Quetiapine Albumin Nanoparticles as an Efficacious Platform for Brain Deposition and Potentially Improved Antipsychotic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation and Evaluation of Quetiapine HSA Nanoparticles
2.2.1. Experimental Design
2.2.2. Preparation of Quetiapine- HSA Nanoparticles (QP-NP)
2.3. Characterization of the Prepared QP-NP
2.3.1. Particle Size Analysis, Polydispersity Index, and Zeta Potential
2.3.2. Entrapment Efficiency (EE%) and Loading Efficiency (LE%)
2.3.3. Transmission Electron Microscope (TEM)
2.3.4. In Vitro Drug Release
2.4. In Vitro Hemolysis Assay of the Optimized QP-NPs
2.5. In Vivo Studies of the Selected QP-NPs
2.5.1. Pharmacokinetic Studies
2.5.2. Pharmacodynamics Studies
Paw Test
Open Field Test in Schizophrenia Rat Model
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Quetiapine HSA Nanoparticles (QP-NPs)
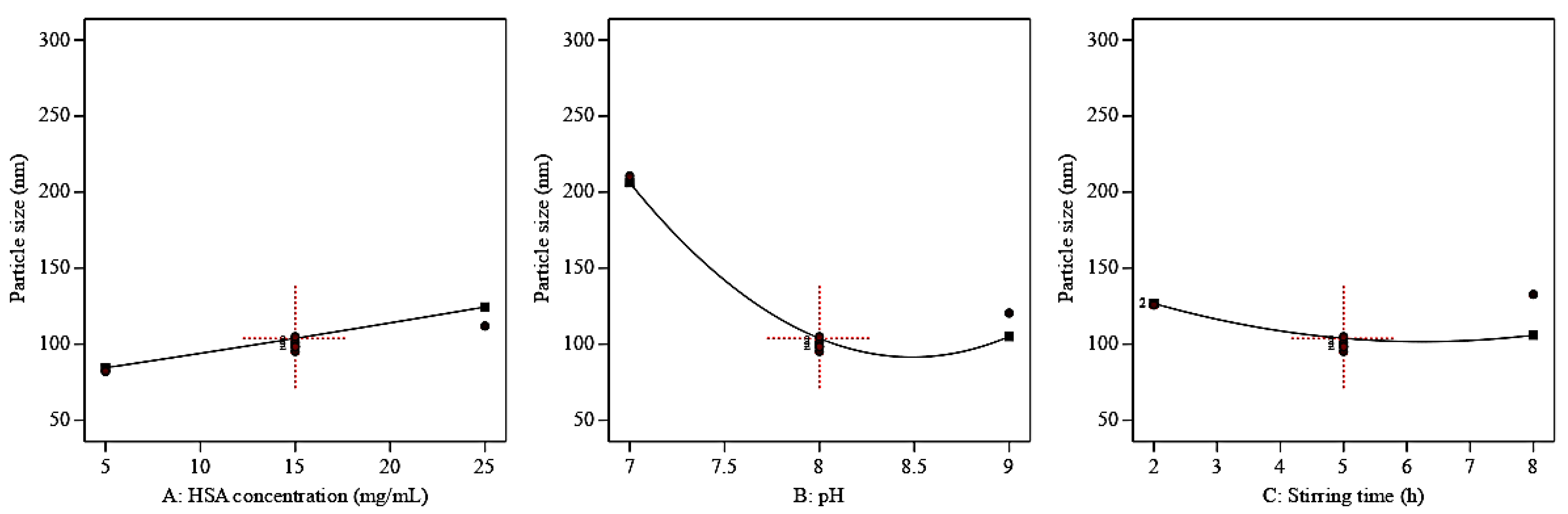
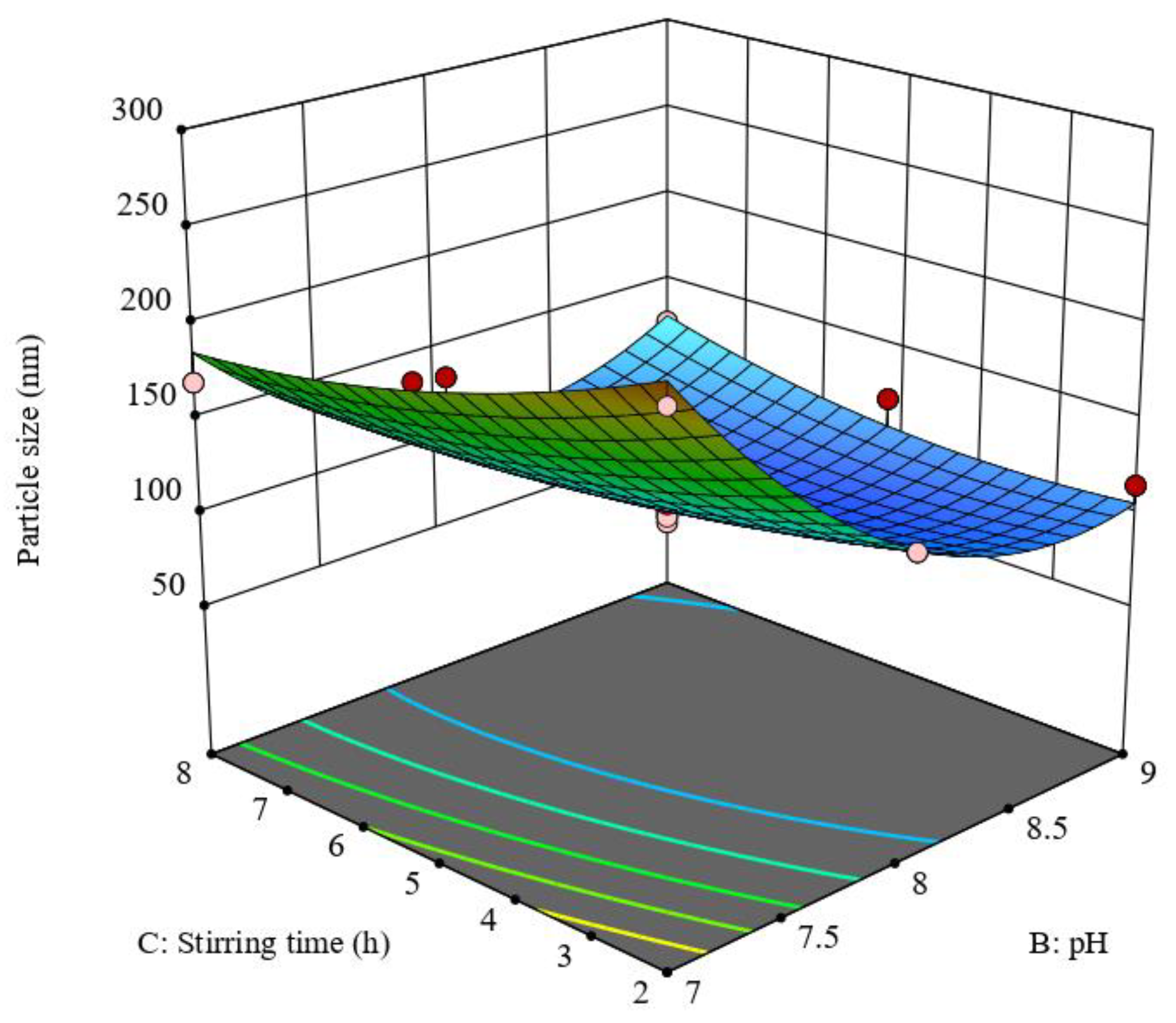
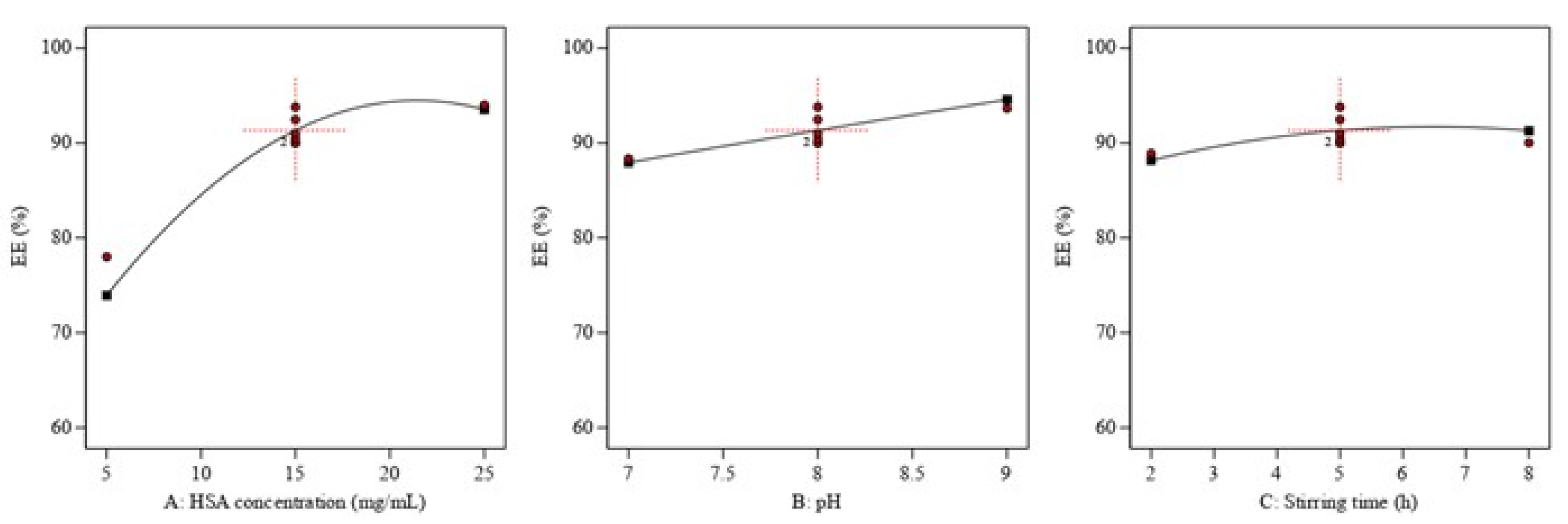
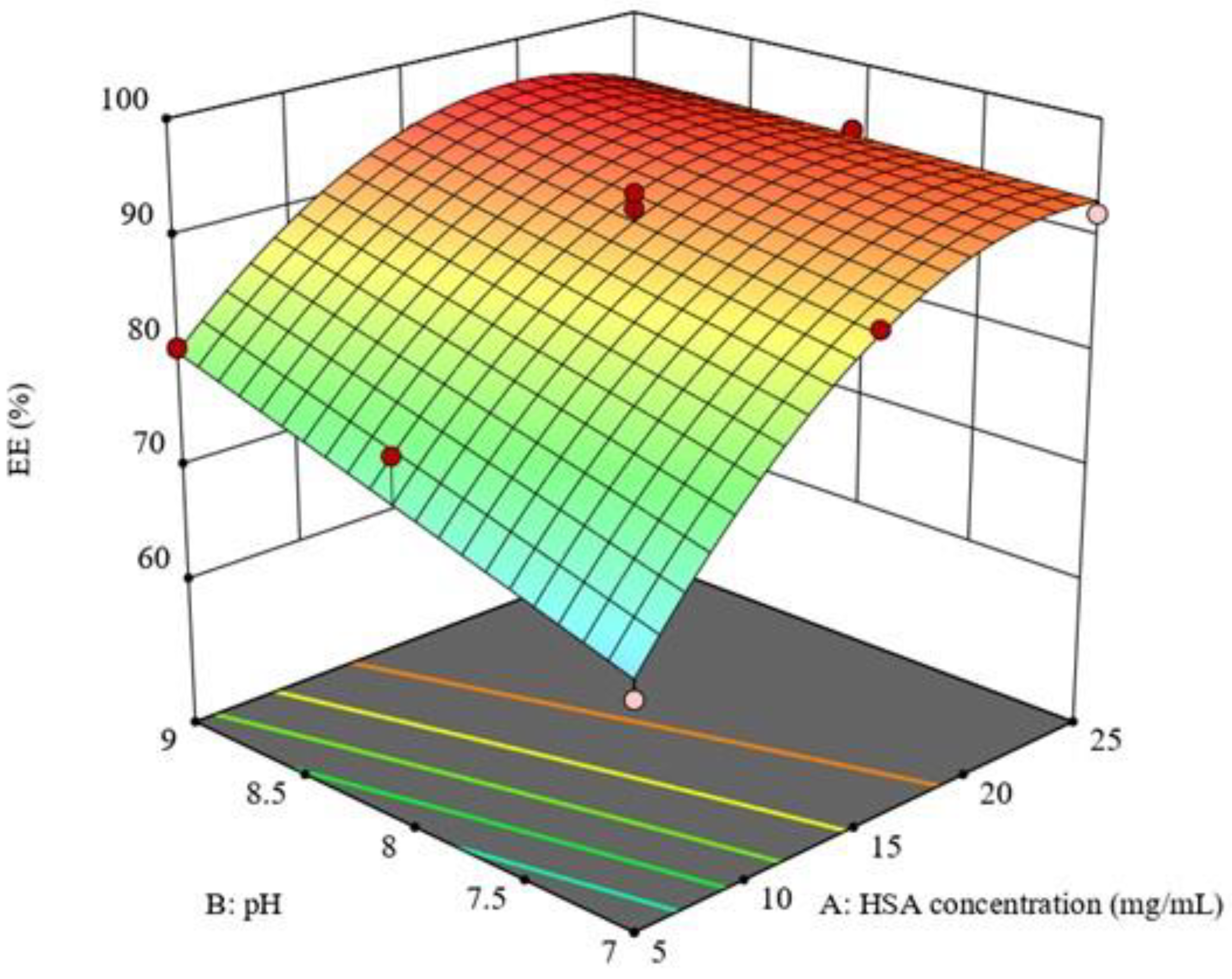
3.2. Optimization of the Prepared QP-NPs
3.3. Morphological Structure of the Optimized, Selected Quetiapine-HSA- Nanoparticles
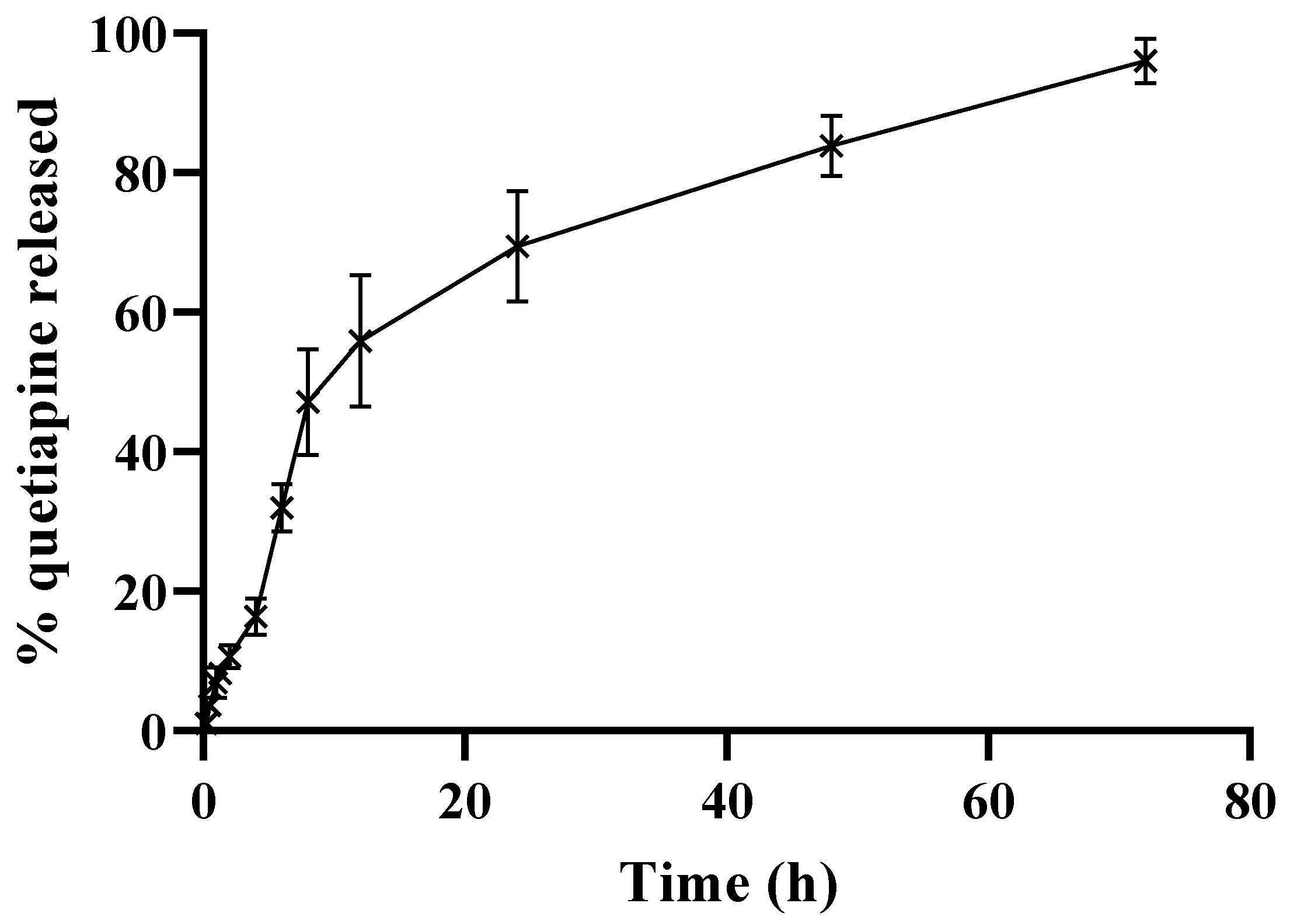
3.4. In Vitro Release of QP from the Selected HSA NPs
3.5. The In Vitro Hemolysis Assay of the Optimized QP-NPs
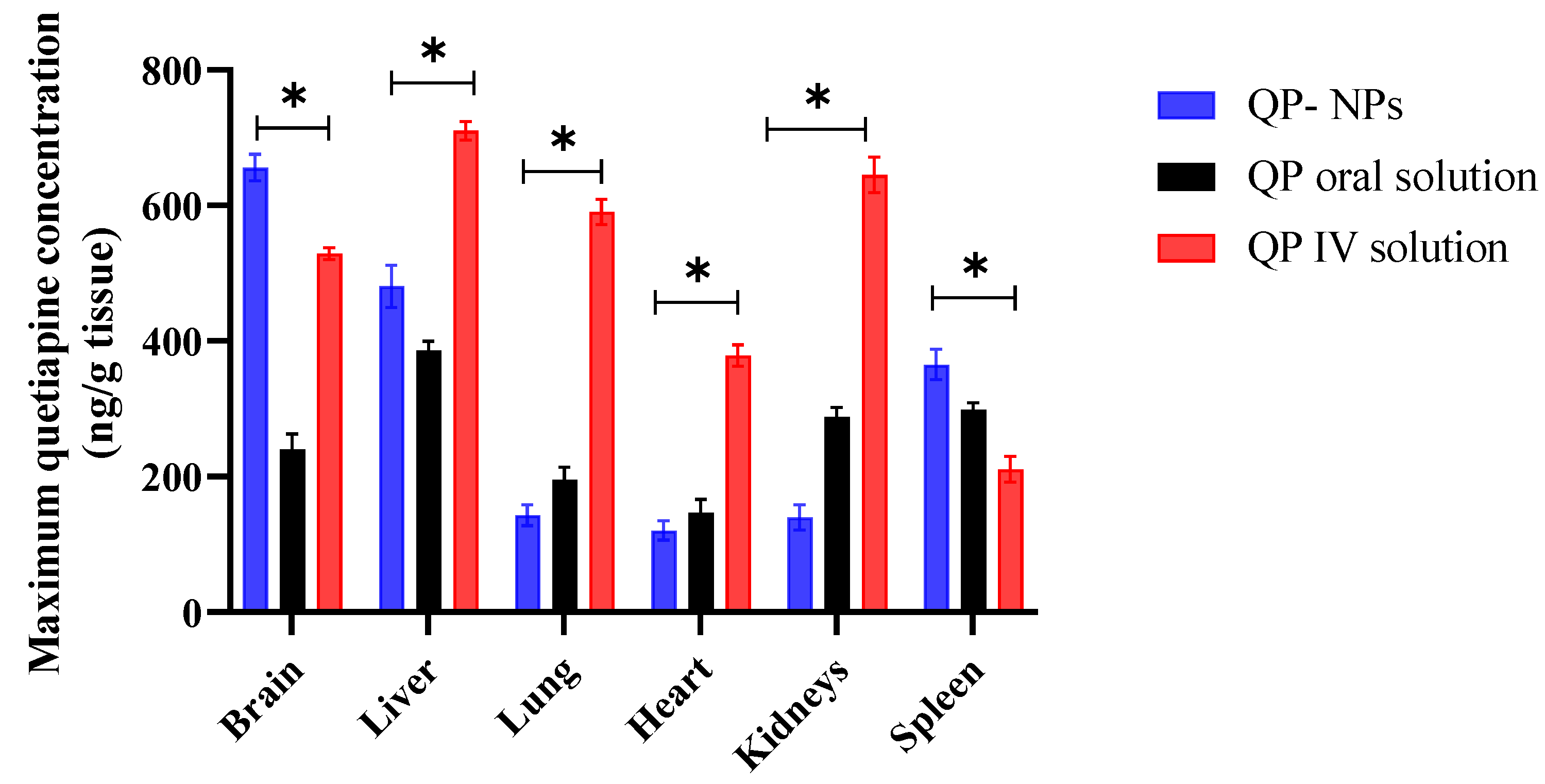
3.6. Pharmacokinetics Study
3.7. Pharmacodynamics Studies
3.7.1. Paw Test
3.7.2. Open Field Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinaldi, R.; Bersani, G.; Marinelli, E.; Zaami, S. The rise of new psychoactive substances and psychiatric implications: A wide-ranging, multifaceted challenge that needs far-reaching common legislative strategies. Hum. Psychopharmacol. Clin. Exp. 2020, 35, e2727. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, Y.; Zaheer, M.R.; Roohi; Iqbal, S.; Iqbal, J. Electron Transfer-Mediated Photodegradation of Phototoxic Antipsychotic Drug Quetiapine. ACS Omega 2021, 6, 30834–30840. [Google Scholar] [CrossRef] [PubMed]
