Iron-Gallic Acid Peptide Nanoparticles as a Versatile Platform for Cellular Delivery with Synergistic ROS Enhancement Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 3,4,5-tri-O-(tert-butoxycarbonyl)-gallic Acid
2.2. General Synthesis of Peptides
2.3. Synthesis of Iron–Gallic Acid Peptide Nanoparticles (IGPNs)
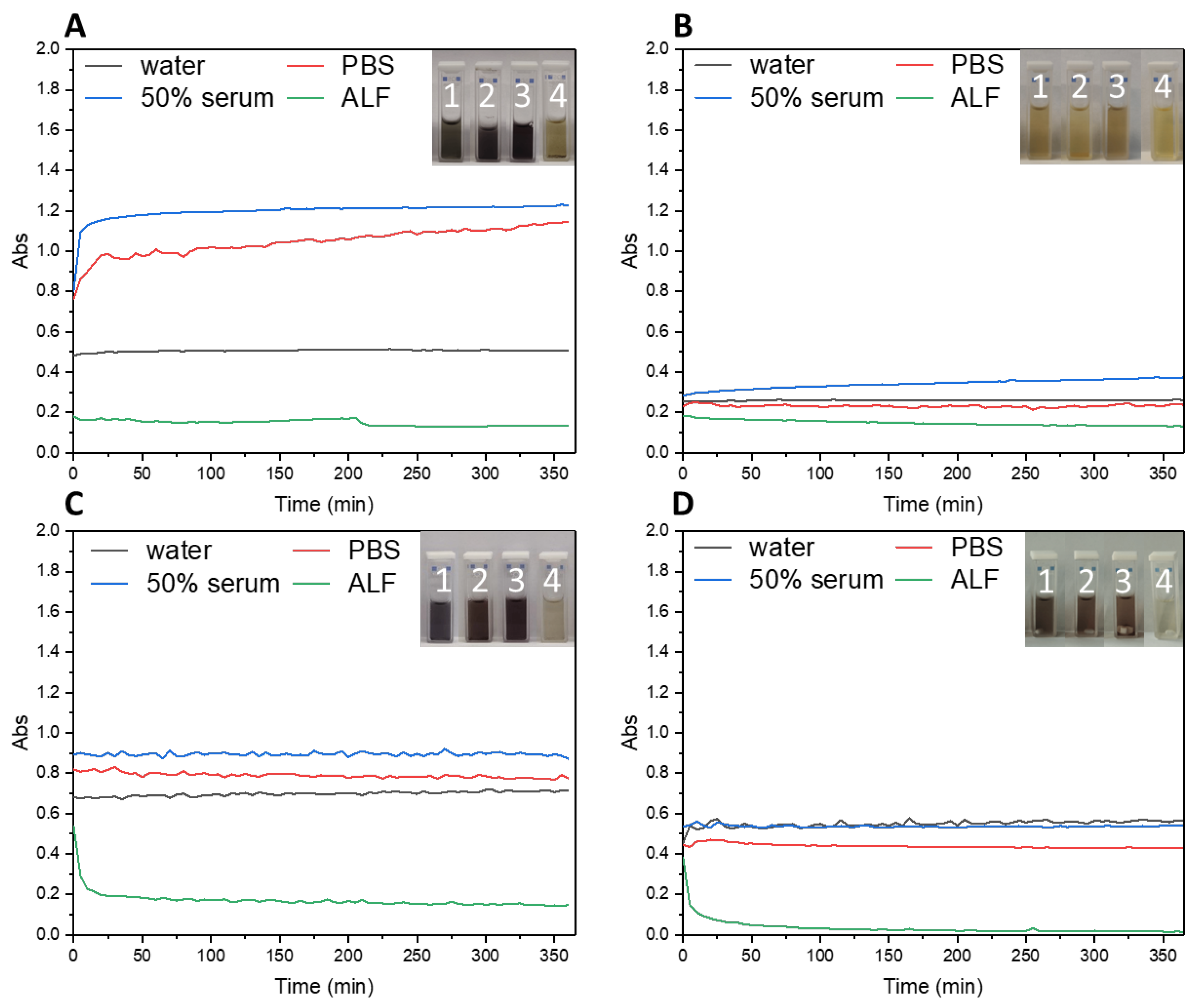
2.4. Investigation of the Stability of IGPNs in Different Media
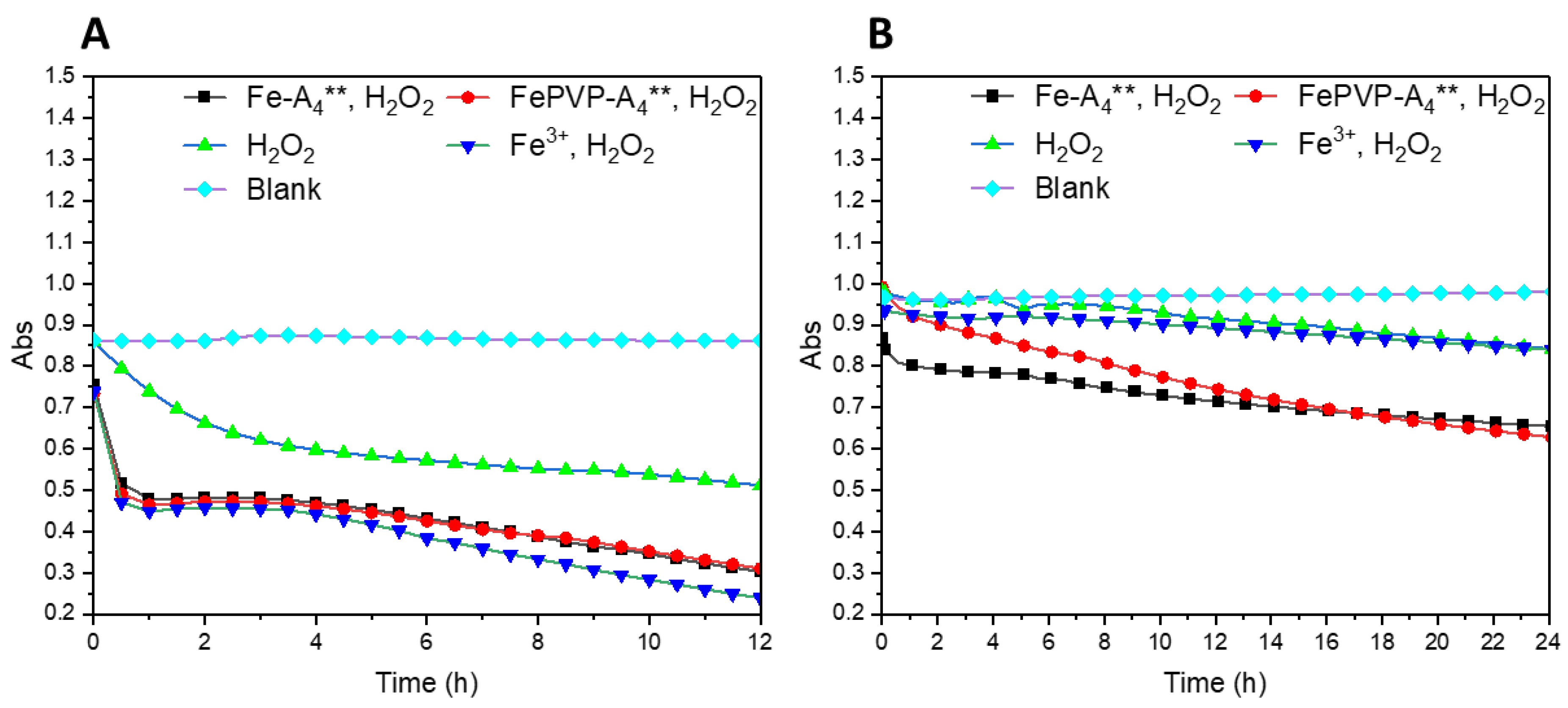
2.5. Methylene Blue Assay
2.6. Cell Culture
2.7. Evaluation of ROS Generation by Flow Cytometry
2.8. Evaluation of ROS Generation by Confocal Laser Scanning Microscopy (CLSM)
2.9. Evaluation of Cellular Uptake by Flow Cytometry
2.10. Evaluation of Cellular Uptake by Confocal Laser Scanning Microscopy (CLSM)
2.11. Evaluation of Cell Viability (CellTiter-Glo Assay)
2.12. Apoptosis Assay
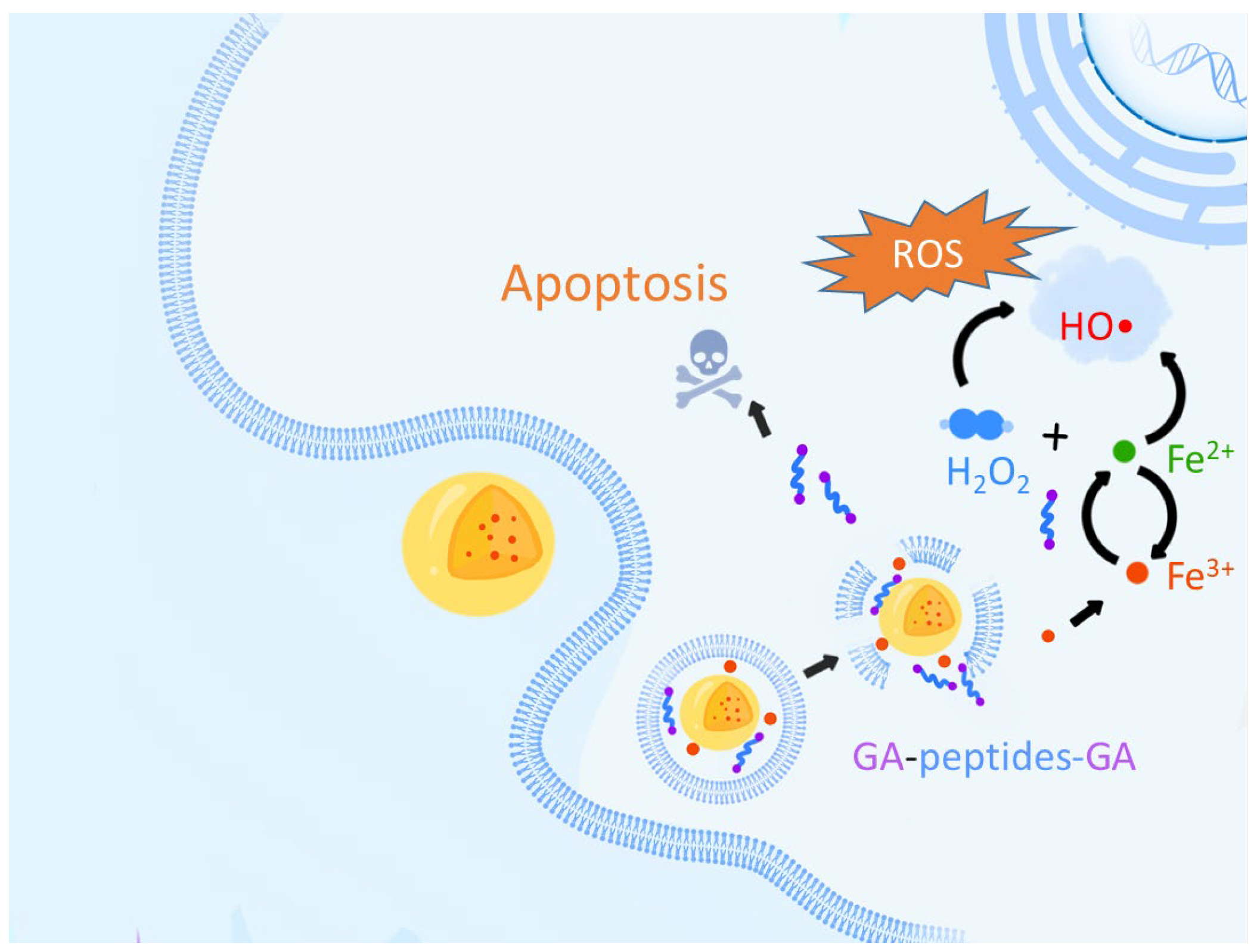
3. Results and Discussion
3.1. Synthesis of Gallic Acid-Tagged Peptides
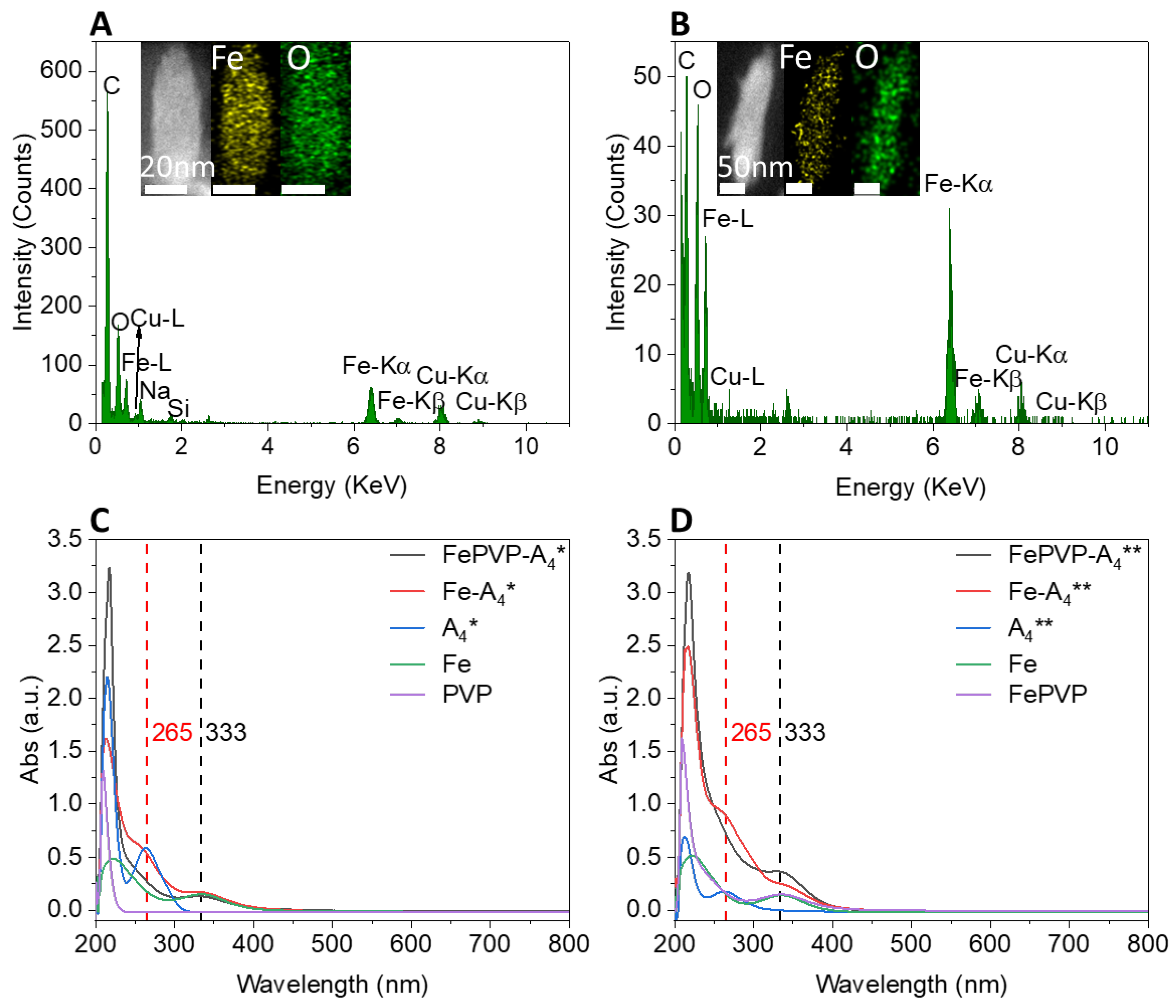
3.2. Synthesis and Characterization of Iron–Gallic Acid Nanoparticles (IGPNs)
3.3. Cellular Uptake of IGPNs
3.4. ROS Production of IGPNs
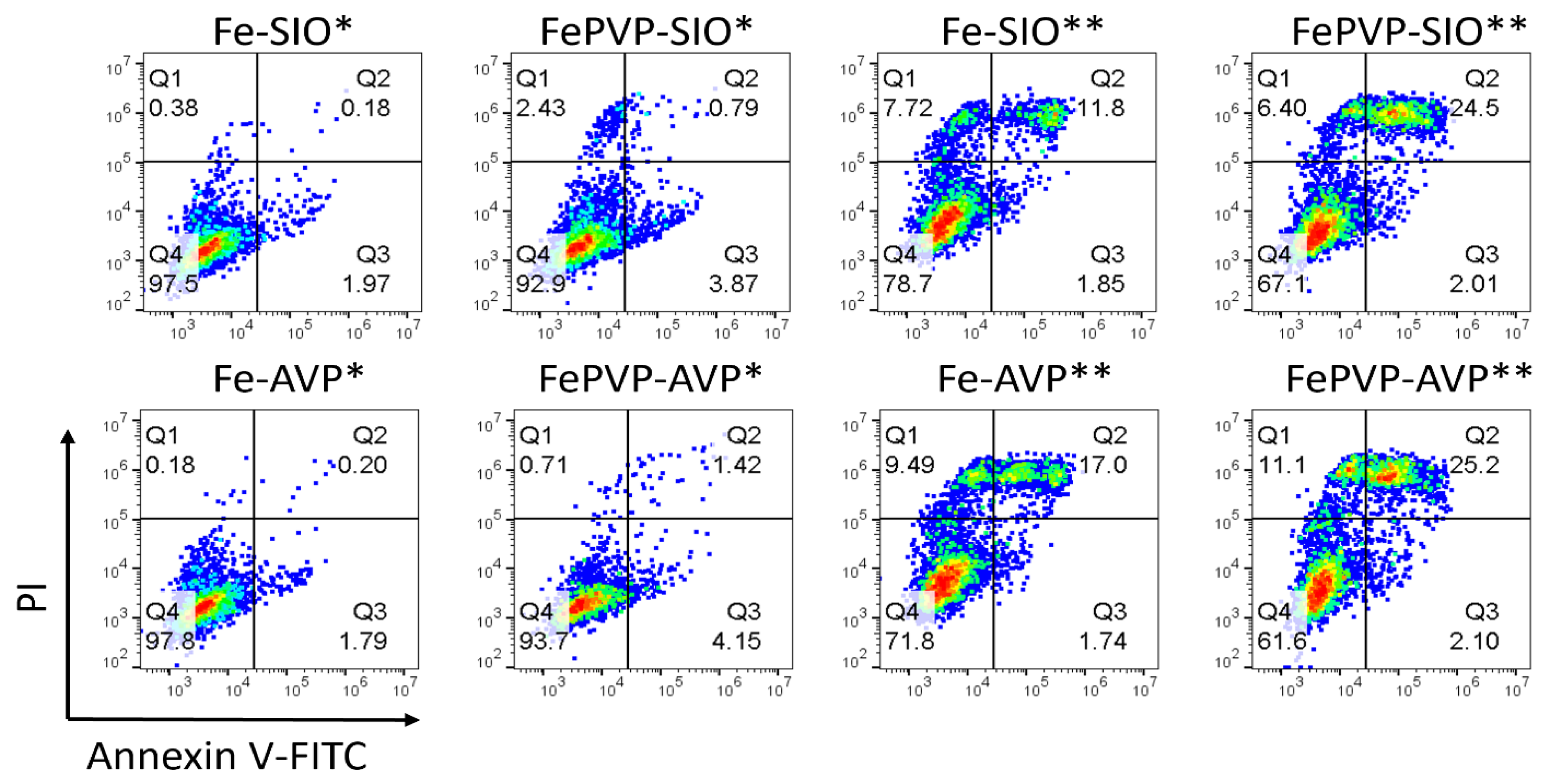
3.5. Bioactivity of IGPNs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holz, E.; Darwish, M.; Tesar, D.B.; Shatz-Binder, W. A Review of Protein- and Peptide-Based Chemical Conjugates: Past, Present, and Future. Pharmaceutics 2023, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.M.; Iegre, J.; O’Donovan, D.H.; Ölwegård Halvarsson, M.; Spring, D.R. Peptides as a Platform