Posttransplant Stages. Front. Pharmacol. 2022, 13, 859351. [Google Scholar] [CrossRef]




| Subject Characteristics | 1000 mg CellCept (n = 12) | Placebo (n = 4) |
|---|---|---|
| Age (range) | 24.8 (18–39) | 27.2 (18–46) |
| Gender (female/male) | 5/7 | 3/1 |
| BMI (kg/m2), mean (range) | 24.41 (18.7–28.9) | 21.85 (20.1–24.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
in ’t Veld, A.E.; Jansen, M.A.A.; de Kam, M.L.; Yavuz, Y.; Moes, D.J.A.R.; Oudhoff, K.A.; van Poelgeest, M.I.E.; Burggraaf, J.; Moerland, M. Immune Monitoring of Mycophenolate Mofetil Activity in Healthy Volunteers Using Ex Vivo T Cell Function Assays. Pharmaceutics 2023, 15, 1635. https://doi.org/10.3390/pharmaceutics15061635
in ’t Veld AE, Jansen MAA, de Kam ML, Yavuz Y, Moes DJAR, Oudhoff KA, van Poelgeest MIE, Burggraaf J, Moerland M. Immune Monitoring of Mycophenolate Mofetil Activity in Healthy Volunteers Using Ex Vivo T Cell Function Assays. Pharmaceutics. 2023; 15(6):1635. https://doi.org/10.3390/pharmaceutics15061635
Chicago/Turabian Stylein ’t Veld, Aliede E., Manon A. A. Jansen, Marieke L. de Kam, Yalҫin Yavuz, Dirk Jan A. R. Moes, Kathalijne A. Oudhoff, Mariette I. E. van Poelgeest, Jacobus Burggraaf, and Matthijs Moerland. 2023. "Immune Monitoring of Mycophenolate Mofetil Activity in Healthy Volunteers Using Ex Vivo T Cell Function Assays" Pharmaceutics 15, no. 6: 1635. https://doi.org/10.3390/pharmaceutics15061635
APA Stylein ’t Veld, A. E., Jansen, M. A. A., de Kam, M. L., Yavuz, Y., Moes, D. J. A. R., Oudhoff, K. A., van Poelgeest, M. I. E., Burggraaf, J., & Moerland, M. (2023). Immune Monitoring of Mycophenolate Mofetil Activity in Healthy Volunteers Using Ex Vivo T Cell Function Assays. Pharmaceutics, 15(6), 1635. https://doi.org/10.3390/pharmaceutics15061635