- Akamine, Y.; Yasui-Furukori, N.; Uno, T. Drug-drug interactions of P-gp substrates unrelated to CYP metabolism. Curr. Drug Metab. 2019, 20, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.; Gervase, M.A.; Evans, J.; Deville, R.; Dong, X. The stability of quetiapine oral suspension compounded from commercially available tablets. PLoS ONE 2021, 16, e0255963. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.J. The pharmacology and toxicology of atypical antipsychotic agents. J. Toxicol. Clin. Toxicol. 2001, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.C.; Paletta, S.; Di Pace, C.; Reggiori, A.; Cirnigliaro, G.; Valli, I.; Altamura, A.C. Clinical pharmacokinetics of atypical antipsychotics: An update. Clin. Pharmacokinet. 2018, 57, 1493–1528. [Google Scholar] [CrossRef]
- Gareri, P.; Segura-García, C.; Manfredi, V.G.L.; Bruni, A.; Ciambrone, P.; Cerminara, G.; De Sarro, G.; De Fazio, P. Use of atypical antipsychotics in the elderly: A clinical review. Clin. Interv. Aging 2014, 9, 1363–1373. [Google Scholar]
- Shah, B.; Khunt, D.; Misra, M. Comparative evaluation of intranasally delivered quetiapine loaded mucoadhesive microemulsion and polymeric nanoparticles for brain targeting: Pharmacokinetic and gamma scintigraphy studies. Future J. Pharm. Sci. 2021, 7, 6. [Google Scholar] [CrossRef]
- Catuogno, S.; Esposito, C.; Quintavalle, C.; Condorelli, G.; de Franciscis, V.; Cerchia, L. Nucleic acids in human glioma treatment: Innovative approaches and recent results. J. Signal Transduct. 2012, 2012, 735135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raval, N.; Mistry, T.; Acharya, N.; Acharya, S. Development of glutathione-conjugated asiatic acid-loaded bovine serum albumin nanoparticles for brain-targeted drug delivery. J. Pharm. Pharmacol. 2015, 67, 1503–1511. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Takayama, K.; Ueda, H.; Machida, Y.; Nagai, T. Preparation of bovine serum albumin nanospheres as drug targeting carriers. Drug Des. Deliv. 1987, 2, 99–107. [Google Scholar] [PubMed]
- Elzoghby, A.O.; Abd-Elwakil, M.M.; Abd-Elsalam, K.; Elsayed, M.T.; Hashem, Y.; Mohamed, O. Natural polymeric nanoparticles for brain-targeting: Implications on drug and gene delivery. Curr. Pharm. Des. 2016, 22, 3305–3323. [Google Scholar] [CrossRef] [PubMed]
- Anhorn, M.G.; Wagner, S.; Kreuter, J.; Langer, K.; von Briesen, H. Specific targeting of HER2 overexpressing breast cancer cells with doxorubicin-loaded trastuzumab-modified human serum albumin nanoparticles. Bioconjugate Chem. 2008, 19, 2321–2331. [Google Scholar] [CrossRef]