for Targeted Therapeutics for Cancer: Peptide-Drug Conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Acar, H.; Ting, J.M.; Srivastava, S.; LaBelle, J.L.; Tirrell, M.V. Molecular Engineering Solutions for Therapeutic Peptide Delivery. Chem. Soc. Rev. 2017, 46, 6553–6569. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Rong, G.; Wang, C.; Chen, L.; Yan, Y.; Cheng, Y. Fluoroalkylation Promotes Cytosolic Peptide Delivery. Sci. Adv. 2020, 6, eaaz1774. [Google Scholar] [CrossRef]
- Soudy, R.; Kimura, R.; Patel, A.; Fu, W.; Kaur, K.; Westaway, D.; Yang, J.; Jhamandas, J. Short Amylin Receptor Antagonist Peptides Improve Memory Deficits in Alzheimer’s Disease Mouse Model. Sci. Rep. 2019, 9, 10942. [Google Scholar] [CrossRef] [Green Version]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, F.; Ji, Y.; Yin, L. Recent Advances in Anti-Cancer Protein/Peptide Delivery. Bioconjug. Chem. 2019, 30, 305–324. [Google Scholar] [CrossRef]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef]
- Wolfe, J.M.; Fadzen, C.M.; Holden, R.L.; Yao, M.; Hanson, G.J.; Pentelute, B.L. Perfluoroaryl Bicyclic Cell-Penetrating Peptides for Delivery of Antisense Oligonucleotides. Angew. Chemie 2018, 130, 4846–4849. [Google Scholar] [CrossRef] [Green Version]
- Röder, R.; Preiß, T.; Hirschle, P.; Steinborn, B.; Zimpel, A.; Höhn, M.; Rädler, J.O.; Bein, T.; Wagner, E.; Wuttke, S.; et al. Multifunctional Nanoparticles by Coordinative Self-Assembly of His-Tagged Units with Metal-Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 2359–2368. