Phosphorylcholine and KR12-Containing Corneal Implants in HSV-1-Infected Rabbit Corneas
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Silico Comparison of KR12 and LL37
2.2. Effect of KR12 on HSV-1 and Corneal Cells
2.2.1. In Vitro Cell Viability Studies
2.2.2. Wound Healing Assay
2.2.3. In Vitro Efficacy Studies of KR12 Peptide
2.3. Silica Nanoparticles Releasing KR12 and Composite RHCIII-MPC Implants with KR12 Activity
2.3.1. KR12 Release Study and Encapsulation Efficacy
2.3.2. Biocompatibility of KR12-Releasing RHCIII-MPC Hydrogels In Vitro
2.4. Rabbit HSV-1 Infections and Surgery
2.4.1. Histopathology and Immunohistochemistry
2.4.2. Real-Time Quantitative PCR to Check HSV-1 Shedding in Rabbit Tears
2.5. Statistical Analysis
3. Results
3.1. In Silico Comparisons of KR12 vs LL37
3.2. KR12 Effects on Cells and HSV-1
Biocompatibility of Composite Implants with KR12
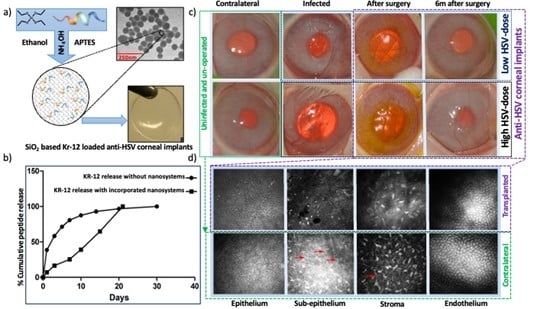
3.3. Effects of Composite K12-Releasing Implants in HSV-1-Infected Rabbit Corneas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| uM | micro Molar |
| α-SMA | Alpha smooth muscle actin |
| β-tubulin | Beta-tubulin |
| ASA | Accessible surface areas |
| ACV | Acyclovir |
| APC | Antigen-presenting cells |
| APS | Ammonium persulphate |
| BMDCs | Bone marrow dendritic cells |
| Bur | Burial |
| CHDPs | Cationic host defense peptides |
| CI | Confidence Interval |
| CD | Cluster of differentiation |
| DNA | Deoxyribonucleic acid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| E. coli | Escherichia coli |
| EDC | N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide |
| EGF | Epidermal growth factor |
| FBS | Fetal bovine serum |
| FITC | Fluorescein isothiocyanate |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GFP | Green fluorescence protein |
| GFP | HCECs Green fluorescence protein transfected human corneal epithelial cells |
| HEPES | (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) |
| H&E | Hematoxylin-eosin |
| HCECs | Human corneal epithelial cells |
| HSV-1 | Herpes Simplex Virus serotype 1 |
| Ig G | Immunoglobulin G |
| IOP | Intraocular pressure |
| IVCM | In vivo confocal microscopy |
| KR12 | KRIVQRIKDFLR |
| KSFM | Keratinocyte serum free media |
| LPS | Lipopolysaccharides |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide |
| MPr | Membrane propensity index |
| Mw | Molecular weight |
| MES | Morpholinoethane sulfonic acid monohydrate |
| MOI | Multiplicity of infection |
| NHS | N-hydroxysuccinimide |
| PFA | Paraformaldehyde |
| PFUs | Plaque-forming units |
| PCR | Polymerase chain reaction |
| PBS | Phosphate-buffered saline |
| PEGDA | Poly (ethylene glycol) diacrylate |
| RHCIII-MPC | Recombinant human collagen type III and 2-methacryloyloxyethyl phosphorylcholine |
| rGb | Residue Given burial |
| RT | Room temperature |
| SiNPs | Silica dioxide nanoparticles |
| TEM | Transmission electron microscopy |
| TEMED | N,N,N,N-tetramethylethylenediamine |
| TCP | Tissue culture plastic (TCP) |
References
- Khan, B.F. Viral Keratitis. In Copeland and Afshari’s Principles and Practice of Cornea; Copeland, R.A., Jr., Ed.; NA Afshari Jaypee-Highlights Medical Publ. Inc.: New Delhi, India, 2013; pp. 265–283. [Google Scholar]