- Su, Z.; Xing, L.; Chen, Y.; Xu, Y.; Yang, F.; Zhang, C.; Ping, Q.; Xiao, Y. Lactoferrin-modified poly (ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol. Pharm. 2014, 11, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Abul Qais, F.; Ahmad, I.; Alam, P.; Hasan Khan, R.; Naseem, I. Multi-spectroscopic and molecular modelling approach to investigate the interaction of riboflavin with human serum albumin. J. Biomol. Struct. Dyn. 2018, 36, 795–809. [Google Scholar] [CrossRef]
- Rabbani, G.; Lee, E.J.; Ahmad, K.; Baig, M.H.; Choi, I. Binding of tolperisone hydrochloride with human serum albumin: Effects on the conformation, thermodynamics, and activity of HSA. Mol. Pharm. 2018, 15, 1445–1456. [Google Scholar] [CrossRef]
- Ko, S.; Gunasekaran, S. Preparation of sub-100-nm β-lactoglobulin (BLG) nanoparticles. J. Microencapsul. 2006, 23, 887–898. [Google Scholar] [CrossRef]
- Khater, S.E.; El-Khouly, A.; Abdel-Bar, H.M.; Al-Mahallawi, A.M.; Ghorab, D.M. Fluoxetine hydrochloride loaded lipid polymer hybrid nanoparticles showed possible efficiency against SARS-CoV-2 infection. Int. J. Pharm. 2021, 607, 121023. [Google Scholar] [CrossRef]
- Govender, T.; Stolnik, S.; Garnett, M.C.; Illum, L.; Davis, S.S. PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water soluble drug. J. Control Release 1999, 57, 171–185. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, X.Y. Temperature and pH-responsive polymeric composite membranes for controlled delivery of proteins and peptides. Biomaterials 2004, 25, 5281–5291. [Google Scholar] [CrossRef]
- Mandrioli, R.; Fanali, S.; Ferranti, A.; Raggi, M.A. HPLC analysis of the novel antipsychotic drug quetiapine in human plasma. J. Pharm. Biomed. Anal. 2002, 30, 969–977. [Google Scholar] [CrossRef]
- Hamdi, M.; Elmowafy, E.; Abdel-Bar, H.M.; ElKashlan, A.M.; Al-Jamal, K.T.; Awad, G.A.S. Hyaluronic acid-entecavir conjugates-core/lipid-shell nanohybrids for efficient macrophage uptake and hepatotropic prospects. Int. J. Biol. Macromol. 2022, 217, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: Formulation, physicochemical and pharmacokinetic consideration. Eur. J. Pharm. Sci. 2016, 91, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Lee, R.J.; Sun, Y.; Cai, G.; Wang, J.; Wang, M.; Lu, J.; Meng, Q.; Teng, L.; Wang, D.; et al. Cabazitaxel-loaded human serum albumin nanoparticles as a therapeutic agent against prostate cancer. Int. J. Nanomed. 