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Sánchez, J.; Argente-García, A.I.; Moliner-Martínez, Y.; Roca-Sanjuán, D.; Antypov, D.; Campíns-Falcó, P.; Rosseinsky, M.J.; Martí-Gastaldo, C. Peptide Metal-Organic Frameworks for Enantioselective Separation of Chiral Drugs. J. Am. Chem. Soc. 2017, 139, 4294–4297. [Google Scholar] [CrossRef] [Green Version]
- Martí-Gastaldo, C.; Warren, J.E.; Stylianou, K.C.; Flack, N.L.O.; Rosseinsky, M.J. Enhanced Stability in Rigid Peptide-Based Porous Materials. Angew. Chem.-Int. Ed. 2012, 51, 11044–11048. [Google Scholar] [CrossRef]
- Katsoulidis, A.P.; Park, K.S.; Antypov, D.; Martí-Gastaldo, C.; Miller, G.J.; Warren, J.E.; Robertson, C.M.; Blanc, F.; Darling, G.R.; Berry, N.G.; et al. Guest-Adaptable and Water-Stable Peptide-Based Porous Materials by Imidazolate Side Chain Control. Angew. Chem.-Int. Ed. 2014, 53, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Lv, J.; Wang, H.; Cheng, Y. A Coordinative Dendrimer Achieves Excellent Efficiency in Cytosolic Protein and Peptide Delivery. Angew. Chem.-Int. Ed. 2020, 59, 4711–4719. [Google Scholar] [CrossRef]
- Yu, X.; Gou, X.; Wu, P.; Han, L.; Tian, D.; Du, F.; Chen, Z.; Liu, F.; Deng, G.; Chen, A.T.; et al. Activatable Protein Nanoparticles for Targeted Delivery of Therapeutic Peptides. Adv. Mater. 2018, 30, 1705383. [Google Scholar] [CrossRef]
- Suma, T.; Cui, J.; Müllner, M.; Fu, S.; Tran, J.; Noi, K.F.; Ju, Y.; Caruso, F. Modulated Fragmentation of Proapoptotic Peptide Nanoparticles Regulates Cytotoxicity. J. Am. Chem. Soc. 2017, 139, 4009–4018. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Liu, M.; Yang, H.; Nezamzadeh-Ejhieh, A.; Lu, C.; Pan, Y.; Liu, J.; Bai, Z. Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications. Pharmaceutics 2023, 15, 1323. [Google Scholar] [CrossRef]