- Koay, P.Y.P.; Lee, W.H.; Figueiredo, F.C. Opinions on Risk Factors and Management of Corneal Graft Rejection in the United Kingdom. Cornea 2005, 24, 292–296. [Google Scholar] [CrossRef]
- Maguire, M.G.; Stark, W.J.; Gottsch, J.D.; Stulting, R.D.; Sugar, A.; Fink, N.E.; Schwartz, A. Risk Factors for Corneal Graft Failure and Rejection in the Collaborative Corneal Transplantation Studies. Ophthalmology 1994, 101, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.S.; Duncan, J. Penetrating Keratoplasty for Herpes Simplex Keratitis. Am. J. Ophthalmol. 1981, 92, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Beekhuis, W.H.; de Lavalette, J.G.C.R.; Van Rij, G.; Schaap, G.J.P. Therapeutic keratoplasty for active herpetic corneal disease: Viral culture and prognosis. Doc. Ophthalmol. 1983, 55, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ghosheh, F.R.; Cremona, F.; Ayres, B.D.; Hammersmith, K.M.; Cohen, E.J.; Raber, I.M.; Laibson, P.R.; Rapuano, C.J. Indications for Penetrating Keratoplasty and Associated Procedures, 2001–2005. Eye Contact Lens: Sci. Clin. Pract. 2008, 34, 211–214. [Google Scholar] [CrossRef]
- White, M.L.; Chodosh, J. Herpes Simplex Virus Keratitis: A Treatment Guideline Adoption of innovation in Herpes Simplex Virus Keratitis. Am. Acad. Ophthalmol. 2014. Available online: https://www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline (accessed on 28 May 2023).
- Whitley, R.; Kimberlin, D.W.; Prober, C.G. Pathogenesis and Disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK47449/ (accessed on 28 May 2023).
- Al-Dujaili, L.J.; Clerkin, P.P.; Clement, C.; McFerrin, H.E.; Bhattacharjee, P.S.; Varnell, E.D.; Kaufman, H.E.; Hill, J.M. Ocular herpes simplex virus: How are latency, reactivation, recurrent disease and therapy interrelated? Future Microbiol. 2011, 6, 877–907. [Google Scholar] [CrossRef] [Green Version]
- Miserocchi, E.; Modorati, G.; Galli, L.; Rama, P. Efficacy of Valacyclovir vs Acyclovir for the prevention of recurrent Herpes Simplex Virus eye disease: A Pilot Study. Am. J. Ophthalmol. 2007, 144, 547–551.e1. [Google Scholar] [CrossRef]
- Wilhelmus, K.R.; Beck, R.W.; Moke, P.S.; Dawson, C.R.; Barron, B.A.; Jones, D.B.; Kaufman, H.E.; Kurinij, N.; Stulting, R.D.; Sugar, J.; et al. Acyclovir for the prevention of recurrent Herpes Simplex Virus eye disease. N. Engl. J. Med. 1998, 339, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Colin, J. Ganciclovir ophthalmic gel, 0.15%: A valuable tool for treating ocular herpes. Clin. Ophthalmol. 2007, 1, 441–453. [Google Scholar] [PubMed]
- Sousa, F.H.; Casanova, V.; Stevens, C.; Barlow, P.G. Antiviral Host Defence Peptides. In Host Defense Peptides and Their Potential as Therapeutic Agents; Epand, R., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human Cathelicidin (LL-37), a Multifunctional Peptide, is Expressed by Ocular Surface Epithelia and has Potent Antibacterial and Antiviral Activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Buznyk, O.; Kuffova, L.; Rajendran, V.; Forrester, J.V.; Phopase, J.; Islam, M.M.; Skog, M.; Ahlqvist, J.; Griffith, M. Cathelicidin