2016, 11, 3451–3459. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Bar, H.M.; Walters, A.A.; Lim, Y.; Rouatbi, N.; Qin, Y.; Gheidari, F.; Han, S.; Osman, R.; Wang, J.T.; Al-Jamal, K.T. An “eat me” combinatory nano-formulation for systemic immunotherapy of solid tumors. Theranostics 2021, 11, 8738–8754. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.M.; Miyakawa, T. Schizophrenia-relevant behavioral testing in rodent models: A uniquely human disorder? Biol. Psychiatry 2006, 59, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Ellenbroek, B.A.; Peeters, B.; Honig, W.; Cools, A. The paw test: A behavioural paradigm for differentiating between classical and atypical neuroleptic drugs. Psychopharmacology 1987, 93, 343–348. [Google Scholar] [CrossRef]
- Abo El-Enin, H.A.; Ahmed, M.F.; Naguib, I.A.; El-Far, S.W.; Ghoneim, M.M.; Alsalahat, I.; Abdel-Bar, H.M. Utilization of Polymeric Micelles as a Lucrative Platform for Efficient Brain Deposition of Olanzapine as an Antischizophrenic Drug via Intranasal Delivery. Pharmaceuticals 2022, 15, 249. [Google Scholar] [CrossRef]
- Pitsikas, N.; Georgiadou, G.; Delis, F.; Antoniou, K. Effects of Anesthetic Ketamine on Anxiety-Like Behaviour in Rats. Neurochem. Res. 2019, 44, 829–838. [Google Scholar] [CrossRef]
- Abdel-Bar, H.M.; Walters, A.A.; Wang, J.T.; Al-Jamal, K.T. Combinatory Delivery of Etoposide and siCD47 in a Lipid Polymer Hybrid Delays Lung Tumor Growth in an Experimental Melanoma Lung Metastatic Model. Adv. Heal. Mater 2021, 10, e2001853. [Google Scholar] [CrossRef]
- Galisteo-González, F.; Molina-Bolívar, J.A. Systematic study on the preparation of BSA nanoparticles. Colloids Surf. B Biointerfaces 2014, 123, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Gajra, B.; Patel, R.R.; Dalwadi, C. Formulation, optimization and characterization of cationic polymeric nanoparticles of mast cell stabilizing agent using the Box-Behnken experimental design. Drug Dev. Ind. Pharm. 2016, 42, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, L.; Xu, C.; Cai, C.; Li, L. Zwitterionic nanocapsules with pH-and thermal-responsiveness for drug-controlled release. Nanotechnology 2023, 34, 155101. [Google Scholar] [CrossRef] [PubMed]