- Geng, H.; Zhong, Q.Z.; Li, J.; Lin, Z.; Cui, J.; Caruso, F.; Hao, J. Metal Ion-Directed Functional Metal-Phenolic Materials. Chem. Rev. 2022, 122, 11432–11473. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, Y.; Zhang, G.; Li, X.; Ni, S.; Cui, J. Targeted Delivery of Fenton Reaction Packages and Drugs for Cancer Theranostics. Appl. Mater. Today 2022, 26, 101353. [Google Scholar] [CrossRef]
- Qin, J.; Liang, G.; Cheng, D.; Liu, Y.; Cheng, X.; Yang, P.; Wu, N.; Zhao, Y.; Wei, J. Controllable Synthesis of Iron-Polyphenol Colloidal Nanoparticles with Composition-Dependent Photothermal Performance. J. Colloid Interface Sci. 2021, 593, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, Y.; Liu, X.; Zhou, Q.; Piao, Y.; Shao, S.; Tang, J.; Zhou, Z.; Xie, T.; Shen, Y. Natural Polyphenols-Platinum Nanocomplexes Stimulate Immune System for Combination Cancer Therapy. Nano Lett. 2022, 22, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, O.; Alivand, M.S.; Zavabeti, A.; Spoljaric, S.; Pan, S.; Chen, D.; Caruso, F.; Suter, H.C.; Mumford, K.A. Assembly of Metal–Phenolic Networks on Water-Soluble Substrates in Nonaqueous Media. Adv. Funct. Mater. 2022, 32, 2111942. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Lin, Y.-C.; Yen, G.-C.; Chen, H.-Y. Preventive Effects of Guava (Psidium guajava L.) Leaves and Its Active Compounds against α-Dicarbonyl Compounds-Induced Blood Coagulation. Food Chem. 2007, 103, 528–535. [Google Scholar] [CrossRef]