LL-37 and HSV-1 Corneal infection: Peptide versus gene therapy. Trans. Vis. Sci. Tech. 2014, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G. Structures of human host defense Cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Davido, D.J.; Morrison, L.A. HSV-1 strain McKrae is more neuroinvasive than HSV-1 KOS after corneal or vaginal inoculation in mice. Virus Res. 2013, 173, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.M.; Buznyk, O.; Reddy, J.C.; Pasyechnikova, N.; Alarcon, E.I.; Hayes, S.; Lewis, P.; Fagerholm, P.; He, C.; Iakymenko, S.; et al. Biomaterials-enabled cornea regeneration in patients at high risk for rejection of donor tissue transplantation. NPJ Regen Med. 2018, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Armitage, W.J.; Goodchild, C.; Griffin, M.D.; Gunn, D.J.; Hjortdal, J.; Lohan, P.; Murphy, C.C.; Pleyer, U.; Ritter, T.; Tole, D.M.; et al. High-risk Corneal Transplantation: Recent Developments and Future Possibilities. Transplantation 2019, 103, 2468–2478. [Google Scholar] [CrossRef] [Green Version]
- Amouzegar, A.; Chauhan, S.K.; Dana, R. Alloimmunity and tolerance in corneal transplantation. J. Immunol. 2016, 196, 3983–3991. [Google Scholar] [CrossRef] [Green Version]
- Kakinoki, S.; Sakai, Y.; Takemura, T.; Hanagata, N.; Fujisato, T.; Ishihara, K.; Yamaoka, T. Gene chip/PCR-array analysis of tissue response to 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer surfaces in a mouse subcutaneous transplantation system. J. Biomater. Sci. Polym. Ed. 2014, 25, 1658–1672. [Google Scholar] [CrossRef]
- Yumoto, H.; Hirota, K.; Hirao, K.; Miyazaki, T.; Yamamoto, N.; Miyamoto, K.; Murakami, K.; Fujiwara, N.; Matsuo, T.; Miyake, Y. Anti-inflammatory and protective effects of 2-methacryloyloxyethyl phosphorylcholine polymer on oral epithelial cells. J. Biomed. Mater. Res. Part A 2015, 103, 555–563. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.-W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 204173141350335. [Google Scholar] [CrossRef] [Green Version]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Bhattacharyya, D.; Banerjee, R. Applications of complementarity plot in error detection and structure validation of proteins. Indian J. Biochem. Biophys. 2014, 51, 188–200. [Google Scholar] [PubMed]
- Basu, S.; Wallner, B. Finding correct protein–protein docking models using ProQDock. Bioinformatics Oxf. Engl. 2016, 32, i262–i270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S. CPdock: The complementarity plot for docking of proteins: Implementing multi-dielectric continuum electrostatics. J. Mol. Model 2018, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Chakravarty, D.; Bhattacharyya, D.; Saha, P.; Patra, H.K. Plausible blockers of Spike RBD in SARS-CoV2—Molecular design and underlying interaction dynamics from high-level structural descriptors. J. Mol. Model 2021, 27, 191. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, S.; Thornton, J.; NACCESS. Computer Program 1993, Department of Biochemistry and Molecular Biology, University College London—Open Access Library. Available online: http://www.oalib.com/references/5299711 (accessed on 1 March 2017).