- Katona, G.; Balogh, G.T.; Dargó, G.; Gáspár, R.; Márki, Á.; Ducza, E.; Sztojkov-Ivanov, A.; Tömösi, F.; Kecskeméti, G.; Janáky, T. Development of meloxicam-human serum albumin nanoparticles for nose-to-brain delivery via application of a quality by design approach. Pharmaceutics 2020, 12, 97. [Google Scholar] [CrossRef] [Green Version]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D.; Neufeld, R. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2841. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.-Z.; Noh, H. Brane tachyon dynamics. Phys. Lett. B 2010, 691, 111905. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Du, Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2003, 250, 215–226. [Google Scholar] [CrossRef]
- Hallan, S.S.; Kaur, P.; Kaur, V.; Mishra, N.; Vaidya, B. Lipid polymer hybrid as emerging tool in nanocarriers for oral drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 334–349. [Google Scholar] [CrossRef]
- Jenita Joseph, J.L.; Chockalingam, V.; Barnabas, W. Development, characterization and evaluation of antiretroviral drug—Didanosine loaded serum albumin nanocarriers for an antiretroviral therapy. J. Pharm. Res. 2013, 7, 712–719. [Google Scholar] [CrossRef]
- Bernabeu, E.; Helguera, G.; Legaspi, M.J.; Gonzalez, L.; Hocht, C.; Taira, C.; Chiappetta, D.A. Paclitaxel-loaded PCL–TPGS nanoparticles: In vitro and in vivo performance compared with Abraxane®. Colloids Surf. B Biointerfaces 2014, 113, 43–50. [Google Scholar] [CrossRef]
- Sebak, S.; Mirzaei, M.; Malhotra, M.; Kulamarva, A.; Prakash, S. Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: Preparation and in vitro analysis. Int. J. Nanomed. 2010, 5, 525–532. [Google Scholar]
- Cao, J.; Chen, Y.W.; Wang, X.; Luo, X.L. Enhancing blood compatibility of biodegradable polymers by introducing sulfobetaine. J. Biomed. Mater. Res. Part A 2011, 97, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control Release 2018, 277, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Barbu, E.; Molnàr, É.; Tsibouklis, J.; Górecki, D.C. The potential for nanoparticle-based drug delivery to the brain: Overcoming the blood–brain barrier. Expert Opin. Drug Deliv. 2009, 6, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Werk, A.; Lefeldt, S.; Bruckmueller, H.; Hemmrich-Stanisak, G.; Franke, A.; Roos, M.; Küchle, C.; Steubl, D.; Schmaderer, C.; Bräsen, J. Identification and characterization of a defective CYP3A4 genotype in a kidney transplant patient with severely diminished tacrolimus clearance. Clin. Pharmacol. Ther. 2014, 95, 416–422. [Google Scholar] [CrossRef]