- De Mejía, E.G.; Chandra, S.; Ramírez-Mares, M.V.; Wang, W. Catalytic Inhibition of Human DNA Topoisomerase by Phenolic Compounds in Ardisia Compressa Extracts and Their Effect on Human Colon Cancer Cells. Food Chem. Toxicol. 2006, 44, 1191–1203. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic Contents and Antioxidant Activities of Bitter Gourd (Momordica charantia L.) Leaf, Stem and Fruit Fraction Extracts in Vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Chang, S.S.; Lee, V.S.Y.; Tseng, Y.L.; Chang, K.C.; Chen, K.B.; Chen, Y.L.; Li, C.Y. Gallic Acid Attenuates Platelet Activation and Platelet-Leukocyte Aggregation: Involving Pathways of Akt and GSK3β. Evid.-Based Complement. Altern. Med. 2012, 2012, 683872. [Google Scholar] [CrossRef] [Green Version]
- Uozaki, M.; Yamasaki, H.; Katsuyama, Y.; Higuchi, M.; Higuti, T.; Koyama, A.H. Antiviral Effect of Octyl Gallate against DNA and RNA Viruses. Antivir. Res. 2007, 73, 85–91. [Google Scholar] [CrossRef]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of Iron with Phenolic Compounds from Soybean Nodules and Other Legume Tissues: Prooxidant and Antioxidant Properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Almeida, N.; González, A.G.; Santana-Casiano, J.M.; González-Dávila, M. Ocean Acidification Effect on the Iron-Gallic Acid Redox Interaction in Seawater. Front. Mar. Sci. 2022, 9, 837363. [Google Scholar] [CrossRef]