- Lee, B.; Richards, F.M. The interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 1971, 55, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Bhattacharyya, D.; Banerjee, R. Self-complementarity within proteins: Bridging the gap between binding and folding. Biophys. J. 2012, 102, 2605–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Assaf, S.S.; Teheux, F.; Rooman, M.; Pucci, F. BRANEart: Identify Stability Strength and Weakness Regions in Membrane Proteins. Front. Bioinform. 2021, 1, 742843. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y. An SV40-Immortalized Human Corneal Epithelial Cell Line and Its Characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Mirazul Islam, M.; Cėpla, V.; He, C.; Edin, J.; Rakickas, T.; Kobuch, K.; Ruželė, Ž.; Bruce Jackson, W.; Rafat, M.; Lohmann, C.P.; et al. Functional fabrication of recombinant human collagen–phosphorylcholine hydrogels for regenerative medicine applications. Acta Biomater. 2015, 12, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, A.; Kehn-Hall, K. Viral concentration determination through plaque assays: Using traditional and novel overlay systems. J. Vis. Exp. 2014, 93, e52065. [Google Scholar]
- Simpson, F.C.; McTiernan, C.D.; Islam, M.M.; Buznyk, O.; Lewis, P.N.; Meek, K.M.; Haagdorens, M.; Audiger, C.; Lesage, S.; Gueriot, F.-X.; et al. Collagen analogs with phosphorylcholine are inflammation-suppressing scaffolds for corneal regeneration from alkali burns in mini-pigs. Commun. Biol. 2021, 4, 608. [Google Scholar] [CrossRef] [PubMed]
- Altmann, S.; Emanuel, A.; Toomey, M.; McIntyre, K.; Covert, J.; Dubielzig, R.R.; Leatherberry, G.; Murphy, C.J.; Kodihalli, S.; Brandt, C.R. A quantitative rabbit model of Vaccinia keratitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Tyler, K.L. Acute Viral Encephalitis. N. Engl. J. Med. 2018, 379, 557–566. [Google Scholar] [CrossRef]
- De Matos, R.; Russell, D.; Van Alstine, W.; Miller, A. Spontaneous fatal Human herpesvirus 1 encephalitis in two domestic rabbits (Oryctolagus cuniculus). J. VET Diagn. Investig. 2014, 26, 689–694. [Google Scholar] [CrossRef] [Green Version]
- Loutsch, J.M.; Sainz, B.; Marquart, M.E.; Zheng, X.; Kesavan, P.; Higaki, S.; Hill, J.M.; Tal-Singer, R. Effect of famciclovir on Herpes Simplex Virus Type 1 corneal disease and establishment of latency in rabbits. Antimicrob. Agents Chemother. 2001, 45, 2044–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Sambanthamoorthy, K.; Palys, T.; Paranavitana, C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter Baumannii. Peptides 2013, 49, 131–137. [Google Scholar] [CrossRef]
- Hackett, J.M.; Lagali, N.; Merrett, K.; Edelhauser, H.; Sun, Y.; Gan, L.; Griffith, M.; Fagerholm, P. Biosynthetic corneal implants for replacement of pathologic corneal tissue: Performance in a controlled rabbit alkali burn model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 651–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, W.C.; Cheung, K.Y.; Orban, J.; Lee, C.J.; Turner, A.P.F.; Griffith, M. Surface-engineered contact lens as an advanced theranostic platform for modulation and detection of viral infection. ACS Appl. Mater. Interfaces 2015, 7, 25487–25494. [Google Scholar] [CrossRef]
- Jacob, B.; Park, I.-S.; Bang, J.-K.; Shin, S.Y. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity: Antimicrobial And Antiendotoxic Activities Of KR-12 Analogs. J. Pept. Sci. 2013, 19, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Ao, H.; Chen, C.; Xie, K.; Zhou, J.; Long, T.; Tang, T.; Yue, B. Covalent immobilization of KR-12 peptide onto a titanium surface for decreasing infection and promoting osteogenic differentiation. RSC Adv. 2016, 6, 46733–46743. [Google Scholar] [CrossRef]