- Nelson, D.R. The Cytochrome P450 Homepage. Hum. Genom. 2009, 4, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Himmerich, H.; Hamilton, A. Mood stabilizers: Side effects, contraindications, and interactions. In NeuroPsychopharmacotherapy; Springer International Publishing: Cham, Switzerland, 2022; pp. 1447–1467. [Google Scholar]
- Gowda, S.M.; Madhupreetha, S.; Kg, V.K. Quetiapine for the Management of Tardive Dyskinesia in Schizoaffective Disorder Comorbid With Diabetes Mellitus and Chronic Kidney Disease. Prim. Care Companion CNS Disord. 2021, 23, 25969. [Google Scholar] [CrossRef]
- Yamada, H.; Katsumori, Y.; Kawano, M.; Mori, S.; Takeshige, R.; Mukai, J.; Imada, H.; Shimoura, H.; Takahashi, H.; Horai, T. Quetiapine-related acute kidney injury requiring transient continuous hemodiafiltration. Intern. Med. 2018, 57, 1763–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, M.; Shirakawa, K.; Matsuoka, N.; Mutoh, S. Combined treatment of quetiapine with haloperidol in animal models of antipsychotic effect and extrapyramidal side effects: Comparison with risperidone and chlorpromazine. Psychopharmacology 2004, 176, 94–100. [Google Scholar] [CrossRef]
- Ellenbroek, B. Treatment of schizophrenia: A clinical and preclinical evaluation of neuroleptic drugs. Pharmacol. Ther. 1993, 57, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Saller, C.F.; Salama, A.I. Seroquel: Biochemical profile of a potential atypical antipsychotic. Psychopharmacology 1993, 112, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.E.; Bruhwyler, J.; Delarge, J.; Géczy, J.; Liégeois, J.-F. The behavioral effects of acute and chronic JL 13, a putative antipsychotic, in Cebus non-human primates. Psychopharmacology 2001, 157, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Cools, A.R. The PAW test: An animal model for neuroleptic drugs which fulfils the criteria for pharmacological isomorphism. Life Sci. 1988, 42, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ampatzis, K.; Björnfors, E.R.; El Manira, A. Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature 2016, 529, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gallegos, D.A.; Pogorelov, V.M.; O’Hare, J.K.; Calakos, N.; Wetsel, W.C.; West, A.E. Parvalbumin interneurons of the mouse nucleus accumbens are required for amphetamine-induced locomotor sensitization and conditioned place preference. Neuropsychopharmacology 2018, 43, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochiai, Y.; Fujita, M.; Hagino, Y.; Kobayashi, K.; Okiyama, R.; Takahashi, K.; Ikeda, K. Therapeutic Effects of Quetiapine and 5-HT1A Receptor Agonism on Hyperactivity in Dopamine-Deficient Mice. Int. J. Mol. Sci. 2022, 23, 7436. [Google Scholar] [CrossRef]




| Critical Process Parameters (Coded Independent Variables) | Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (1) | |
| A: HSA concentration (mg/mL) | 5 | 15 | 25 |
| B: pH | 7 | 8 | 9 |
| C: Stirring time (h) | 2 | 5 | 8 |
| Critical Quality attributes (Responses) | Quality target product profile (Constrains) | ||
| Y1: Particle size (nm) | Minimum | ||
| Y2: Entrapment efficiency EE (%) | Maximize | ||
| Run | Critical Process Parameters (CPPs) | Critical Quality Attributes (CQAs) | |||
|---|---|---|---|---|---|
| A: HSA Concentration (mg/mL) | B: pH | C: Stirring Time (h) | Particle Size (nm) a,c | EE% b,c | |
| 1 | 5 | 7 | 8 | 153.2 ± 3.25 | 70.12 ± 1.25 |
| 2 | 15 | 8 | 5 | 98.5 ± 2.5 | 90.05 ± 2.35 |
| 3 | 5 | 8 | 8 | 82.41 ± 3.21 | 72.55 ± 1.45 |
| 4 | 15 | 8 | 5 | 98.3 ± 1.25 | 91 ± 2.65 |
| 5 | 25 | 7 | 2 | 274 ± 3.47 | 89.78 ± 3.45 |
| 6 | 15 | 8 | 5 | 101.74 ± 2.65 | 92.47 ± 2.47 |
| 7 | 15 | 8 | 5 | 95.3 ± 4.98 | 93.78 ± 2.36 |
| 8 | 15 | 9 | 2 | 115.2 ± 5.11 | 90.06 ± 2.14 |
| 9 | 25 | 7 | 8 | 205.4 ± 6.78 | 90.78 ± 3.45 |
| 10 | 5 | 7 | 2 | 230.7 ± 8.45 | 60.78 ± 2.54 |
| 11 | 5 | 8 | 2 | 105.41 ± 3.21 | 67.71 ± 1.45 |
| 12 | 15 | 8 | 8 | 132.78 ± 2.15 | 90.03 ± 2.65 |
| 13 | 25 | 8 | 2 | 151 ± 1.36 | 88.73 ± 3.56 |
| 14 | 15 | 9 | 8 | 125.94 ± 2.51 | 91.45 ± 2.78 |
| 15 | 15 | 8 | 5 | 100.5 ± 2.41 | 89.97 ± 3.45 |
| 16 | 15 | 8 | 5 | 105 ± 2.54 | 90.45 ± 2.45 |
| 17 | 15 | 9 | 5 | 120.5 ± 2.36 | 93.65 ± 2.14 |
| 18 | 25 | 8 | 5 | 112 ± 3.14 | 94 ± 3.24 |
| 19 | 5 | 7 | 5 | 196 ± 2.45 | 65.89 ± 2.45 |
| 20 | 25 | 8 | 8 | 142.65 ± 3.14 | 95.78 ± 1.47 |
| 21 | 15 | 7 | 2 | 240 ± 2.45 | 87.65 ± 3.24 |
| 22 | 25 | 9 | 2 | 132.87 ± 2.54 | 94.78 ± 2.54 |
| 23 | 15 | 7 | 5 | 210.6 ± 2.35 | 88.34 ± 2.45 |
| 24 | 15 | 8 | 2 | 125.78 ± 3.14 | 88.9 ± 3.24 |