- Ma, P.; Xiao, H.; Yu, C.; Liu, J.; Cheng, Z.; Song, H.; Zhang, X.; Li, C.; Wang, J.; Gu, Z.; et al. Enhanced Cisplatin Chemotherapy by Iron Oxide Nanocarrier-Mediated Generation of Highly Toxic Reactive Oxygen Species. Nano Lett. 2017, 17, 928–937. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Nakamura, Y. Reactive Oxygen Species and Angiogenesis NADPH Oxidase As. Cancer Lett. 2008, 266, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Stockwell, B.R. The Role of Iron and Reactive Oxygen Species in Cell Death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application to Protect Brain from Injury in Ischemic Stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew. Chem.-Int. Ed. 2016, 55, 2101–2106. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.; Zhang, M.; Yu, W.; Gao, F.; Li, C.; Wang, S.B.; Feng, J.; Zhang, X.Z. Ferrous-Supply-Regeneration Nanoengineering for Cancer-Cell-Specific Ferroptosis in Combination with Imaging-Guided Photodynamic Therapy. ACS Nano 2018, 12, 12181–12192. [Google Scholar] [CrossRef]
- Biessen, E.A.L.; Appeldoorn, C.C.M.; Bonnefoy, A.; van Berkel, T.J.C.; Johan Kuiper, M.F.H. Polyhydroxy Phenols and Their Use in Bindng P-Selectin. U.S. Patent US 7,273,951 B2, 25 September 2007. [Google Scholar]
- Yang, L.; Mashima, T.; Sato, S.; Mochizuki, M.; Sakamoto, H.; Yamori, T.; Oh-hara, T.; Tsuruo, T. Predominant Suppression of Apoptosome by Inhibitor of Apoptosis Protein in Non-Small Cell Lung Cancer H460 Cells: Therapeutic Effect of a Novel Polyarginine-Conjugated Smac Peptide. Cancer Res. 2003, 63, 831–837. [Google Scholar]