- Thennarasu, S.; Tan, A.; Penumatchu, R.; Shelburne, C.E.; Heyl, D.L.; Ramamoorthy, A. Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys. J. 2010, 98, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Li, X.R.; Xu, X.Y.; Lv, Y.F.; Li, Z.Y.; Shan, A.S.; Wang, J.L. Characterization of bactericidal efficiency, cell selectivity, and mechanism of short interspecific hybrid peptides. Amino Acids 2018, 50, 453–468. [Google Scholar] [CrossRef]
- Al Tall, Y.; Abualhaijaa, A.; Alsaggar, M.; Almaaytah, A.; Masadeh, M.; Alzoubi, K.H. Design and characterization of a new hybrid peptide from LL-37 and BMAP-27. Infect. Drug Resist. 2019, 12, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Gwyer Findlay, E.; Currie, S.M.; Davidson, D.J. Cationic host defence peptides: Potential as antiviral therapeutics. BioDrugs 2013, 27, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Gronberg, A.; Mahlapuu, M.; Stahle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: A randomized, placebo-controlled clinical trial. Wound Repair Regen 2014, 22, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Molchanova, N.; Herlan, C.; Fortkort, J.A.; Lin, J.S.; Figgins, E.; Bopp, N.; Ryan, L.K.; Chung, D.; Adcock, R.S.; et al. Potent Antiviral Activity against HSV-1 and SARS-CoV-2 by Antimicrobial Peptoids. Pharmaceuticals 2021, 14, 304. [Google Scholar] [CrossRef] [PubMed]






| Target | Antibody | Dilution Factor |
|---|---|---|
| CD11c | Brilliant Violet 650™ anti-mouse CD11c, Clone: N418, IsoType: Armenian Hamster IgG, Format: BV650, APP: FC, BioLegend (San Diego, CA, USA), 117339 | 1:600 |
| CD40 | CD40, APC, clone: 1C10, eBioscience™ (Carlsbad, CA, USA), 501129392 | 1:400 |
| CD80 | PE anti-mouse CD80, Clone: 16-10A1, IsoType: Armenian Hamster IgG, Biolegend (San Diego, CA, USA), 104708 | 1:1600 |
| CD86 | FITC anti-mouse CD86, Clone: GL-1, IsoType: Rat IgG2a,κ, Biolegend (San Diego, CA, USA), 105006 | 1:50 |
| Target | Antibody | Dilution Factor |
|---|---|---|
| herpes simplex virus serotype 1 | Anti-HSV1 [20.7.1], ab860 Abcam, Cambridge, UK | 1:100 |
| activated stromal cells or myofibroblast | Anti-SMA, [1A4] ab7817, Abcam, Cambridge, UK | 1:50 |
| vascular endothelium | Anti-CD31, ab199012, Abcam, Cambridge, UK | 1:50 |
| lymphatic vessels | Anti-Lyve 1, ab14917, Abcam, Cambridge, UK | 1:100 |
| Macrophages | Anti-MAC 387, ab22506, Abcam, Cambridge, UK | 1:100 |
| Nerves | neuron-specific Anti-beta III Tubulin [Tu-20], ab7751, Abcam, Cambridge, UK | 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, K.; Buznyk, O.; Islam, M.M.; Edin, E.; Basu, S.; Groleau, M.; Dégué, D.S.; Fagerholm, P.; Fois, A.; Lesage, S.; et al. Phosphorylcholine and KR12-Containing Corneal Implants in HSV-1-Infected Rabbit Corneas. Pharmaceutics 2023, 15, 1658. https://doi.org/10.3390/pharmaceutics15061658
Malhotra K, Buznyk O, Islam MM, Edin E, Basu S, Groleau M, Dégué DS, Fagerholm P, Fois A, Lesage S, et al. Phosphorylcholine and KR12-Containing Corneal Implants in HSV-1-Infected Rabbit Corneas. Pharmaceutics. 2023; 15(6):1658. https://doi.org/10.3390/pharmaceutics15061658
Chicago/Turabian StyleMalhotra, Kamal, Oleksiy Buznyk, Mohammad Mirazul Islam, Elle Edin, Sankar Basu, Marc Groleau, Delali Shana Dégué, Per Fagerholm, Adrien Fois, Sylvie Lesage, and et al. 2023. "Phosphorylcholine and KR12-Containing Corneal Implants in HSV-1-Infected Rabbit Corneas" Pharmaceutics 15, no. 6: 1658. https://doi.org/10.3390/pharmaceutics15061658
APA StyleMalhotra, K., Buznyk, O., Islam, M. M., Edin, E., Basu, S., Groleau, M., Dégué, D. S., Fagerholm, P., Fois, A., Lesage, S., Jangamreddy, J. R., Šimoliūnas, E., Liszka, A., Patra, H. K., & Griffith, M. (2023). Phosphorylcholine and KR12-Containing Corneal Implants in HSV-1-Infected Rabbit Corneas. Pharmaceutics, 15(6), 1658. https://doi.org/10.3390/pharmaceutics15061658