| 25 | 25 | 9 | 5 | 97.8 ± 2.45 | 92.47 ± 2.87 |
| 26 | 15 | 7 | 8 | 170 ± 2.14 | 90.79 ± 3.47 |
| 27 | 5 | 9 | 8 | 120 ± 1.45 | 80.79 ± 3.15 |
| 28 | 25 | 9 | 8 | 135.69 ± 3.65 | 93.23 ± 2.84 |
| 29 | 25 | 7 | 5 | 254.87 ± 4.12 | 92 ± 3.78 |
| 30 | 5 | 8 | 5 | 81.98 ± 3.25 | 78 ± 4.15 |
| 31 | 5 | 9 | 5 | 95.68 ± 2.45 | 80.48 ± 1.25 |
| 32 | 5 | 9 | 2 | 80.98 ± 3.12 | 78.94 ± 3.12 |
| HSA Concentration (mg/mL) | pH | Stirring Time (h) | Particle Size (nm) a,e | PDI a,c | Zeta Potential (mV) b,e | EE% c,e | LE % d,e |
|---|---|---|---|---|---|---|---|
| 18 | 8.6 | 5 | 103.54 ± 2.36 | 0.152 ± 0.005 | −21.35 ± 1.54 | 96.32 ± 3.98 | 7.11 ± 0.32 |
| Parameter | Plasma | Brain | ||||
|---|---|---|---|---|---|---|
| QP-NPs | IV QP Solution | Oral QP Solution | QP-NPs | IV QP Solution | Oral QP Solution | |
| Cmax (ng/mL) | - | - | 410.62 ± 17.51 | 655.93 ± 19.53 | 528.83 ± 8.77 | 310.62 ± 23.67 |
| Tmax (h) | - | - | 0.5 | 2 | 1 | 4 |
| AUC0–24h (µg/mL.h) | 7.48 | 5.58 | 1.81 | 7.24 | 1.87 | 1.72 |
| AUC0–∞ (µg/mL.h) | 9.05 | 6.36 | 1.87 | 8.94 | 1.96 | 1.82 |
| MRT (h) | 10.15 | 8.71 | 5.97 | 11.25 | 7.12 | 7.4 |
| Kel (h−1) | 0.08 | 0.09 | 0.14 | 0.076 | 0.14 | 0.13 |
| Absolute bioavailability % | 141.5 | 100 | 29.4 | - | - | - |
| Relative bioavailability % | 483.95 | 340.11 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Bar, H.M.; Tulbah, A.S.; Darwish, H.W.; Salama, R.; Naguib, I.A.; Yassin, H.A.; Abo El-Enin, H.A. Quetiapine Albumin Nanoparticles as an Efficacious Platform for Brain Deposition and Potentially Improved Antipsychotic Activity. Pharmaceutics 2023, 15, 1785. https://doi.org/10.3390/pharmaceutics15071785
Abdel-Bar HM, Tulbah AS, Darwish HW, Salama R, Naguib IA, Yassin HA, Abo El-Enin HA. Quetiapine Albumin Nanoparticles as an Efficacious Platform for Brain Deposition and Potentially Improved Antipsychotic Activity. Pharmaceutics. 2023; 15(7):1785. https://doi.org/10.3390/pharmaceutics15071785
Chicago/Turabian StyleAbdel-Bar, Hend Mohamed, Alaa S. Tulbah, Hany W. Darwish, Rania Salama, Ibrahim A. Naguib, Heba A. Yassin, and Hadel A. Abo El-Enin. 2023. "Quetiapine Albumin Nanoparticles as an Efficacious Platform for Brain Deposition and Potentially Improved Antipsychotic Activity" Pharmaceutics 15, no. 7: 1785. https://doi.org/10.3390/pharmaceutics15071785