- Zhang, Z.; Sun, L.; Zhou, G.; Xie, P.; Ye, J. Sepia Ink Oligopeptide Induces Apoptosis and Growth Inhibition in Human Lung Cancer Cells. Oncotarget 2017, 8, 23202–23212. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Yang, Z.; Cheng, S.; Wang, Z.; Zhang, R.; Zhu, G.; Wang, Z.; Yung, B.C.; Tian, R.; Jacobson, O.; et al. Toxic Reactive Oxygen Species Enhanced Synergistic Combination Therapy by Self-Assembled Metal-Phenolic Network Nanoparticles. Adv. Mater. 2018, 30, 1704877. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tian, S.; Ning, P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, S.S.; Li, C.X.; Xu, L.; Cheng, H.; Zhang, X.Z. An Adenosine Triphosphate-Responsive Autocatalytic Fenton Nanoparticle for Tumor Ablation with Self-Supplied H2O2 and Acceleration of Fe(III)/Fe(II) Conversion. Nano Lett. 2018, 18, 7609–7618. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhu, W.; Jiang, D.; Zhang, R.; Kutyreff, C.J.; Engle, J.W.; Huang, P.; Cai, W.; Liu, Z.; Cheng, L. Ultra-Small Iron-Gallic Acid Coordination Polymer Nanoparticles for Chelator-Free Labeling of 64Cu and Multimodal Imaging-Guided Photothermal Therapy. Nanoscale 2017, 9, 12609–12617. [Google Scholar] [CrossRef] [PubMed]
- Fazary, A.E.; Taha, M.; Ju, Y.H. Iron Complexation Studies of Gallic Acid. J. Chem. Eng. Data 2009, 54, 35–42. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Stopford, W.; Turner, J.; Cappellini, D.; Brock, T. Bioaccessibility Testing of Cobalt Compounds. J. Environ. Monit. 2003, 5, 675–680. [Google Scholar] [CrossRef]






| Code | Sequence a | Molecular Weight (Da) | Function |
|---|---|---|---|
| K * K ** | K(GA) GA-K(GA) | 298.3 450.0 | minimal peptide motif |
| A4 A4 * A4 ** | AAAA AAAAK(GA) K(GA)AAAAK(GA) | 300.2 582.6 862.4 | neutral model peptides |
| (KA)2 (KA)2 * (KA)2 ** | KAKA KAKAK(GA) K(GA)KAKAK(GA) | 416.3 696.8 977.1 | cationic model peptides |
| (EA)2 (EA)2 * (EA)2 ** | EAEA EAEAK(GA) K(GA)EAEAK(GA) | 418.4 698.7 979.0 | anionic model peptides |
| SIO SIO * SIO ** | QPK QPK(GA) K(GA)QPK(GA) | 371.4 523.5 803.8 | pro-apoptotic SIO peptides |
| AVP AVP * AVP ** | AVPIAQK AVPIAQK(GA) K(GA)AVPIAQK(GA) | 725.9 878.0 1158.3 | pro-apoptotic AVP peptides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, F.; Lin, Y.; Höhn, M.; Luo, X.; Döblinger, M.; Wagner, E.; Lächelt, U. Iron-Gallic Acid Peptide Nanoparticles as a Versatile Platform for Cellular Delivery with Synergistic ROS Enhancement Effect. Pharmaceutics 2023, 15, 1789. https://doi.org/10.3390/pharmaceutics15071789
Shen F, Lin Y, Höhn M, Luo X, Döblinger M, Wagner E, Lächelt U. Iron-Gallic Acid Peptide Nanoparticles as a Versatile Platform for Cellular Delivery with Synergistic ROS Enhancement Effect. Pharmaceutics. 2023; 15(7):1789. https://doi.org/10.3390/pharmaceutics15071789
Chicago/Turabian StyleShen, Faqian, Yi Lin, Miriam Höhn, Xianjin Luo, Markus Döblinger, Ernst Wagner, and Ulrich Lächelt. 2023. "Iron-Gallic Acid Peptide Nanoparticles as a Versatile Platform for Cellular Delivery with Synergistic ROS Enhancement Effect" Pharmaceutics 15, no. 7: 1789. https://doi.org/10.3390/pharmaceutics15071789





